Summary
A 67-year-old man with medically refractory vertebrobasilar insufficiency and short segment occlusions of the intracranial vertebral arteries was treated with angioplasty and stent placement. Fifteen hours after the procedure the patient developed symptoms of posterior fossa ischemia and repeat angiography showed thrombus formation within the stent which was treated with thrombolytic and aggressive antiplatelet therapy. Angiography revealed lysis of the clot, but concerns regarding the mechanism of the thrombotic phenomenon prompted frequency-domain optical coherence tomography (FDOCT) assessment. FDOCT provided excellent visualization of the stent and vessel wall interactions, as well as excluding residual flow-limiting stenosis, obviating the need for further intervention. The potential utility of FDOCT in the evaluation of intracranial atherosclerotic disease and additional intracranial applications are discussed.
Keywords: Atherosclerosis, Catheter, Stent, Technology, Vessel Wall
Background
Intravascular frequency-domain optical coherence tomography (FDOCT), a relatively new light-based imaging modality approved by the FDA for use in the coronary vasculature, delivers the highest resolution (10–15 µm) among currently available imaging techniques. While FDOCT accurately evaluates vessel wall structure and lumen (ie, stenosis severity), its unique axial resolution coupled with sharp delineation of lumen borders enables unprecedented assessments of vessel dissections, tissue prolapse, and stent–vessel interactions.1 Besides its use in the coronary vasculature, FDOCT assessment of the carotid arteries has recently been described,2––4 opening a new avenue for expanding its indications to other vascular territories. We report the novel use of FDOCT for the evaluation of periprocedural neurologic deficit following intracranial angioplasty and stent placement for medically refractory vertebrobasilar insufficiency. The potential utility of FDOCT in evaluating recurrent/residual stenosis, guiding further treatment, and additional intracranial applications will be discussed.
Case presentation
A 67-year-old man presented with medically refractory vertebrobasilar insufficiency and repetitive ‘drop attacks’ upon standing and walking. The patient was symptomatic despite dual antiplatelet therapy with clopidigrel and aspirin (PRU 133) and anticoagulation therapy (rivaroxaban for asymptomatic atrial fibrillation). Digital subtraction angiography showed short segment occlusions of the bilateral intracranial vertebral arteries with tiny collateral vessels reconstituting the basilar artery (figure 1).
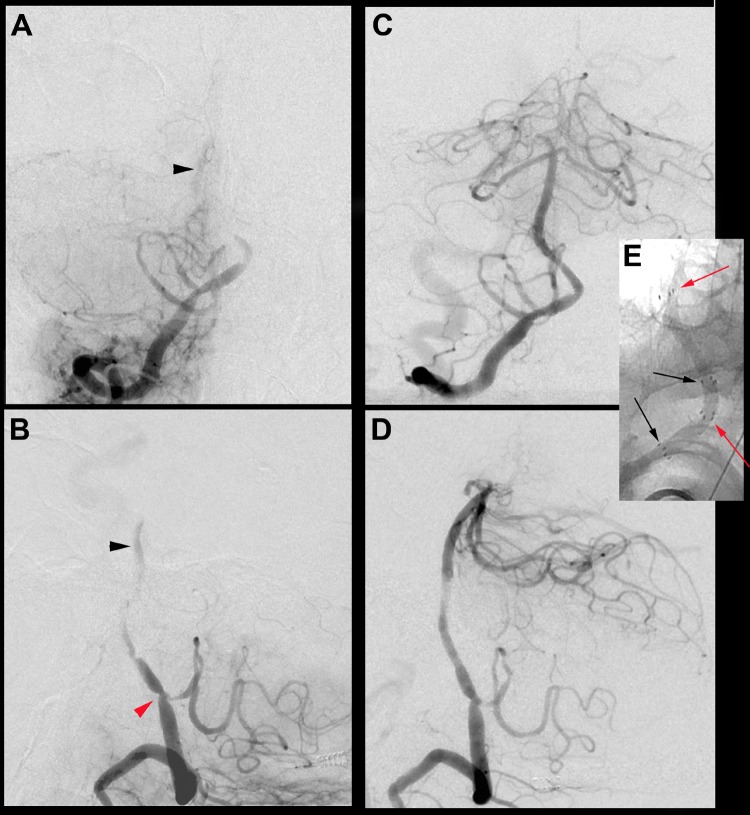
Figure 1.
Anterior-posterior (A) and lateral (B) projections from the digital subtraction angiogram (DSA) showing short segment occlusion of the right vertebral artery at the vertebrobasilar junction with only faint opacification of the basilar artery (black arrowheads). There is a lesser degree of stenosis present at the level of the posterior inferior cerebellar artery (PICA) (red arrowhead). Post-intervention DSA in the anterior-posterior (C) and lateral (D) projections showing restoration of near normal lumen and flow within the right vertebrobasilar system following angioplasty/stent placement. The lesser stenosis at the level of the PICA was treated solely with balloon angioplasty. Unsubtracted anterior-posterior magnified view (E) showing overlapping segments of the proximal (black arrows) and distal (red arrows) stents, visible by the four radiopaque markers on the proximal/distal aspects of the stents.
Treatment
Revascularization of the right vertebral artery and vertebrobasilar system was achieved with angioplasty and stent placement using overlapping 3.5 mm Wingspan stents (Target Therapeutics, Fremont, California, USA) (figure 1). Overlapping stents were deemed necessary to cover the entire segment of previously occluded artery and maintain patency. The patient awoke from anesthesia and was without new deficit, but 15 h after the procedure he developed sudden onset of right-sided weakness, nystagmus, and respiratory failure. Repeat angiography showed non-occlusive thrombus within the proximal stent (figure 2). Intra-arterial tissue plasminogen activator (2 mg) was administered via microcatheter followed by weight-based single bolus (180 μg/kg) and maintenance intravenous eptifibatide. Angiography showed lysis of the thrombus, but concerns regarding the mechanisms that potentially led to the thrombotic phenomenon remained unclear. Although residual stenosis within the proximal segment of the stented region or an area of stenosis at the origin of the posterior inferior cerebellar artery (PICA), previously treated solely with balloon angioplasty, may have contributed to the prothrombotic vascular milieu, the need for further intervention was uncertain based on angiography. We therefore decided to perform FDOCT assessment in the region of interest in order to better evaluate whether there was a flow-limiting residual stenosis or any other ‘complicated’ feature such as dissections that would demand additional intervention (ie, balloon angioplasty, stent) in order to maintain patency of the vertebrobasilar system.
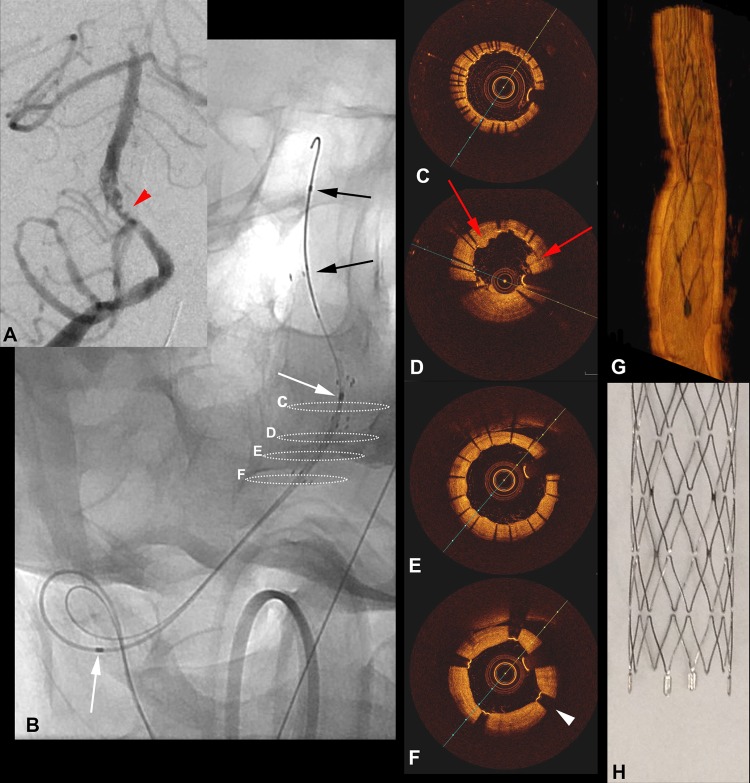
Figure 2.
Anterior-posterior (A) digital subtraction angiography image obtained 15 h after completion of the initial procedure showing non-occlusive thrombus (red arrowheads) within the mid portions of the stent construct. Anterior-posterior radiograph (B) demonstrating the position of the frequency-domain optical coherence tomography (FDOCT) catheter over the microwire, with delineation of the imaging length of the catheter (white arrows) and the monorail segment (black arrows). Dashed circles in (B) correspond to the cross-sections from the FDOCT acquisition (C–F). In (C) there is excellent wall apposition of overlapping segments of both stents. In (D) a small amount of tissue prolapse through the stent struts is present (red arrows). In (E) the stent struts from the proximal stent are easily visualized with excellent apposition and expansion. In (F) the four radiopaque markers (black arrowheads) delineating the edges of the stent are distinguishable from the stent struts. The entirety of the vessel wall is visible in all sections. Three-dimensional FDOCT (G) shows excellent FDOCT correlation in the appearance of the stent and proximal markers compared with a high-resolution photograph (H).
With a 6 Fr long sheath positioned in the mid-cervical right vertebral artery, an Excelsior SL10 microcatheter (Target) was advanced over a Transend EX (Target) microwire through the right vertebrobasilar stent construct and into the distal basilar artery. An exchange length Transend (Target) floppy microwire was then advanced into the distal basilar artery and the Excelsior microcatheter was exchanged for a 2.7 Fr Dragonfly FDOCT imaging catheter (St Jude Medical, St Paul, Minnesota, USA). A standard ‘monorail’ approach with a short 0.014 wire might have sufficed in this case, but we elected to advance the FDOCT catheter over support of the exchange length wire. The short ‘monorail’ design of the FDOCT catheter would not permit imaging of the entirety of the basilar artery and stent construct without advancement into the posterior cerebral artery, but would suffice for imaging the area of concern within the proximal stent and at the PICA origin (figure 2). A single FDOCT acquisition was then performed with hand injection of 15 mL undiluted iodixanol 320 to transiently clear red blood cells from the imaging area in a similar fashion to that previously reported in the cervical carotid vasculature.2 Briefly, FDOCT uses an internalized automated ‘pull-back’ method that does not require catheter manipulation while acquiring images. Red blood cells reflect light and must be cleared from the imaging area during image acquisition; this is typically achieved with either a contrast or saline flush. While our group has successfully used saline flush in the evaluation of cervical carotid disease,4 our anecdotal experience of saline utilization in the smaller coronary vessels (akin to the intracranial vasculature) has been challenging without balloon occlusive technique. Post-processing and immediate analysis of the data was performed on a commercially available FDOCT system (Ilumien Optis Imaging System, St Jude Medical).
FDOCT imaging demonstrated excellent visualization of the stents and strut wall apposition, with only minimal tissue prolapse through the stent struts (figure 2). Using the mid-basilar artery as the reference ‘normal’ vessel (most distal vessel imaged with FDOCT), the proximal stent segment and the vertebral artery (at the level of the PICA) revealed a maximal 28% residual stenosis (FDOCT-derived) without further abnormal findings, so no additional intervention was performed (figure 3). Given the lack of flow-limiting stenosis on FDOCT, it was deemed that the local clot formation within the stent would require more aggressive antiplatelet therapy and the patient was continued on intravenous eptifibatide therapy (2 μg/kg/min) for several days. The symptoms of nystagmus and right-sided weakness quickly resolved and follow-up CT angiography 3 days later (not shown) revealed a widely patent stent and resolution of thrombus.
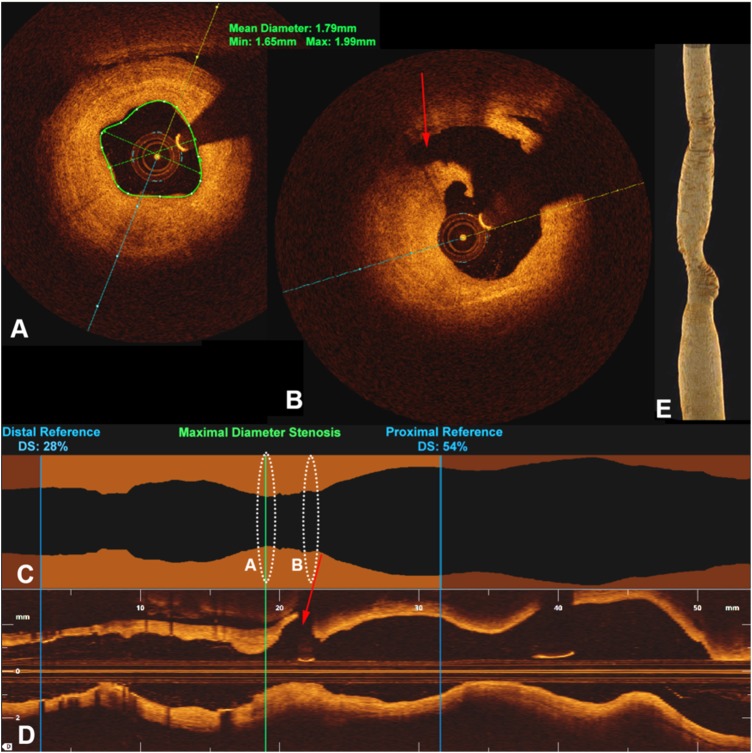
Figure 3.
Cross-sectional frequency-domain optical coherence tomography (FDOCT) images within the right vertebral artery (at the area of prior balloon angioplasty) just distal to (A) and at the level of the posterior inferior cerebellar artery (PICA) (B) show pathologic intimal thickening corresponding to a 28% residual stenosis. Stenosis analysis (C) and longitudinal views (D) show the severity of the stenosis based on proximal and distal reference vessels. The origin of the PICA (red arrows) is shown just proximal to the stenosis. Dashed circles in (C) and (D) correspond to the cross-sections in (A) and (B). (E) Three-dimensional FDOCT reconstruction demonstrates the luminal contour of the stent-vessel construct without significant stenosis.
Outcome and follow-up
The patient spent several weeks in hospital but was ambulating and without focal deficit at the time of discharge.
Discussion
The role of angioplasty and stent placement in the treatment of intracranial atherosclerotic disease (ICAD) has become less clear in recent years since the publication of the SAMMPRIS trial.5 Regional perforator infarction is cited as one of the frequent causes for periprocedural ischemic events in SAMMPRIS,6 and remains a concern during intervention for ICAD. While the ‘snow-plowing’ effect and smashing plaque to cover regional perforators has been implicated as a mechanism of ischemia in some cases,6–8 local platelet aggregation and clot formation within the stent and unstable or complicated plaque with active thrombus formation are additional plausible mechanisms. The coronary literature describes complicated plaque (AHA type VI) as those with areas of intimal disruption, intramural hemorrhage, or associated thrombus.9 The concept of unstable or complicated plaque is a recognized risk factor for intervention in both the extracranial carotid and intracranial circulation,9 with active thrombus formation a likely key contributor. Intracranial stenosis possessing complicated plaque would be at a higher risk for ischemic complications during intervention secondary to plaque and/or thrombus coverage of regional perforators during angioplasty and/or stent deployment (snow-plowing), as well as a higher risk for distal thromboembolic events. It is imperative, particularly in the post-SAMMPRIS era, that strides continue to be taken with regard to procedural/periprocedural risk reduction, which includes improved recognition of complicated plaque. Based on the coronary literature1 and our personal experience in the extracranial carotid,2–4 FDOCT more frequently identifies complicated plaque and thrombus formation that might pose a higher risk for perforator injury with intervention, a finding which might lead one to delay treatment until stabilization of the plaque.
To our knowledge, this represents the first in vivo application of FDOCT in the intracranial vasculature, and several observations can be drawn from this novel use. Despite limitations imposed by the ‘monorail’ design of current FDOCT catheters, this report does illustrate the feasibility of use within the intracranial vasculature. Lopes et al10 described the use of an intermediate catheter to effectively ‘unsheath’ the FDOCT catheter as an option for overcoming the monorail design in a cadaveric model, a technique that may be necessary for navigating the carotid siphon/anterior circulation with the current design of FDOCT catheters, but this was not necessary in our case. Based on our limited experience, it appears an ‘over-the-wire’ design would be advantageous for intracranial use, albeit not currently commercially available.
FDOCT has proved useful in the coronary vasculature for evaluation of indeterminate areas of stenosis, stent–vessel interactions, evaluating edge dissections, and assessing intimal healing of stents to help guide the duration of antiplatelet therapy.1 Stent–vessel wall interactions, particularly strut wall apposition, has proved vital in the long-term patency of coronary interventions, an assessment optimally evaluated with FDOCT. Such interactions, while not well studied in the cerebral vasculature, might hold equal importance in the long-term patency and success of ICAD interventions. The importance of adequate stent wall apposition in the case of flow-diverter therapy in the treatment of cerebral aneurysms is well recognized and the utility of FDOCT in this evaluation is particularly intriguing.
Additional observations made from this single case report include the apparent ability to visualize the entirety of the vessel wall along its intradural course owing to cerebrospinal fluid encasing the vessel (figure 2). This represents a contradistinction from the coronary vessels, which are surrounded by muscle, making it challenging to discriminate the outer wall from muscle. Vessel wall imaging has immense potential in evaluating cerebral aneurysms and rupture risk (assessing for areas of thinning of the aneurysm wall), evaluating ‘healing’ at the aneurysm following treatment (intimal coverage of aneurysm neck), as well as possibly differentiating inflammatory conditions (ie, vasculitis) from atherosclerosis. The utility of OCT in assessing intimal healing of aneurysms following coil embolization has been previously postulated based on ex vivo animal models.11
Potential drawbacks of the use of FDOCT in the intracranial vasculature would include risks inherent to intracranial interventions—namely, wire/catheter injury to the blood vessels and dislodgement of thrombus/plaque with distal emboli. While future studies will need to be performed to validate the safety of FDOCT, this novel use does illustrate its feasibility in the cerebral vasculature and speculates as to its future utility with regard to evaluation of ICAD, stent–vessel interactions, and pre/post-treatment aneurysm evaluation. In our case, FDOCT was of particular use in obviating the need for further intervention following angioplasty/stent placement for medically refractory vertebrobasilar insufficiency.
Learning points.
Frequency-domain optical coherence tomography (FDOCT) is feasible in the intracranial vasculature.
FDOCT has potential intracranial applications, particularly in the evaluation of intracranial atherosclerotic disease (ICAD) and cerebral aneurysm therapy.
FDOCT may provide better understanding of ICAD and plaque morphology with potential implications on ICAD intervention and risk reduction.
Footnotes
Competing interests: Given: consultant for product development (non-related) for Stryker Medical (Target Therapeutics); Ramsey: no competing interests; Attizani: serves on Speakers’ Bureau of St Jude Medical and consultant for St Jude Medical; Jones: serves on Speakers’ Bureau of St Jude Medical; Brooks - no competing interests; Bezerra: research support from St Jude Medical and is a medical advisor for St Jude Medical; Costa: serves on Speakers’ Bureau of St Jude Medical and has received research support from St. Jude Medical.
Patient consent: Obtained.
Ethics approval: Ethics approval was obtained from the local IRB.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bezerra HG, Costa MA, Guagliumi G, et al. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv 2009;2:1035–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones MR, Attizzani GF, Given CA, et al. Intravascular frequency-domain optical coherence tomography assessment of atherosclerosis and stent-vessel interactions in human carotid arteries. AJNR Am J Neuroradiol 2012;33:1494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attizzani GF, Jones MR, Given CA, II, et al. Frequency-domain optical coherence tomography assessment of very late vascular response after carotid stent implantation. J Vasc Surg 2013;58:201–4 [DOI] [PubMed] [Google Scholar]
- 4.Given CA, Attizzani GF, Jones MR, et al. Frequency-domain optical coherence tomography assessment of human carotid atherosclerosis using saline flush for blood clearance without balloon occlusion. AJNR Am J Neuroradiol 2013;34:1414–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derdeyn CP, Fiorella D, Lynn MJ, et al. Mechanisms of stroke after intracranial angioplasty and stenting in the SAMMPRIS trial. Neurosurgery 2013;72:777–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy EL, Hanel RA, Boulos AS, et al. Comparison of periprocedure complications resulting from direct stent placement compared with those due to conventional and staged stent placement in the basilar artery. J Neurosurg 2003;99:653–60 [DOI] [PubMed] [Google Scholar]
- 8.Abou-Chebl A, Steinmetz H. Critique of “Stenting versus aggressive medical therapy for intracranial arterial stenosis” by Chimowitz et al in the New England Journal of Medicine. Stroke 2012;43:616–20 [DOI] [PubMed] [Google Scholar]
- 9.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation 1995;92:1355–74 [DOI] [PubMed] [Google Scholar]
- 10.Lopes DL, Johnson AK. Evaluation of cerebral artery perforators and the Pipeline embolization device using optical coherence tomography. J Neurointerv Surg 2012;4:291–4 [DOI] [PubMed] [Google Scholar]
- 11.Thorell WE, Chow MM, Prayson RA. Optical coherence tomography: a new method to assess aneurysm healing. J Neurosurg 2005;102:348–54 [DOI] [PMC free article] [PubMed] [Google Scholar]