Abstract
Spondylodiscitis of the lumbar spine is described in a 67-year-old-man receiving chronic haemodialysis via a central venous catheter for diabetic nephropathy. He also had a forearm arteriovenous fistula created 1 month earlier. Clinical, MRI and surgical findings are described. The patient died despite spinal surgery and 1 month of antibiotic therapy from suspected pulmonary embolism. Early recognition by MRI or other imaging technique, prompt antibiotic therapy and often surgery are necessary for a successful outcome in this increasingly recognised complication in patients on chronic haemodialysis. Diabetes mellitus may be an added risk factor.
Background
Spondylodiscitis in dialysed patients is a rare complication that is being increasingly recognised. It may be dismissed as non-specific back pain especially in the elderly. Both bacterial and fungal infections can occur. Reports are few.1–6 We highlight such a case in a patient with diabetes, which resulted in death and highlight the use of MRI in diagnosis.
Case presentation
A 78-year-old man with known end-stage renal failure and a 15-year history of type 2 diabetes, hypertension, dyslipidaemia and primary hypothyroidism presented with a 1-month history of worsening low back pain. He was admitted for investigation as the pain was unresponsive to simple analgesics. He had no history of previous vascular events apart from a transient ischaemic attack 6 years previously.
A forearm arteriovenous fistula had been created a month before admission but the patient was receiving twice weekly haemodialysis sessions via an infraclavicular tunnelled central venous permcath for 4 months.
His medications included nifedipine 20 mg daily, carvedilol 12.5 mg twice daily, clopidogrel 75 mg daily, human recombinant insulin (70/30) 20 units morning and 10 units evening, levothyroxine 0.1 mg daily, multivitamins, rosuvastatin 20 mg daily and α cholecalciferol 0.25 µg daily.
On admission, he was afebrile and other vital signs were normal. The physical examination showed normal cognition, consciousness, tone, power and reflexes with absence of cerebellar signs. However, there was evidence of a sensorimotor neuropathy below the ankles bilaterally. The cardiorespiratory system and abdomen were unremarkable. Bowel and bladder functions were normal. He had no wounds or leg ulceration.
Investigations
On admission, the following laboratory values were obtained: haemoglobin 12.5 g/dL, white cell count 14 000 /µL, platelet count 361 000 /µL, serum sodium 131 mmol/L, serum potassium 5.18 mmol/L, serum chloride 98.4 mmol/L, serum calcium 8.3 mg/dL, inorganic phosphorous 8.3 mg/dL, uric acid 8.4 mg/dL, alkaline phosphatase 211.7 U/L, serum magnesium 2.0 mEq/L, total proteins 6.5 g/dL, albumin 3.42 g/dL, globulin 3.08 g/dL, HIV 1 and 2 were negative by ELISA, hepatitis BsAg and hepatitis C virus IgM and G antibody tests were negative, prostatic-specific antigen 5.0 ng/dL and serum creatinine 7 mg/dL. The C-reactive protein was elevated at 259.3 (0–5.00 mg/L) and the erythrocyte sedimentation rate was elevated at 125 mm/h. A recent glycosylated haemoglobin level was 64 mmol/mol.
Serum protein electrophoresis showed a polyclonal increase in the γ region. Blood cultures revealed no growth for aerobic and anaerobic bacteria. Fungal cultures were not carried out. Chest X-ray was normal apart from mild cardiomegaly and the presence of a permcath without evidence of tuberculosis. ECG showed left ventricular hypertrophy and significant Q waves in leads III and aVF.
Ultrasound of the abdomen showed bilateral shrunken kidneys, 7.1 cm on the right and 7.4 cm on the left. Echocardiogram showed an ejection fraction of 49%, with normal valves, inferior wall hypokinesis, no vegetations and no pericardial effusion. MRI of the spine showed an L2, L3 spondylodiscitis with an epidural abscess and diffuse degenerative disc disease (figure 1).
Figure 1.
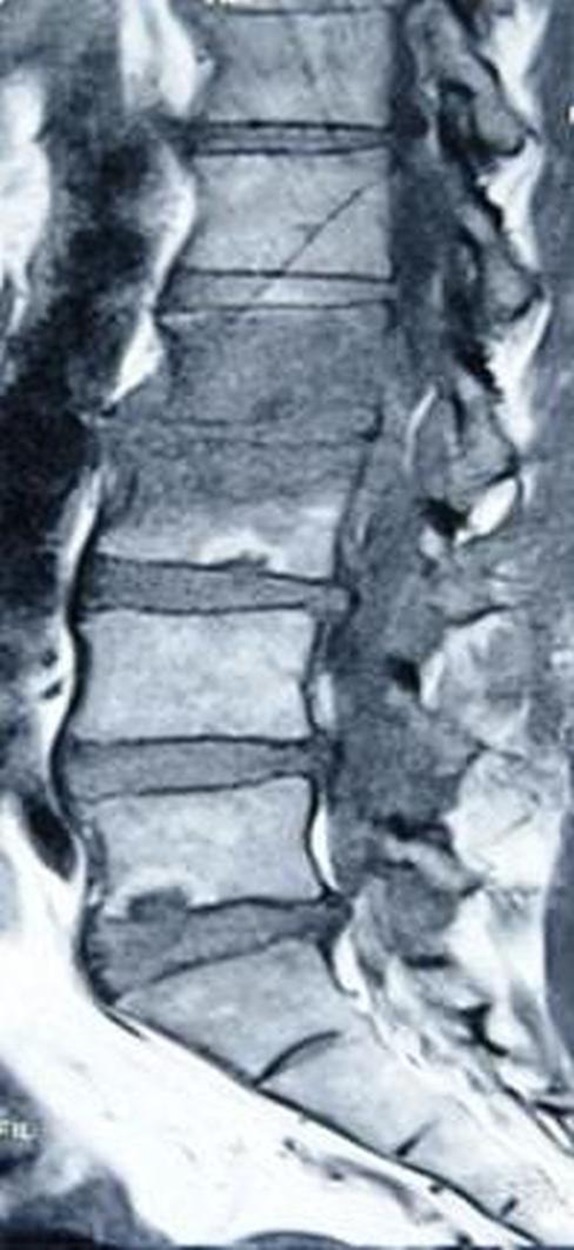
Sagittal T1-weighted MRI showing L2 and 3 spondylodiscitis with adjacent empyaema, diffuse degenerative disc disease and spinal stenosis (see arrows).
Treatment
He was started on intravenous metronidazole and ceftriaxone over the next 15 days and had temperature elevations on four occasions. He continued to receive dialysis during this period.
On the 15th day of hospitalisation, the patient developed bilateral lower limb weakness and, over a 3-day period, had grade 4/5 power (MRC scale). Lumbar surgery was performed as his condition had deteriorated. The thecal sac was exposed via L2 and he had an L2 and partial L3 laminectomy. A thin film of 3 mm of purulent fluid was encountered. Two swabs were taken for culture and sensitivity and the rest was removed by suction. Closure was routine. Three days postoperatively, power in his lower limbs increased to 5/5 (MRC scale) and there was significant pain relief.
The purulent fluid was negative on Gram staining and no bacterial growth was obtained on anaerobic and aerobic cultures. Fungal cultures were unavailable.
Outcome and follow-up
After 16 days postoperatively with continuous antibiotics and intermittent haemodialysis, the patient died suddenly with clinical features of a pulmonary embolism. A postmortem was not performed.
Discussion
Spondylodiscitis in patients on dialysis is being increasingly recognised. Bacteraemia via the portal of entry of the vascular access allows ingress of pathogens which gain access to the discs in the lumbar area and other organs in 50.8% of cases.7 Risk factors have included bacteraemia, receipt of blood products, invasive procedures and the establishment of vascular access.8 Diabetes mellitus in dialysed patients, as noted in this case, has not been frequently highlighted as a risk factor even though the propensity for sepsis and spondylodiscitis in the non-dialysed patient with diabetes has recently been highlighted.9 10 In dialysed patients however, one report described that three of five patients with spondylodiscitis as having diabetes mellitus from a cohort of 830 patients with central venous lines for haemodialysis.4
It is possible that bacteria may have gained access via the permcath or at the time of fistula creation. Blood cultures in our case were negative probably due to prior antibiotic use but the surgical findings suggested a pyogenic process.
Culture-negative spondylodiscitis has been recognised and various empirical intravenous antibiotic regimes have been advocated including fluoroquinolones with a β-lactam or fosfomycin.11 More recently, vancomycin and gentamycin use have been suggested as best in a series where 8 of 11 patients grew Staphylococcus aureus.2 Commentators have lamented the absence of controlled trials and lack of guidelines for treatment of spondylodiscitis in general.12 In our patient, there was no evidence of tuberculosis on clinical, radiological or intraoperative evidence. Moreover, fungal infections, while a well-recognised cause of spondylodiscitis, have not been commonly reported in patients on haemodialysis.5
Our patient's back pain was unresponsive to simple analgesics and, with his elevated inflammatory markers, should have served as red flags for the presence of sinister pathology necessitating MRI or other imaging modality.9 10 With the increasing number of patients receiving dialysis by central venous catheters and with a rising global incidence of diabetic nephropathy, we hope to highlight this condition thus encouraging earlier diagnosis and appropriate treatment.
Learning points.
Persistent low back pain can have a sinister cause.
Patients on haemodialysis with intravascular access lines are prone to spondylodiscitis.
Elevated erythrocyte sedimentation rate and/or C reactive protein should have prompted MRI of lumbosacral spine or other available imaging technique.
Patients with diabetes on haemodialysis with intravascular access lines are a high risk group.
Central venous lines should be left in place for the minimum time possible.
Acknowledgments
The authors thank Consultant Physicians, Dr Neal Bhagwandass and Dr Joel Teelucksingh, for critically reviewing the manuscript.
Footnotes
Contributors: KR and LC conceived the report and prepared the manuscript. BM prepared the nephrology component and worked on the final manuscript. RN performed spinal surgery, contributed the neurosurgical component and worked on the final manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ramírez-Huaranga MA, Sánchez de la Nieta-García MD, Anaya-Fernández S, et al. Spondylodiscitis, nephrology department's experience. Nefrologia 2013;33:250–5 [DOI] [PubMed] [Google Scholar]
- 2.Faria B, Canto Moreira N, Sousa TC, et al. Spondylodiscitis in hemodialysis patients: a case series. Clin Nephrol 2011;76:380–7 [DOI] [PubMed] [Google Scholar]
- 3.Cervan AM, Colmenero Jde D, Del Arco A, et al. Spondylodiscitis in patients under hemodialysis. Int Orthop 2012;36:421–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobo Sánchez JL, Gándara Revuelta M, Cuadrado Mantecón ME, et al. Infectious spondylodiscitis in patients with central venous catheters for hemodialysis: a retrospective study. J Ren Care 2012;38:147–50 [DOI] [PubMed] [Google Scholar]
- 5.Palmisano A, Benecchi M, De Filippo M, et al. Candida sake as the causative agent of spondylodiscitis in a hemodialysis patient. Spine J 2011;11:e12–6 [DOI] [PubMed] [Google Scholar]
- 6.Afshar M, Reilly RF, Spondylodiscitis in a patient on chronic hemodialysis. Nat Rev Nephrol 2011;7:599–604 [DOI] [PubMed] [Google Scholar]
- 7.Kessler M, Hoen B, Mayeux D, et al. Bacteremia in patients on chronic hemodialysis. A multicenter prospective survey. Nephron 1993;64:95–100 [DOI] [PubMed] [Google Scholar]
- 8.Helewa RM, Embil JM, Boughen CG, et al. Risk factors for infectious spondylodiscitis in patients receiving hemodialysis. Infect Control Hosp Epidemiol 2008;29:567–71 [DOI] [PubMed] [Google Scholar]
- 9.Kapsalaki E, Gatselis N, Stefos A, et al. Spontaneous spondylodiscitis: presentation, risk factors, diagnosis, management and outcome. Int J Infect Dis 2009;13: 564–9 [DOI] [PubMed] [Google Scholar]
- 10.Cechurová D, Lacigová S, Zourek M, et al. Spondylodiscitis and epidural empyema as a complication of diabetic foot. Vnitr Lek 2013;59:412–15 [PubMed] [Google Scholar]
- 11.Gillard J, Boutoille D, Varin S, et al. Suspected disc space infection with negative microbiological tests—report of eight cases and comparison with documented pyogenic discitis. Joint Bone Spine 2005;72:156–62 [DOI] [PubMed] [Google Scholar]
- 12.Gouliouris T, Aliyu SH, Brown NM, Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010;65:11–24 [DOI] [PubMed] [Google Scholar]


