Abstract
Hepatitis E virus genotype 3 is not rare in developed countries, and may cause chronic hepatitis in immunocompromised patients. This may not only lead to abnormalities in liver test and malaise, but to severe neurological symptoms as well. In this case, chronic hepatitis E infection caused encephalopathy, an atactic gait, Lhermitte's sign, incomplete bladder emptying and peripheral sensory neuropathy in a renal transplant recipient. The diagnosis was not performed until years after the onset of first symptoms and several months after the onset of neurological symptoms. If treated adequately, viral load can be reduced in over two-thirds of patients and neurological symptoms are often resolved. More widespread knowledge about this virus and its extrahepatic manifestations may lead to a quicker diagnosis, and may limit pathology. Serological screening should be added to standard pretransplant virological screening, so that, in the future, patients without antibodies could be vaccinated.
Background
Although many clinicians in developed countries are not familiar with hepatitis E virus (HEV) infection, it is a diagnosis to be considered in western countries.
Currently, there are four known HEV genotypes infecting humans.1 2 Genotypes 1 and 2 are human viruses. They are transmitted through contaminated water and cause epidemics of acute hepatitis in developing countries. Genotypes 3 and 4 are swine viruses that infect humans as accidental host.3 HEV genotype 4 occurs mainly in southeast Asia, although rare cases have been described in western Europe. Genotype 3 occurs worldwide, in developing and in developed countries.
Recently, HEV genotype 3 (HEV3) infection has increasingly been reported in Europe and North America. In southern France, HEV3 is hyperendemic. Research among blood donors in Toulouse, in the South of France, showed a seroprevalence of 52%.4 A study among organ transplant recipients in that region showed an incidence of HEV3 infection of 3.2% per year.5
In most cases, HEV infection is asymptomatic and self-limiting, but immunocompromised patients are at risk for developing chronic hepatitis E. The symptoms of chronic HEV are non-specific. Malaise and arthralgia are common symptoms.6 Neurological involvement is uncommon and if it occurs, symptoms are usually rather non-specific. As demonstrated in this case report, chronic HEV infection can be accompanied by severe neurological symptoms that are hard to classify.
Case presentation
A 66-year-old woman was followed in the outpatient transplant clinic. In 2007, she underwent a pre-emptive kidney transplantation due to progressive renal impairment caused by glomerulonephritis of unknown aetiology. The transplant functioned immediately, with an estimated glomerular filtration rate of 52 mL/min. Immunosuppression was started with cyclosporin A (CsA), mycophenolate mofetil (MMF) and prednisolone. In 2008, she developed hypertrichosis, for which CsA was converted to tacrolimus and the steroids were gradually withdrawn. In 2009, she had a positive PCR for JC virus and she suffered from severe gastroenteritis due to cryptosporidium. Owing to these infections, MMF was discontinued. Her travelling history was unremarkable: she never travelled outside Europe. She spent most summers in the South of France.
In 2009, she developed malaise, arthralgia and showed abnormalities in liver test, mainly transaminase elevation (table 1). Standard virology tests on Epstein-Barr virus, Cytomegalovirus and hepatitis A, B and C virus were negative. Autoimmune serology was negative. Abdominal ultrasound showed a homogenous aspect of the liver, without any focal liver lesions. Liver biopsy showed signs of chronic inflammation (figure 1). At that point in time, all drugs but the tacrolimus were discontinued. Owing to lack of improvement, tacrolimus was stopped and immunosuppression was continued with MMF (750 mg twice daily) and prednisolone (10 mg once daily). Initially, this led to a slight improvement. However, afterwards, malaise and arthralgia continued. Lowering the dose of the steroids did not reduce her symptoms.
Table 1.
Liver function tests have improved after reduction of immunosuppression and antiviral therapy with ribavirin, which was initiated in September 2012
| Measurement | Normal value | 22 June 2009 | 21 September 2012 | 23 November 2012 | 12 February 2013 |
|---|---|---|---|---|---|
| Total bilirubin (µmol/L) | 0–17 | 11 | 8 | 8 | 7 |
| γGT (U/L) | 0–35 | 252 | 417 | 125 | 49 |
| Alkaline phosphatase (U/L) | 0–120 | 132 | 114 | 83 | 60 |
| ALAT (U/L) | 0–31 | 299 | 80 | <7 | <7 |
| ASAT (U/L) | 0–31 | 130 | 66 | 20 | 19 |
| LDH (U/L) | 0–250 | 433 | 224 | 145 | 206 |
ALAT, alanine transaminase; ASAT, aspartate aminotransferase; γGT, γ-glutamyl transpeptidase; LDH, lactate dehydrogenase.
Figure 1.
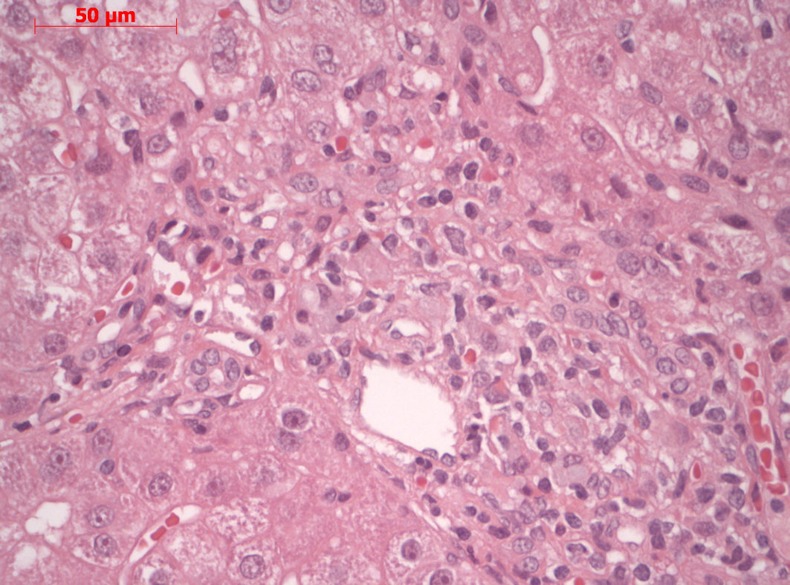
Liver biopsy showed signs of chronic inflammation, possibly toxic drug-induced hepatitis.
In 2011, the patient developed progressive sensory symptoms in all limbs and a gait disorder. MRI showed a lesion in the cervical cord caused by a mild mid-cervical stenosis. Electromyografical (EMG) studies showed no signs of neuropathy. In 2012, the atactic gait worsened, the patient developed Lhermitte's sign, had incomplete bladder emptying and became drowsy and confused in a few weeks’ time. The cervical stenosis could only explain part of her symptoms. Analysis of all standard infectious agents in serum and cerebrospinal fluid (CSF), including bacterial culture, serology and PCR, and a new EMG and MRI of the neuraxis did not yield a diagnosis. EEG showed a slow wave pattern.
Investigations
Recent publications about HEV drew attention to this virus. Our patient tested positive for IgM and IgG antibodies as well as for viral RNA by PCR; genotyping showed the presence of genotype 3. PCR analysis for hepatitis E in the CSF was positive, without a cellular CSF response or elevated protein level. With HEV RNA and serology being positive in an archived blood sample, the diagnosis of chronic hepatitis E was confirmed. Since other causes of encephalopathy and increased ataxia had been excluded, signs were attributed directly to central nervous system infection with hepatitis E.
Treatment
The patient was treated by lowering immunosuppression—MMF was reduced to 500 mg twice daily and prednisolone to 5 mg once daily—and with antiviral therapy with ribavirin, in a dose of 400 mg once daily.
Outcome and follow-up
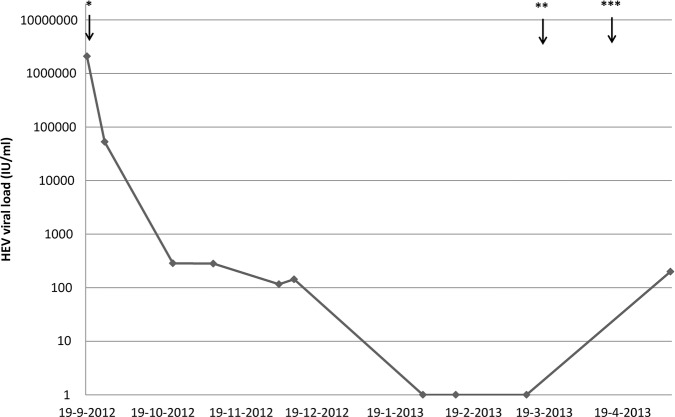
This led to a gradual reduction in the viral load in serum (figure 2), and to an improvement of encephalopathic signs, normalisation of the slow-wave pattern on the EEG and improved liver tests (table 1).
Figure 2.
Hepatitis E virus viral load decreased after reduction of immunosuppression and antiviral therapy with ribavirin in September 2012. *Start of ribavirin 400 mg once daily on 19 September 2012; **Reduction of ribavirin to 200 mg twice daily on 18 March 2013; ***Discontinuation of ribavirin on 16 April 2013.
However, after an initial reduction, after 1 month HEV viral load stabilised. At that point, the dose of ribavirin was increased to 600 mg/day, titrated on blood levels of ribavirin. Thereafter, viral RNA gradually decreased to undetectable levels after 4 months. Spontaneous voiding had recovered. However, the gait disorder persisted. After 6 months of therapy, our patient was able to walk again, with support of a walking aid. At that point in time, ribavirin treatment was discontinued. As a consequence of ribavirin therapy, she developed hearing loss and haemolytic anaemia, which was not restored by darbepoetin (150 μg once a week) and for which several red blood cell transfusions were necessary. The transplant function remained well throughout the treatment period. One month after discontinuation of ribavirin, however, HEV returned at a low level, so far asymptomatic.
Discussion
We present a case of chronic HEV3 infection in a renal transplant recipient. In this case, the infection caused not only malaise, arthralgia and abnormalities in liver test, but also cerebellar symptoms and encephalopathic features. The diagnosis was not performed until several years after the onset of the first symptoms.
HEV3 can be transmitted by exposure to pigs and consumption of undercooked meat, and by consumption of shellfish and crops that have been exposed to infected water. Direct human transmission has only been described via blood transfusions and organ transplantation.7 8 Our patient spent many holidays in southern France, a region with the highest seroprevalence of HEV3 reported in the industrialised world.4 It is, therefore, very well possible that she became infected there. However, HEV3 is endemic in the Netherlands as well. Cross-sectional analysis of 1200 Dutch transplant recipients revealed 1% positive patients. The majority of these patients, 11 of 12, appeared to have a chronic infection.9 Research among blood donors in the Netherlands demonstrated an HEV3 seropositivity of 27%.10
According to case series from southern France and southwest England, 5.5% of patients with chronic HEV develop neurological symptoms.11 Recently, Cheung et al12 reviewed the neurological manifestations of this virus. Most described that neurological symptoms in chronic HEV are peripheral nerve disorders, such as Guillain-Barré syndrome and brachial neuritis. However, cranial nerve palsy, meningitis, transverse myelitis, seizure, encephalitis and intracranial hypertension have been described. The time between infection and onset of symptoms ranged from 18 months to 3 years. The pathogenesis of this virus leading to neurological symptoms remains unclear. One suggested mechanism is an autoimmune disorder, involving antiganglioside antibodies.12 A humoral mechanism instead of a classical meningoencephalitis would comply with the absence of a cellular response in the CSF in this case.
In patients with chronic HEV infection, the first step of treatment is reducing immunosuppressive drugs. This results in viral clearance in approximately 30% of patients.1 If immunosuppression cannot be reduced, or if reduction does not lead to viral clearance, off-label antiviral therapy could be considered. Suggested antiviral drugs are ribavirin and interferon α, as monotherapy or in combination.1 Since interferon α is associated with acute rejection in kidney-transplant recipients,13 ribavirin is the drug of choice for these patients. Treatment with ribavirin leads to a reduction of viral load in more than 65% of patients.1 The advised dose for patients with normal kidney function is 600–800 mg/day during at least 3 months.2 It is advised to continue treatment until the viral RNA becomes undetectable and to monitor viral RNA after discontinuation of antiviral medication, because of possible recurrence. However, further studies to determine the optimal duration of treatment are required. Currently, neither ribavirin nor interferon is approved for the treatment of chronic HEV infection. When treatment is successful, the neurological symptoms often resolve within several months.
In the near future, it may be possible to vaccinate patients at risk for HEV. Two candidate recombinant HEV vaccines have been successfully tested in clinical trials.14 15 However, it needs to be investigated whether these vaccines protect against HEV3 as well. If so, it would be advisable to add HEV to the standard pretransplant virological screening, so that patients without antibodies can be vaccinated prior to transplantation.
Learning points.
Hepatitis E virus (HEV) genotype 3 is not rare.
HEV genotype may cause chronic hepatitis in immunocompromised patients, which may lead not only to abnormalities in liver test and malaise, but also to severe neurological sequelae that can either involve the central or the peripheral nervous system.
More widespread knowledge about this virus and its symptoms may lead to a quicker diagnosis, and may limit pathology.
Diagnosis can be established by serology and PCR testing in serum and CSF.
Acknowledgments
The authors thank Ms J Roodbol and Dr A van der Eijck for their help with the manuscript.
Footnotes
Contributors: MAdV and JMMB have contributed to the description of the case from the Internal Medicine point of view. RdM has contributed from the hepatology and virology point of view. JPAS has contributed to this case report from the neurological point of view. In addition, all the authors have revised the article and gave final approval of the version to be published.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet 2012;379:2477–88 [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med 2012;367:1237–44 [DOI] [PubMed] [Google Scholar]
- 3.Meng X-J. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res 2011;161:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansuy J-M, Bendall R, Legrand-Abravanel F, et al. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis 2011;17:2309–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legrand-Abravanel F, Kamar N, Sandres-Saune K, et al. Hepatitis E virus infection without reactivation in solid-organ transplant recipients, France. Emerg Infect Dis 2011;17:30–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton HR, Bendall R, Ijaz S, et al. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis 2008;8:698–709 [DOI] [PubMed] [Google Scholar]
- 7.Matsubayashi K, Kang J-H, Sakata H, et al. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion 2008;48:1368–75 [DOI] [PubMed] [Google Scholar]
- 8.Boxall E, Herborn A, Kochethu G, et al. Transfusion-transmitted hepatitis E in a “nonhyperendemic” country. Transfus Med 2006;16:79–83 [DOI] [PubMed] [Google Scholar]
- 9.Pas SD, de Man RA, Mulders C, et al. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis 2012;18:869–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slot E, Hogema B, Riezebos-Brilman A, et al. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill 2013;18:pii: 20550. [DOI] [PubMed] [Google Scholar]
- 11.Kamar N, Bendall RP, Peron JM, et al. Hepatitis E virus and neurologic disorders. Emerg Infect Dis 2011;17:173–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung MCM, Maguire J, Carey I, et al. Review of the neurological manifestations of hepatitis E infection. Ann Hepatol 2012;11:618–22 [PubMed] [Google Scholar]
- 13.Wéclawiack H, Kamar N, Mehrenberger M, et al. Alpha-interferon therapy for chronic hepatitis C may induce acute allograft rejection in kidney transplant patients with failed allografts. Nephrol Dial Transplant 2008;23:1043–7 [DOI] [PubMed] [Google Scholar]
- 14.Zhu F-C, Zhang J, Zhang X-F, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010;376:895–902 [DOI] [PubMed] [Google Scholar]
- 15.Shrestha MP, Scott RM, Joshi DM, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med 2007;356:895–903 [DOI] [PubMed] [Google Scholar]