Abstract
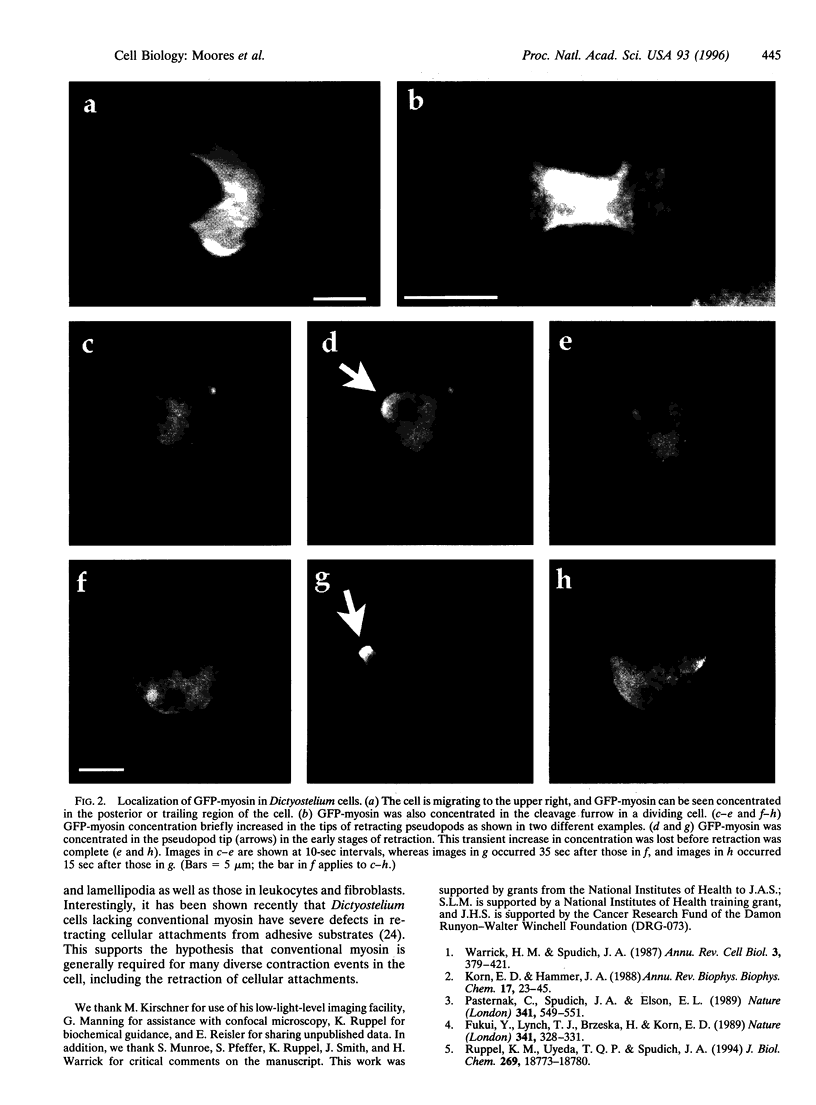
Conventional myosin plays a key role in the cytoskeletal reorganization necessary for cytokinesis, migration, and morphological changes associated with development in nonmuscle cells. We have made a fusion between the green fluorescent protein (GFP) and the Dictyostelium discoideum myosin heavy chain (GFP-myosin). The unique Dictyostelium system allows us to test the GFP-tagged myosin for activity both in vivo and in vitro. Expression of GFP-myosin rescues all myosin null cell defects. Additionally, GFP-myosin purified from these cells exhibits the same ATPase activities and in vitro motility as wild-type myosin. GFP-myosin is concentrated in the cleavage furrow during cytokinesis and in the posterior cortex of migrating cells. Surprisingly, GFP-myosin concentration increases transiently in the tips of retracting pseudopods. Contrary to previous thinking, this suggests that conventional myosin may play an important role in the dynamics of pseudopods as well as filopodia, lamellipodia, and other cellular protrusions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Titus M. A., Manstein D. J., Ruppel K. M., Spudich J. A. Molecular genetic tools for study of the cytoskeleton in Dictyostelium. Methods Enzymol. 1991;196:319–334. doi: 10.1016/0076-6879(91)96029-q. [DOI] [PubMed] [Google Scholar]
- Fisher A. J., Smith C. A., Thoden J., Smith R., Sutoh K., Holden H. M., Rayment I. Structural studies of myosin:nucleotide complexes: a revised model for the molecular basis of muscle contraction. Biophys J. 1995 Apr;68(4 Suppl):19S–28S. [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Lynch T. J., Brzeska H., Korn E. D. Myosin I is located at the leading edges of locomoting Dictyostelium amoebae. Nature. 1989 Sep 28;341(6240):328–331. doi: 10.1038/341328a0. [DOI] [PubMed] [Google Scholar]
- Jay P. Y., Pham P. A., Wong S. A., Elson E. L. A mechanical function of myosin II in cell motility. J Cell Sci. 1995 Jan;108(Pt 1):387–393. doi: 10.1242/jcs.108.1.387. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Hammer J. A., 3rd Myosins of nonmuscle cells. Annu Rev Biophys Biophys Chem. 1988;17:23–45. doi: 10.1146/annurev.bb.17.060188.000323. [DOI] [PubMed] [Google Scholar]
- Pasternak C., Spudich J. A., Elson E. L. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989 Oct 12;341(6242):549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Peltz G., Spudich J. A., Parham P. Monoclonal antibodies against seven sites on the head and tail of Dictyostelium myosin. J Cell Biol. 1985 Apr;100(4):1016–1023. doi: 10.1083/jcb.100.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher D. C., Eckenrode V. K., Ward W. W., Prendergast F. G., Cormier M. J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992 Feb 15;111(2):229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Ruppel K. M., Uyeda T. Q., Spudich J. A. Role of highly conserved lysine 130 of myosin motor domain. In vivo and in vitro characterization of site specifically mutated myosin. J Biol Chem. 1994 Jul 22;269(29):18773–18780. [PubMed] [Google Scholar]
- Ruppert C., Kroschewski R., Bähler M. Identification, characterization and cloning of myr 1, a mammalian myosin-I. J Cell Biol. 1993 Mar;120(6):1393–1403. doi: 10.1083/jcb.120.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Kron S. J., Spudich J. A. Myosin step size. Estimation from slow sliding movement of actin over low densities of heavy meromyosin. J Mol Biol. 1990 Aug 5;214(3):699–710. doi: 10.1016/0022-2836(90)90287-V. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Ruppel K. M., Spudich J. A. Enzymatic activities correlate with chimaeric substitutions at the actin-binding face of myosin. Nature. 1994 Apr 7;368(6471):567–569. doi: 10.1038/368567a0. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Spudich J. A. A functional recombinant myosin II lacking a regulatory light chain-binding site. Science. 1993 Dec 17;262(5141):1867–1870. doi: 10.1126/science.8266074. [DOI] [PubMed] [Google Scholar]
- Warrick H. M., Spudich J. A. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Yumura S., Fukui Y. Reversible cyclic AMP-dependent change in distribution of myosin thick filaments in Dictyostelium. Nature. 1985 Mar 14;314(6007):194–196. doi: 10.1038/314194a0. [DOI] [PubMed] [Google Scholar]
- Yumura S., Mori H., Fukui Y. Localization of actin and myosin for the study of ameboid movement in Dictyostelium using improved immunofluorescence. J Cell Biol. 1984 Sep;99(3):894–899. doi: 10.1083/jcb.99.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]