Abstract
Objective:
The release of toxic metal ions from orthodontic alloys has induced concerns regarding the biocompatibility of fixed appliances. This study investigated the genotoxic effect of metal appliances in a sample of patients undergoing fixed orthodontic treatment.
Materials and Methods:
The study included twenty-five healthy individuals requiring orthodontic therapy in both jaws. The patients were treated by stainless steel orthodontic brackets and nickel-titanium or stainless steel arch wires. The oral mucosa cells were gathered just before the appliance placement and 9 months later. The cells were centrifuged, fixed and dropped onto slides. After staining, the micronucleus (MN) assay was used to determine genome alteration. The data were analyzed by paired sample t-test.
Results:
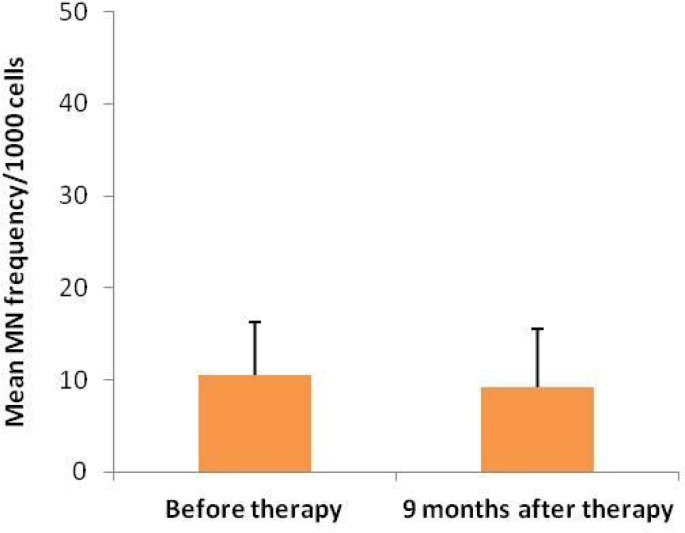
The mean micronuclei frequency in the buccal mucosa was 10.6 ± 5.7 per 1000 cells before the appliance placement and 9.2 ± 6.37 per 1000 cells 9 months later. No significant difference was found in the MN count before and 9 months after therapy (p=0.336).
Conclusion:
Under the conditions used in this study, application of fixed orthodontic appliances did not expose healthy individuals to increased risk of DNA damage in oral mucosa cells.
Keywords: Orthodontic Appliances, DNA Damage, Micronucleus Test, Biocompatibility, Genotoxicity
INTRODUCTION
The orthodontic patients are exposed to a noticeable amount of metal alloys in the mouth. The thermal, microbiologic and aqueous properties of the oral environment combined with the fluctuation in pH and intake of various drinks, food and mouthwashes facilitate corrosion and result in the release of metallic ions from appliances into oral tissues and biologic fluids of patients undergoing fixed orthodontic treatment. Nickel, chromium, cobalt and other metal ions that are released from orthodontic appliances have been demonstrated to cause biologic health hazards including contact dermatitis, hypersensitivity and cytotoxicity in several studies [1–4]. A more hazardous effect of metal alloys is the possibility of causing DNA damage (genotoxicity) in human cells. The genotoxic effect of metal alloys may be due to the generation of oxidative DNA damage (direct interaction) or interference with DNA replication (indirect interaction) [5–7]. Cellular repair is an important factor in preventing persistent DNA damage, and the metal ions can also inhibit DNA repair in oral tissues [5–7]. Despite the low release of ions from metal appliances, these can be taken up by the adjacent oral tissues [7–9] over the long period of orthodontic treatment and may possibly lead to genome alteration in the oral tissues of patients wearing them.
The studies on the biocompatibility of orthodontic appliances reported controversial findings. The corrosion eluates obtained from orthodontic alloys indicated genotoxic damage in a previous study [10] while other studies found no DNA damage in vitro [11–13]. Pereira et al. [14] reported that bracket placement produced a decrease in nuclear size and an increase in cytoplasm in buccal mucosa cells adjacent to brackets, but the alterations did not suggest malignancy. Faccioni et al. [7] and Hafez et al. [8] found that orthodontic appliances induced DNA breakage in buccal tissues of patients undergoing fixed orthodontic treatment. In contrast, the study conducted by Angelieri et al. [15] revealed that orthodontic therapy did not generate DNA damage and it was not able to enhance cytotoxicity.
Two assays are commonly used to determine DNA damage: the single cell gel (comet) assay and the micronucleus (MN) assay. The micronucleus assay is a mutagenic test system that is frequently used in in-vitro and invivo toxicological screening for detecting potential genotoxic compounds that lead to the induction of small DNA fragments (micronuclei) in the cytoplasm of the dividing cells. Micronuclei can be observed as chromosome fragments produced by DNA strand breakage, or as whole chromosomes that have been formed during the anaphase of mitosis or meiosis when they were not able to migrate with the rest of the chromosomes towards the spindle poles. These chromatin masses are surrounded by individual membranes and appear as one small nucleus or several small nuclei in the cytoplasm instead of the main nuclei of the daughter cells.
This study investigated the possible genetic damage to buccal tissues of subjects undergoing fixed orthodontic therapy by employing the micronucleus assay.
MATERIALS AND METHODS
The sample consisted of 25 subjects, 15 females and 10 males, attending the Department of Orthodontics at Mashhad Dental School, Mashhad University of Medical Sciences, Mashhad, Iran.
They ranged in age from 12 to 20 years and required fixed orthodontic treatment in both arches. The patients had no previous orthodontic therapy and did not use medicine or any supplements. None of the study subjects had amalgam fillings, sharp edge restorations and oral or systemic diseases and none reported allergy to jewelry or other products that contain nickel and chromium. The patients were all non smokers and no one consumed alcoholbased mouthwashes or drinks. The healthy oral mucosa was confirmed in all subjects through clinical examination.
The Ethics committee of Mashhad University of Medical Sciences approved the study protocol. The purposes of the study were fully explained for the participants and an informed consent was obtained from each subject before sampling.
This was a prospective study to determine DNA damage in patients undergoing fixed orthodontic treatment.
The orthodontic appliances consisted of 4 bands with buccaltubes (Dentaurum, Ispringen, Germany) on the upper and lower first molars and 16 to 20 brackets (standard edge-wise, 0.018-in slot; Dentaurum, Ispringen, Germany). The bands and brackets were made of stainless steel.
The arch wires used over the course of this study included 0.014-in nickel-titanium (NiTi; Ortho Technology, Inc., Tampa, Florida, USA), 0.016-in stainless steel (Denturum) and 0.016×0.022-in stainless steel (Dentaurum). The arch wires were held with elastomeric ligatures (Ortho Technology, Inc).
Sampling of buccal mucosa cells
The initial sampling was performed before the appliance placement. The subjects were asked to rinse the mouth several times with tap water to eliminate the exfoliated dead cells. Then, the epithelial cells from oral mucosa were collected by gentle scraping of the internal side of the right and left cheeks with a metal spatula in a sweeping motion. The cells were transferred to a plastic tube containing 5 ml PRMI-1640 medium (GibcoBRL, Grand Island, NY, USA). The sampling was repeated 9 months after the appliance placement. The cells were centrifuged, fixed in 3:1 v/v methanol: Glacial acetic acid, and dropped onto clean glass slides. The slides were air-dried and stained with May Grunwald Giemsa. The standard protocol of Fenech et al. [16] was used for micronucleus assay.
One thousand cells from each subject for each sampling time were scored under a light microscope at the magnification of × 400.
Statistical Analysis
After the normal distribution of the data was confirmed by the Kolmogorov Smirnov test, paired sample t-test was used to delineate any significant difference in the frequency of micronucleated cells before and nine months after therapy.
The statistical analysis was performed using SPSS software (Statistical Package for the Social Sciences, Version 11.5, SPSS Inc, Chicago, Ill) at the 95% confidence interval of the difference.
RESULTS
The mean age of the patients was 16.3 ± 3.6 years. The mean frequency of micronucleated cells in the buccal mucosa of patients before appliance placement was 10.6 ± 5.7 per 1000 cells. Nine months after therapy, the MN frequency was measured as 9.2 ± 6.37 per 1000 cells.
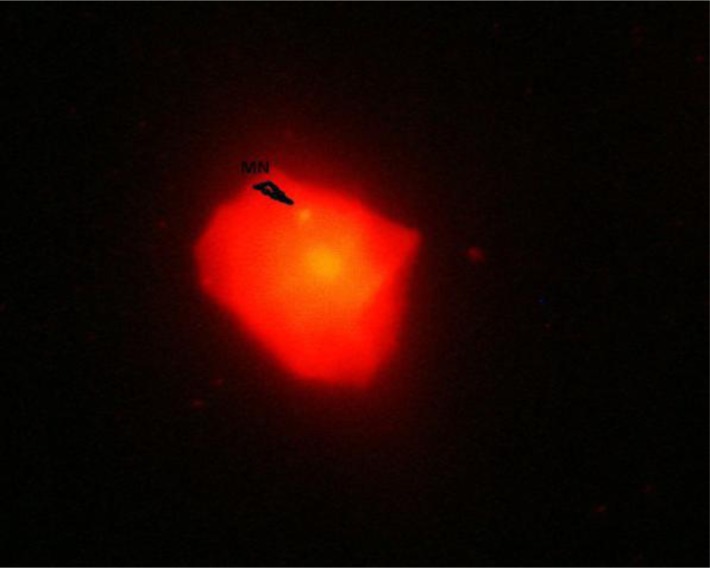
Figure 1 depicts the frequencies of micronucleated cells in subjects undergoing orthodontic treatment. An example of micronucleated cell has been illustrated in Figure 2. Statistical comparison by the paired sample t-test revealed no significant difference in the MN count between the two sampling dates (p=0.336).
Fig 1.
Micronuleus frequency before and 9 months after placement of orthodontic appliances
Fig 2.
A micronucleated cell (arrow) at × 400 magnification
DISCUSSION
The micronucleus test used in this study is a completely noninvasive and reliable technique that is suitable to determine chromosome damage in the oral cavity, nasal cavity, esophagus, bronchi, cervix and urinary tract. Although single cell gel (comet) assay can also be used in the oral mucosa cells to detect DNA damage in individual cells, it has been shown that there is not a close relationship between DNA migration in the comet assay and mutagenesis [15], and therefore it is assumed that the micronucleus assay is more suitable than the single cell gel (comet) assay for the detection of DNA lesions [15].
The basal layer of the epithelium is responsible for cell division. The turnover rate of human oral epithelial cells is varied from 7 to 21 days and some authors consider 14 days as the median [17–18]. Since the formation of micronuclei should take place in the progenitor cells of the epithelium during mitosis, the presence of micronuclei in the exfoliated epithelial cells reflects chromosomal damage that has occurred in the basal cell layer 1–3 weeks earlier. The buccal mucosa cells that are frequently used for sampling are in close contact with the appliances and they may experience damage because of trauma [14] or due to continuous uptake of toxic ions released from the metal alloys [9,7]. Furthermore, buccal cells have a limited potential for DNA repair and thus they are more suitable to reveal genome instability compared to cells that repair DNA damage more efficiently [19].
The present study reports no significant difference in the frequency of micronucleated cells before the appliance placement and 9 months later. This indicates that the placement of fixed orthodontic appliances does not increase the risk of genotoxic damage in human oral epithelium over the time period evaluated. Our findings are in contrast with those of Natarajan et al. [7], who found a significantly higher MN count in the experimental group at the day of debonding compared to the control group without appliances, although the MN frequency reverted to the normal values over the 30 days.
The study carried out by Hafez et al. [8] proved the cytotoxicity and genotoxicity of orthodontic appliances remained in the mouth for 6 months. Faccioni et al. [7] also reported decreased cellular viability and DNA breakage in buccalmuscosa cells of orthodontic patients compared to a control group without appliances. Westphalen et al. [20] reported a significant increase in MN cells after 30 days of orthodontic treatment, but the comet assay failed to show a significant genotoxic effect in the same patients after 10 days of treatment. The findings of this study, however, corroborate the results of a study performed by Angelieri et al. [15] who found no significant differences in the micronucleus frequencies before, during, and after orthodontic therapy, indicating the lack of clastogenic and/or aneugenic effects from orthodontic appliance exposure to oral mucosa cells.
The controversy observed between the results of different studies may be related to several factors. The period of appliance placement was different among the studies, as some evaluated DNA damage in orthodontically treated patients at the day of debonding [17], while others used shorter observation periods of 10 or 30 days following appliance placement [6, 20]. Furthermore, some studies compared the experimental group with an untreated group (cross sectional study) [7, 8, 17], while others evaluated the genotoxic effects longitudinally in the same patients before and after orthodontic treatment [6, 15, 20]. The sample size, the method used to detect genotoxic effects, the materials involved in the treatment, the age range of patients, and the risk factors that the patients were exposed to also vary among studies.
The release of toxic metal ions from orthodontic appliances has been demonstrated in several in vitro [4, 10, 21] and in vivo [22–26] studies, but the measurable concentrations were far from the toxic concentrations [23, 27–29]. Recent studies have focused on measuring metal ion content in human mucosa cells and its correlation to cytotoxic or genotoxic alterations in the buccal epithelium. The results of studies in this field are contradictory, with some reporting a significant correlation between DNA damage and cellular nickel and cobalt concentrations [7] while others found that there is no cause and effect relationship between the cellular content of any metal ion and genome alteration [6,8,17].
It should be noted that subtoxic exposures to metal ions could also induce cellular damage in human tissues [5, 30–31]. In addition, the synergistic effect between the low concentrations of different metal ions [6] as well as the irritation and trauma from orthodontic appliances [14] may play important roles in the biologic effects of orthodontic appliances.
This in vivo study did not report any genotoxic effect caused by fixed appliances on human epithelial cells of healthy subjects 9 months after orthodontic treatment.
The results of this study, however, should be interpreted cautiously. It is possible that any DNA damage induced by orthodontic appliances would re-pair in healthy individuals, but a decrease in repair capacity or alterations in the immune system may allow the DNA damage to remain and be expressed as genome alteration and DNA mutations.
Older age, presence of systemic diseases and risk factors such as tobacco smoke may also aggravate the harmful effects of fixed appliances. Further studies are required to determine the biologic effects of orthodontic appliances in a larger population with longer treatment period and by employing other test systems.
CONCLUSION
The findings of this study indicate that orthodontic appliances do not expose healthy patients to an increased risk of genotoxic damage in oral mucosa cells, as evidenced by the micronuleus assay.
Acknowledgments
The authors would like to thank the Vice-Chancellor for Research of Mashhad University of Medical Sciences for the financial support of this project (grant number 87089).
REFERENCES
- 1.Summer B, Fink U, Zeller R, Rueff F, Maier S, Roider G, et al. Patch test reactivity to a cobalt-chromium-molybdenum alloy and stainless steel in metal-allergic patients in correlation to the metal ion release. Contact Dermatitis. 2007 Jul;57(1):35–9. doi: 10.1111/j.1600-0536.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 2.Burrows D. Hypersensitivity to mercury, nickel and chromium in relation to dental materials. Int Dent J. 1986 Mar;36(1):30–4. [PubMed] [Google Scholar]
- 3.Lu X, Bao X, Huang Y, Qu Y, Lu H, Lu Z. Mechanisms of cytotoxicity of nickel ions based on gene expression profiles. Biomaterials. 2009 Jan;30(2):141–8. doi: 10.1016/j.biomaterials.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 4.David A, Lobner D. In vitro cytotoxicity of orthodontic archwires in cortical cell cultures. Eur J Orthod. 2004 Aug;26(4):421–6. doi: 10.1093/ejo/26.4.421. [DOI] [PubMed] [Google Scholar]
- 5.Hartwig A. Current aspects in metal genotoxicity. Biometals. 1995 Jan;8(1):3–11. doi: 10.1007/BF00156151. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Minano E, Ortiz C, Vicente A, Calvo JL, Ortiz AJ. Metallic ion content and damage to the DNA in oral mucosa cells of children with fixed orthodontic appliances. Biometals. 2011 Oct;24(5):935–41. doi: 10.1007/s10534-011-9448-z. [DOI] [PubMed] [Google Scholar]
- 7.Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofacial Orthop. 2003 Dec;124(6):687–93. doi: 10.1016/j.ajodo.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Hafez HS, Selim EM, Kamel Eid FH, Tawfik WA, Al-Ashkar EA, Mostafa YA. Cytotoxicity, genotoxicity, and metal release in patients with fixed orthodontic appliances: a longitudinal in-vivo study. Am J Orthod Dentofacial Orthop. 2011 Sep;140(3):298–308. doi: 10.1016/j.ajodo.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Amini F, Borzabadi Farahani A, Jafari A, Rabbani M. In vivo study of metal content of oral mucosa cells in patients with and without fixed orthodontic appliances. Orthod Craniofac Res. 2008 Feb;11(1):51–6. doi: 10.1111/j.1601-6343.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz AJ, Fernandez E, Vicente A, Calvo JL, Ortiz C. Metallic ions released from stain-less steel, nickel-free, and titanium orthodontic alloys: toxicity and DNA damage. Am J Orthod Dentofacial Orthop. 2011 Sep;140(3):e115–22. doi: 10.1016/j.ajodo.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Angelieri F, Marcondes JP, de Almeida DC, Salvadori DM, Ribeiro DA. Genotoxicity of corrosion eluates obtained from orthodontic brackets in vitro. Am J Orthod Dentofacial Orthop. 2011 Apr;139(4):504–9. doi: 10.1016/j.ajodo.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 12.Eliades T, Pratsinis H, Kletsas D, Eliades G, Makou M. Characterization and cytotoxicity of ions released from stainless steel and nickel-titanium orthodontic alloys. Am J Orthod Dentofacial Orthop. 2004 Jan;125(1):24–9. doi: 10.1016/j.ajodo.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Tomakidi P, Koke U, Kern R, Erdinger L, Kruger H, Kohl A, et al. Assessment of acute cyto- and genotoxicity of corrosion eluates obtained from orthodontic materials using monolayer cultures of immortalized human gingival keratinocytes. J Orofac Orthop. 2000;61(1):2–19. doi: 10.1007/BF02340928. [DOI] [PubMed] [Google Scholar]
- 14.Pereira BR, Tanaka OM, Lima AA, Guariza-Filho O, Maruo H, Camargo ES. Metal and ceramic bracket effects on human buccal mucosa epithelial cells. Angle Orthod. 2009 Mar;79(2):373–9. doi: 10.2319/021508-92.1. [DOI] [PubMed] [Google Scholar]
- 15.Angelieri F, Carlin V, Martins RA, Ribeiro DA. Biomonitoring of mutagenicity and cytotoxicity in patients undergoing fixed orthodontic therapy. Am J Orthod Dentofacial Orthop. 2011 Apr;139(4 Suppl):e399–404. doi: 10.1016/j.ajodo.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 16.Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. 1993 Jan;285(1):35–44. doi: 10.1016/0027-5107(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 17.Natarajan M, Padmanabhan S, Chitharanjan A, Narasimhan M. Evaluation of the genotoxic effects of fixed appliances on oral mucosal cells and the relationship to nickel and chromium concentrations: an in-vivo study. Am J Orthod Dentofacial Orthop. 2011 Sep;140(3):383–8. doi: 10.1016/j.ajodo.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr. 2001;(29):7–15. doi: 10.1093/oxfordjournals.jncimonographs.a003443. [DOI] [PubMed] [Google Scholar]
- 19.Borthakur G, Butryee C, Stacewicz-Sapuntzakis M, Bowen PE. Exfoliated buccal mucosa cells as a source of DNA to study oxidative stress. Cancer Epidemiol Biomarkers Prev. 2008 Jan;17(1):212–9. doi: 10.1158/1055-9965.EPI-07-0706. [DOI] [PubMed] [Google Scholar]
- 20.Westphalen GH, Menezes LM, Pra D, Garcia GG, Schmitt VM, Henriques JA, et al. In vivo determination of genotoxicity induced by metals from orthodontic appliances using micronucleus and comet assays. Genet Mol Res. 2008;7(4):1259–66. doi: 10.4238/vol7-4gmr508. [DOI] [PubMed] [Google Scholar]
- 21.Barrett RD, Bishara SE, Quinn JK. Biode-gradation of orthodontic appliances. Part I. Biodegradation of nickel and chromium in vitro. Am J Orthod Dentofacial Orthop. 1993 Jan;103(1):8–14. doi: 10.1016/0889-5406(93)70098-9. [DOI] [PubMed] [Google Scholar]
- 22.Petoumenou E, Arndt M, Keilig L, Reimann S, Hoederath H, Eliades T, et al. Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am J Orthod Dentofacial Orthop. 2009 Jan;135(1):59–65. doi: 10.1016/j.ajodo.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Agaoglu G, Arun T, Izgi B, Yarat A. Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod. 2001 Oct;71(5):375–9. doi: 10.1043/0003-3219(2001)071<0375:NACLIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Singh DP, Sehgal V, Pradhan KL, Chandna A, Gupta R. Estimation of nickel and chromium in saliva of patients with fixed orthodontic appliances. World J Orthod. 2008 Fall;9(3):196–202. [PubMed] [Google Scholar]
- 25.Fors R, Persson M. Nickel in dental plaque and saliva in patients with and without orthodontic appliances. Eur J Orthod. 2006 Jun;28(3):292–7. doi: 10.1093/ejo/cji091. [DOI] [PubMed] [Google Scholar]
- 26.Menezes LM, Quintao CA, Bolognese AM. Urinary excretion levels of nickel in orthodontic patients. Am J Orthod Dentofacial Orthop. 2007 May;131(5):635–8. doi: 10.1016/j.ajodo.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Bishara SE, Barrett RD, Selim MI. Biode-gradation of orthodontic appliances. Part II. Changes in the blood level of nickel. Am J Orthod Dentofacial Orthop. 1993 Feb;103(2):115–9. doi: 10.1016/S0889-5406(05)81760-3. [DOI] [PubMed] [Google Scholar]
- 28.Eliades T, Trapalis C, Eliades G, Katsavrias E. Salivary metal levels of orthodontic patients: a novel methodological and analytical approach. Eur J Orthod. 2003 Feb;25(1):103–6. doi: 10.1093/ejo/25.1.103. [DOI] [PubMed] [Google Scholar]
- 29.Sfondrini MF, Cacciafesta V, Maffia E, Massironi S, Scribante A, Alberti G, et al. Chromium release from new stainless steel, recycled and nickel-free orthodontic brackets. Angle Orthod. 2009 Mar;79(2):361–7. doi: 10.2319/042108-223.1. [DOI] [PubMed] [Google Scholar]
- 30.Noda M, Wataha JC, Lockwood PE, Volkmann KR, Kaga M, Sano H. Low-dose, long-term exposures of dental material components alter human monocyte metabolism. J Biomed Mater Res. 2002 Nov;62(2):237–43. doi: 10.1002/jbm.10065. [DOI] [PubMed] [Google Scholar]
- 31.Wataha JC, Lockwood PE, Schedle A. Effect of silver, copper, mercury, and nickel ions on cellular proliferation during extended, low-dose exposures. J Biomed Mater Res. 2000 Nov;52(2):360–4. doi: 10.1002/1097-4636(200011)52:2<360::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]