Abstract
Patient: Female, 41
Final Diagnosis: Ovarian carcinoma
Symptoms: Ascites • hepatomegaly • weight loss
Medication: —
Clinical Procedure: —
Specialty: Oncology
Objective:
Unusual or unexpected effect of treatment
Background:
The aim of this case report is to present the results of treatment of end-stage ovarian carcinoma in a 41-year-old women using weight loss therapy.
Case Report:
We describe the case of a female aged 41 years with epithelial invasive ovarian cancer of III–IV stage, T3N2M1. Concurrent diseases were: abdominal carcinomatosis; hepatomegaly; ascites; condition after laparocentesis and skin-abdominal fistula; condition after 6 courses of neo-adjuvant polychemotherapy; hypertension II stage, risk factor of 3–4; dyslipidemia; and metabolic syndrome. A weight loss method based on a very-low-calorie diet and physical activity was used. Body weight was reduced from 74 kg to 53 due to loss of adipose tissue after 6 months of therapy. At the same time, the percentages of water and muscle tissue were increased significantly. While overweight was reducing, clinical, laboratory, and instrumental results were improving. As a result of the weight loss therapy, about ≈100 mm-sized ovarian cancer was transformed into smaller-sized ovarian cysts.
Conclusions:
An analgesic effect was also achieved without use of narcotic or non-narcotic analgesics. These cyto-reversible processes were documented by laboratory and instrumental data.
The mechanisms behind these differences remain to be elucidated. Future research with a larger study cohort and longer follow-up is needed to further investigate the role of caloric restriction diet in cancer cell changes in ovarian cancer.
MeSH Keywords: Ovarian Neoplasms, Weight Loss, Metabolic Syndrome X
Background
According to the American Society of Clinical Oncology, the prevalence of cancer is increasing globally [1]. Kazakhstan also has a similar trend [2]. Oncologic diseases have multiple causes. Different treatment methods are used, sometimes contradictory to each other [3,4]. Cost-effectiveness studies of cancer treatment reveal that it is among the most costly diseases in medicine [5]. Therefore, every new treatment method that can lead to the regression of the tumor process should merit attention [6].
In recent years, many researchers consider neoplastic process in the body as a systemic disease [7,8]. Therefore, research attention focusses on methods of cancer treatment that lead to normalization of metabolic disorders [9,10].
The aim of this case report is to present the results of treatment of end-stage ovarian carcinoma in the 41-year-old women using weight loss therapy.
Case Report
E.E.A. was a 41-year-old female patient, case history number 53 (the opening date was August 19, 2012).
The patient life-history and disease. She considered herself as being sick beginning January 2012 when there was right-sided abdominal pain near the tumor, abdominal distension, and severe weakness. According to her patient history there were recurrent abdominal pains in the last 6 months and weight loss of 12 kg during 3 months (from 86 kg to 74 kg). She applied to a polyclinic in the community in February 21, 2012, where she was redirected to the polyclinic of a municipal Oncology Center of Almaty City (MOCA), where laboratory and instrumental investigations were conducted. The results of abdominal ultrasound on February 21, 2012 were: diffuse changes in the liver parenchyma (fatty) and pancreas (fatty), and free liquid in the peritoneal cavity. The results of abdominal MRI on February 24, 2012: ovarian carcinoma. Esophagogastroduodenoscopy results on March 14, 2012: pyloroduodenitis; superficial gastritis with focal atrophy; first-degree reflux esophagitis; lower esophageal sphincter insufficiency.
In addition to the anamnesis: The last 6 years was she had a pollen allergy treated by Diprospan. Menstruation ceased in the middle of 2011 (6 months before the abdominal pain began). She has a daughter, and had 2 abortions in last 5 years. The results of pelvic ultrasound on November 19, 2010: the right and left ovaries dimensions of 23×19 mm and 24×22 mm, respectively; in the behind the uterus space is inhomogeneous formation with calcifications and a cavernous component up to 24 mm, which is not associated with the intestine; signs of bilateral salpingo-oophoritis.
The patient received hospital treatment in the MOCA hospital for further examination, confirmation of the diagnosis, and treatment from March 15, 2012 to March 28, 2012.
The results of survey chest radiography on March 15, 2012: a small amount of pleural effusion in the right sinus.
CT scan results of the abdominal and pelvic organs on March 17, 2012: conglomerate cystic-solid formation in the pelvis; the formation of perirectal tissue with involvement in the process of the rectum upper ampulla; lymphadenopathy of pelvic and retroperitoneal lymph nodes, metastases signs; carcinomatosis of the abdominal cavity and pelvis; hepatomegaly; ascites; incidental uretero-hydronephrosis. CA 125 ovarian cancer biomarker in the blood on February 24, 2012 was 623 U/ml (normal is 0–35).
March 16, 2012 laparocentesis was performed in the hospital for the purpose of cytology of the pelvic formation. About 5 liters transparent and straw-yellow liquid was withdrawn from the peritoneal cavity. The result of the cytology (No 8897-99) on March 26, 2012 was squamous cell carcinoma, and the cytogram was typical for ovarian carcinoma metastases in the abdominal cavity.
The complete blood count results on March 25, 2012 were: hemoglobin 136 g/l, erythrocytes 4.2×1012/l, leukocytes 5.8×109/l, and platelets 335×109/l.
Immunosorbent assay on HIV, RW, and HBsAg, HCV on March 7, 2012 (#1604479, #201, and #1604479, respectively) were negative.
The patient was given neo-adjuvant polychemotherapy (NAPCT) before surgery. The first NAPCT course started March 20, 2012: Kemocarb injection (carboplatin) 450 mg per course and Paclitaxel 230 mg with antiemetic (Osetron, Metoclopramide) and infusion (Aminoplasmal, glucose-insulin-potassium infusion) and immunocorrective (Prednisolone, Dexamethasone) therapy.
The patient was discharged in moderately severe condition caused by the oncologic disease under observation of the MOCA polyclinic with concluding diagnosis of epithelial invasive ovarian cancer, stage III (T3N2M1). Abdominal carcinomatosis was present. Recommendations were observation in the MOCA polyclinic, hepatoprotectors, and continuing NAPCT after 3 weeks.
From March 20, 2012 to June 27, 2012 5 courses of NAPCT were administered with 3-week intervals between courses. Three courses were conducted by the scheme of Kemocarb 450 mg and Paclitaxel 230 mg. The fourth course was by the scheme of Kemocarb 600 mg and Taxotere (docetaxel) 120 mg. The fifth course (from June 19, 2012) was by the scheme of Gemzar (gemcitabine) 1200 mg on the 1st and 8th days and Caelyx (doxorubicin) 50 mg.
The results of abdominal and pelvic ultrasound after the 5 NAPCT courses were (on June 21, 2012): blurred diffuse changes in the liver parenchyma (fatty) and pancreas (fatty); incidental moderately marked urinary stasis (pyelectasis up to 21×23 mm, calicectasis up to 12 mm); residual slight ascites; single carcinomatosis lesions in the upper abdomen; cancer of both ovaries in the NAPCT process with signs of increasing size; carcinomatosis lesions in the lower abdomen; metastases in retroperitoneal lymph nodes with pathomorphosis signs.
CA 125 ovarian cancer biomarker on May 6, 2012 was 705 U/ml.
From July 23, 2012 to August 7, 2012 the patient received the second hospital treatment with concluding diagnosis: Epithelial invasive ovarian cancer, stage of III–IV (T3N2M1). Abdominal carcinomatosis was present. Condition after 6 of NAPCT courses: the cancer was in progression.
Biochemical blood test results: total protein 69.5 g/l, urea 5 mmol/l, glucose 4.4 mmol/l, ALT level 51 U/l, AST level 43 U/l, total bilirubin 16.4 umol/l, thymol test 3.6 U.
The result of electrocardiography on 17 July, 2012 was sinus tachycardia, heart rate 95 beats/min, normal electrical heart axis.
The complete blood count on July 17, 2012 was unremarkable except platelets 200×109/l, ESR 44 mm/hour. Urine analysis result was unremarkable.
CA 125 ovarian cancer biomarker on July 16, 2012 was 563 U/ml.
CT findings of the abdominal and pelvic organs on July 25, 2012 were: ovarian cancer with conglomerate formation of carcinogenesis of pelvis (on the right 2 solid formations with bumpy contours with size of 102×52×77 mm and 85×79×68 mm, on the left a formation with size of 83×57×95 mm, density from +34 to 55 HU) on the background of NAPCT; formation of perirectal tissue with involving in this process of the rectum upper ampulla and of the distal sigmoid colon; signs of abdomen and pelvis carcinomatosis; hepatomegaly. In comparison with data from the CT scan from March 17, 2012, there was marked ascites regression with growth of the formation in the pelvis.
Beginning July 26, 2012 the 6th NAPCT course was carried out by the scheme of: Gemzar (gemcitabine) 1400 mg on the 1st and 8th days, Caelyx (doxorubicin) 60 mg with detoxification (saline solution #12, glucose-insulin-potassium infusion #3) and antiemetic (Ondem (ondansetron) 8 mg, #3, Metoclopramide 2.0 ml, #20), corticosteroids (dexamethasone 8 mg, #7, prednisolone 30 mg, #3) therapy.
The complete blood count on August 2, 2012 was unremarkable except leukocytes were 9.9×109/l, platelets were 145×109/l, ESR was 38 mm/hour. She was discharged in moderately severe condition caused by the oncologic disease under observation of the MOCA polyclinic.
During the NAPCT course the patient began complaining of partial and then complete alopecia, diarrhea, teeth vacillation, severe decline in working capacity, and sleep disturbance. Oncologic surgeon consultation results: because of metastatic disease progression on the upper sections of the colon, palliative surgery was recommended with moving of colostomy to the anterior abdominal side. The patient refused the surgery. She was discharged at home under observation of a local therapist.
August 19, 2012 the patient consulted with oncologists of a research group working in the Republican Scientific Center for Emergency Medicine at the National Medical Holding Center. Considering the disease history, moderately severe condition, and informed consent to the treatment, we decided to use the technique of “analimentary detoxification” (ANADET) based on the principle of weight loss therapy [11,12].
The mechanism of ANADET is based on a weight loss method using a very low-calorie diet and physical activity, in which the body becomes able to recycle unwanted cells, including changed/tumor cells, as a source of power/energy [13,14]. Duration of the ANADET is 10 to 35 days, depending on the disease and patient characteristics.
One of the main provisions of the treatment is to maintain the target body weight. Reducing diet to 300–400 kcal/day, physical activity is at least 10 000 steps/day. The reduced diet includes very low calorie vegetables. A Hoffmann-La Roche pedometer was used for measuring walking activity.
At the time (August 19, 2012) the patient was in moderately severe condition caused by the progressive oncologic disease. There was a painful syndrome, becoming worse 1.5–2 hours after eating. The laparocentesis site became profusely wet and formed the skin-peritoneal fistula from 16 March 2012. Blood pressure was 145/90 mm Hg. Body weight was 74 kg, height was 175 cm, and body mass index (BMI) was 24.2 kg/m2. Skin turgor index (thickness of subcutaneous fat) was: mean inside of the forearm was 56 mm (normally it is up to 10 mm), the mesogastric umbilical region was 100 mm (normal is up to 20 mm), and the lumbar region was 120 mm (normal is up to 20 mm).
The results of abdominal ultrasound on August 24, 2012 were: cancer of both ovaries, on the right a hyperechoic component with size of 100×90×60 mm with an uneven outline; on the left an anechoic component size 48 mm in diameter with a smooth contour (Figure 1).
Figure 1.
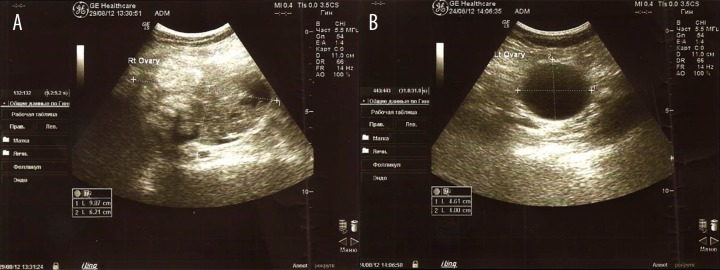
Ultrasound images of the right (A) and left (B) ovaries on August 24, 2012.
The patient diagnosis was oncologic disease: epithelial invasive ovarian cancer (from the result of the cytology (No 8897-99) from March 26, 2012), stage of III–IV, T3N2M1. There were abdominal carcinomatosis, hepatomegaly;, and ascites. Condition after laparocentesis and skin-abdominal fistula and condition after 6 of NAPCT course: hypertension II stage, risk factor of 3–4, dyslipidemia, and metabolic syndrome.
Dynamics and results
Clinical and laboratory results after 10 days of treatment: Body mass was 67 kg. The complete blood count on August 29, 2012 was hemoglobin 132 g/l, erythrocytes 4.1×1012/l, leukocytes 4.2×109/l, platelets 250×109/l, ESR 20 mm/hour. Urine analysis result was a dark color, muddy, density 1025, mucus ++, leukocytes 10–15, single erythrocytes. Biochemical blood test results: total protein 73.3 g/l, urea 2.4 mmol/l, creatinine 79 umol/l, glucose 2.7 mmol/l, ALT 30 U/l, AST 32 U/l, total bilirubin 11.9 umol/l, and cholesterol 5.6 mmol/l. Coprogram was unformed, no mucus or pus, fat+.
During the first 10–15 days of the treatment, the patient complained of headache, nausea, ichorrhea from the genital tract, skin itching, muddy urine with occasional dysuria with stranguria, and fever up to 38.3°C, which were stopped in 2–3 days due to compliance with the treatment recommendations. There was a healing of the skin-abdominal fistula site, which became dry.
Clinical and laboratory results after 1 month of therapy: Body mass was 61 kg (minus 13 kg [17.5% of the initial]). Blood pressure was 115/80 mm Hg. The complete blood count on October 20, 2012: hemoglobin 146 g/l, erythrocytes 4.3×1012/l, leukocytes 5.8×109/l, platelets 230×109/l, ESR 10 mm/hour. Urine analysis results were unremarkable. Biochemical blood test: total protein 69 g/l, glucose 5.3 mmol/l, cholesterol 4.5 mmol/l, and the rest were unremarkable. CA 125 ovarian cancer biomarker on October 4, 2012 was 451 U/ml.
The results of pelvic ultrasound on September 19, 2012 were: Ovarian cancer was present during the treatment. In the right of the ovarian projection there was an anechoic formation with smooth and clear outline with size of 71×48 mm, inside of the formation was a hyperechoic inclusion with diameter up to 7 mm. In the left ovary there was a cystic solid formation with size of 82×37 mm with uneven contour. There were positive dynamics, reducing the size and regression some lesions.
The results of pelvic ultrasound on October 16, 2012 were: ovarian cancer was present during the treatment. In the right of the ovarian projection there was a cystic formation with size of 58×46×65 mm with single hyperechoic inclusions with size up to 8×6 mm with uneven contour. In the left ovary there was a cystic solid formation with size of 75×46 mm with uneven contour. Above of the uterine fundus, in the right iliac region, there was a solid formation with size of 58×44×47 mm predominantly, and there was stabilization of the oncologic process.
Clinical and laboratory results after 2 months of therapy: Blood pressure 115/80 mm Hg. Body mass 56 kg (minus 18 kg [24.3% of the initial]). The complete blood count on November 15, 2012: hemoglobin 153 g/l, erythrocytes 4.5×1012/l, leukocytes 5.0×109/l, platelets 220×109/l, ESR 16 mm/hour. Biochemical blood test: total protein 73 g/l, cholesterol 4.8 mmol/l, and the rest were unremarkable. Urine analysis results were unremarkable. CA 125 ovarian cancer biomarker on November 27, 2012 was 418 U/ml. The site of the skin-abdominal fistula was dry. The patient complained of smearing bleeding from the genital tract. Tumor cells were not found in the smear from the genital tract secretions.
MRI scan results of pelvic organs on November 14, 2012: MRI image of a pelvic cystic formation (a single chamber formation with size of 62×56×47 mm and on the inner contour there was a tissue inclusion up to 9 mm). Metastatic lesion of iliac lymph nodes on both sides (in the iliac region on the left and on the right were determined conglomerates of hyperplastic lymph nodes with size of 66×55×50 mm and 60×56×53 mm, respectively).
The results of pelvic ultrasound on November 23, 2012: Ovarian cancer was present during the treatment. In the right iliac region there was a cystic formation with size of 55×43×63 mm with single hyperechoic inclusions. In the left iliac region there was a cystic formation with size of 64×40 mm with single internal partitions. In the right of the uterus in the iliac region there was defined a cystic structure with the presence of elongated internal partition with size of 59×28×63 mm. There was stabilization of the oncologic process. Along the left iliac vessels there was defined a hypoechoic formation with size of 31×19 mm, and on the right a hypoechoic inhomogeneous formation with size of 45×40 mm with the presence of multiple calcifications (Figure 2).
Figure 2.
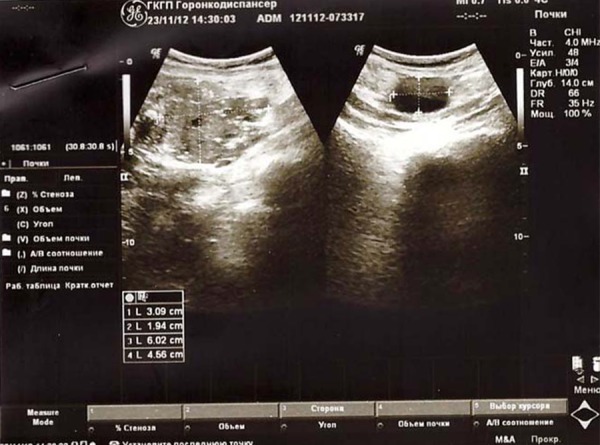
Ultrasound images of the right and left ovaries on November 23, 2012.
Clinical, laboratory, and instrumental results after 6 months therapy: Body mass was 53 kg (minus 21 kg [28% of the initial]). Blood pressure was 115/80 mm Hg. On the basis of the achieved positive clinical and laboratory parameters, we recommended the patient achieve a body weight in the range of 52–54 kg. The complete blood count on February 28, 2013: hemoglobin 136 g/l, platelets 224×109/l, ESR 10 mm/hour. Biochemical blood test: total protein 74 g/l, urea 6.5 mmol/l, cholesterol 4.0 mmol/l, and the rest were unremarkable. Urine analysis was in the normal range. CA 125 ovarian cancer biomarker on February 7, 2013 was 384 U/ml. The patient had no complaints and her performance was fully restored.
The results of pelvic ultrasound on February 8, 2013: Ovarian cancer was present after the treatment. In the right of the ovarian projection there was a retained anechoic liquid formation with size of 43×63 mm with single hyperechoic inclusions, in the left projection an anechoic formation with size of 63×31 mm; on the right of the uterus we defined an anechoic elongated formation with the internal partition with size of 36×63 mm. Along the iliac vessels there was a retained hypoechoic formation with size of 58×43 mm on the right side and 56×38 mm on the left side, which had many calcifications and destruction cavities inside. This marks the stabilization of the oncologic process.
The treatment results after 1 year: Body mass was 53 kg. Blood pressure was 115/80 mm Hg. The complete blood count on September 3, 2013: hemoglobin 153 g/l, erythrocytes 4.6×1012/l, color index of blood 0.9, leukocytes 7.3×109/l, platelets 240×109/l, ESR 5 mm/hour.
Biochemical blood test: total protein 73.3 g/l, cholesterol 4.6 mmol/l, and the rest were unremarkable. Urine analysis was unremarkable. CA 125 ovarian cancer biomarker the October 2, 2013 was 338 U/ml. No patient complaints.
The results of pelvic ultrasound on September 12, 2013: Ovarian cancer after the treatment. In the right of ovarian projection there was a retained anechoic liquid formation with size of 34×47 mm with single hyperechoic inclusions; in the left projection an anechoic formation with size of 52×33 mm; in the right and posterior of the uterus there was a retained multi-chamber liquid elongated formation with size of 28×72 mm with the presence of suspended anechoic matter. Along the iliac vessels there was a hypoechoic formation with size of 47×23 mm on the right side and 52×33 mm on the left side with many calcifications and destruction cavities inside. This represents the stabilization of the oncologic process (Figure 3).
Figure 3.
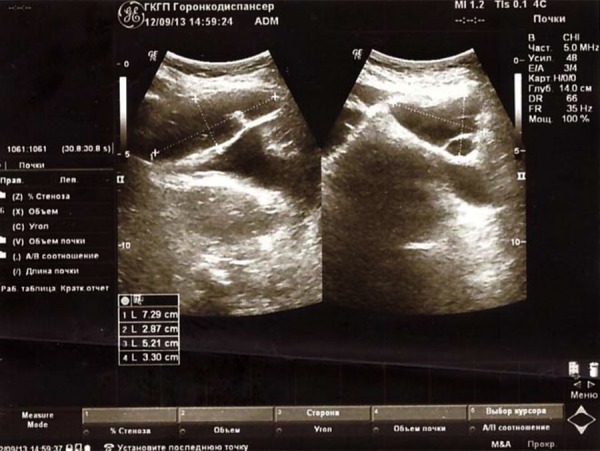
Ultrasound images of the right and left ovaries on September 12, 2013.
While overweight was reduced, it was essential to determine what kind of tissue the weight loss was occurring in. Comparative results obtained using a Tanita SC-330 body composition analyzer (Japan) before and after 6 months of the treatment are shown in Table 1.
Table 1.
Comparative data characteristics of the body composition analyzer “Tanita SC-330” before and after 6 months of the treatment.
| Indicators | Before treatment | After 6 months treatment |
|---|---|---|
| Body mass (kg) | 74 | 53 |
| BMI (kg/m2) | 24.2 | 17.3 |
| Fat (%) | 29.48 | 20.27 |
| Fat (kg) | 27.30 | 8.02 |
| Visceral fat level (Units) | 12.3 | 8.02 |
| Metabolicage (years) | 55.8 | 36.6 |
| Lean mass (kg) | 46.70 | 44.98 |
| Water (kg) | 36.1 | 30.8 |
| Water (%) | 48.8 | 58.1 |
| Muscle mass (kg) | 43.8 | 42.1 |
| Muscle mass (%) | 59.19 | 79.43 |
| Bone mass (kg) | 2.9 | 2.88 |
| Basal metabolic (kkal per day) | 1794.76 | 1398.12 |
| Impedance (OM) | 503.4 | 469.5 |
Table 1 data shows that the weight loss of the patient was due to adipose tissue. At the same time the percentage of water and muscle tissue were increased significantly, whereas an actual increase of muscle mass did not happen. Water mass decreased, which is apparently connected with the decreased ascites and peripheral edema. Importantly, while there was reduction of fat mass, there was no change in lean body mass (lean tissue). These data may indicate that the weight loss achieved through the ANADET methodology takes place only due to reduced body fat mass, while the absolute level of lean mass, including muscle and bone mass, is unchanged.
As shown in Table 1, the overweight reduction led to improving indicators such as metabolic age, basal metabolic rate, and impedance.
It is interesting to note that during the observation period (17 months) after the start of the treatment, the tumor growth was observed as body mass increased (ultrasound data), which was due to non-adherence to the recommended diet. However, while the patient was losing the gained body mass, tumors shrank or disappeared (ultrasound data).
Thus, as a result of our treatment method, there was reversion of end-stage epithelial invasive ovarian carcinoma in ovarian cysts.
Discussion
Of all female genital organ cancers, ovarian cancer is the leading cause of mortality [15]. The majority of women with metastatic ovarian cancer die with minimal (2 cm) residual intraperitoneal tumor remaining after initial laparotomy and current cytoreductive therapy. They have a 5-year survival of only 20% [16].
In most cases, ovarian cancer develops in women over 50 years old [17]. Ovarian cancer developing in young women is characterized by chemoresistance, malignant current, and low survival [18]. One-year overall survival changed from 73% (95% confidence interval (CI): 69–78) in 2000–2002 and to 69% (95% CI: 63–73) in 2009–2011 [19]. Five-year survival changed only slightly during the study period, from 37% (95% CI: 32–42) in 2000–2002 to 39% (95% CI: 34–44) in 2009–2011. According to other authors, indicators of 5-year survival of women with ovarian cancer are below 27% [20].
Based on symptoms before the development of ovarian cancer, such as irregular menstruation and then amenorrhea, and overweight, we can assume that polycystic ovaries syndrome (PCOS) can precede ovarian cancer. The criteria for the PCOS diagnosis are hyperandrogenism, ultrasound definition of cysts, hormonal disorders, and overweight [21]. Overweight and dysmetabolic disorders are constant companions of PCOS [22].
Medline and Embase databases (1968–2008) including 19 studies showed the association between PCOS and gynecological cancers. A meta-analysis of the data suggests that women with PCOS are more likely to develop cancer of the endometrium (OR 2.70, 95% CI 1.00–7.29) and ovarian cancer (OR 2.52, 95% CI 1.08–5.89) [23].
The risk factors increased proportionally to BMI, indicating that the metabolic profile of obese women with PCOS is more unfavorable than that of non-obese patients [24].
Weight loss is one of the basic methods of PCOS therapy [25]. In recent years, more and more researchers pay attention to methods of weight loss as a main factor to control both PCOS and its complications [26].
Many studies show the relationship between the development of neoplastic diseases of female genitals (ovary and uterus) and presence of overweight [27,28].
Approximately 60% to 90% of patients with ovarian cancer and endometrial cancer have overweight or obesity [29,30]. Some studies have indicated obesity is a negative prognostic indicator for survival [31,32], while others did not show a significant difference in overall outcomes [33]. Large cohorts of ovarian cancer patients have demonstrated that the risk of ovarian cancer mortality is increased among those with higher BMI [34,35].
Obesity increases risk of several cancers, including breast, endometrium, kidney, esophageal adenocarcinoma, and colon cancers [36]. Overweight or obese cancer survivors may suffer from obesity-related comorbidities, including type II diabetes, hypertension, cardiovascular disease, osteoarthritis, and pulmonary disease [37].
In the last several years, researchers have begun to pay attention to the therapeutic power of the factors leading to weight loss in cancer diseases [38,39], including ovarian cancer [40].
Cancer development is accompanied by decrease of body weight [41]. One population-based cohort study suggested that weight loss pre- to post-diagnosis may increase risk of mortality in women with breast cancer [42]. This reduction of body weight is associated with growth and metastasis of the malignant tumor process in which, even in overweight patients, muscle loss occurs [43]. If body weight of a patient is reduced due only to reduced fat mass, the cancer cells will experience a lack of nutrients for their growth, which can lead to a cytoreductive process [44].
In 2006, the American Cancer Society recommended that patients with cancer maintain normal weight, increase physical activity, and eat a diet low in fat and refined carbohydrates and high in vegetables and fruits, as a potential aid to some aspects of prognosis, acknowledging the lack of definitive data [45].
A positive effect of a weight loss therapy on cancer is described in a broad review article [46]. Evidence that diet can prevent cancer or the recurrence of cancer is mounting. In the Women’s Intervention Nutrition Study, involving breast cancer patients who were on curative therapy, a low-fat diet was associated with reduced risk for cancer recurrence, particularly in those with estrogen-receptor negative cancers [47].
What is the mechanism of healing of a patient using a low-calorie diet for treatment of cancer? One of explanation could be that the cancer cells, for their own growth and reproduction, use the glucose, fatty acids, ketones, lactate, cholesterol, and other metabolites of fats and carbohydrates metabolism [48,49]. Hyperglycemia and hyperlipidemia contribute to the growth of tumor cells [50,51]. Consequently, if intake of food calories is restricted, conditions are created that will limit “fuel” for cancer cells [52]. In addition, because the body needs nutrients, a “search engine” activates metabolic substances inside own body [53,54], in which the tumor cells can serve as a source of organic substances [55,56].
The very-low-carbohydrate diet strategy is aimed at starving cancer cells that are dependent on glucose, which has both direct and indirect effects on tumor proliferation [57].
In recent years, interest in dietary manipulations in the treatment of cancer has increased, but the optimal strategies are not known. In preclinical studies, metabolic dietary therapies, such as calorie restriction, fasting, and ketogenic diets, have been shown to slow the growth of cancer, but few human clinical trials have been conducted [58,59].
Several studies have investigated the relationship between weight gain after diagnosis and prognosis [60], of which 3 reported increased recurrence risk or decreased survival with weight gain [61].
As the link between obesity and metabolic syndrome and cancer becomes clearer, the need to determine the optimal way to incorporate dietary manipulation into the treatment of cancer patients becomes increasingly important [62]. Metabolic-based therapies, such as caloric restriction, intermittent fasting, and a ketogenic diet, have the ability to decrease the incidence of spontaneous tumors and slow the growth of primary tumors, and may have an effect on distant metastases.
For the first time ever, a randomized controlled trial that uses calorie restriction as a treatment for cancer – and measures a cancer-related outcome – was approved by the institutional review board at Duke University in Durham, North Carolina, and is on its way to the clinic [63–65].
Conclusions
The patient in this case report and treatment program had been previously regarded as incurable by existing official methods of specific oncological treatment.
On the basis of the treatment, ovarian cancer was changed to small ovarian cysts. An analgesic effect was also achieved, without use of narcotic or non-narcotic analgesics. The cytoreversible processes have been documented by our laboratory and instrumental data.
The evidence for weight loss therapy and cancer risk is inadequate and inconclusive. However, we have obtained some positive evidence for risk reduction of ovarian cancer by using weight loss therapy.
The mechanisms behind these differences remain to be elucidated. Further research is also needed to determine the various amounts and intensities of caloric restriction diet and physical exercise required for optimum cancer prevention, recovery, and survival.
Future research with a larger study cohort and longer follow-up is needed to further investigate the role of caloric restriction diet in cancer cell changes in women with ovarian cancer. Further research is also needed to evaluate the potential effects of body composition changes on ovarian cancer outcomes.
Footnotes
Funding
This work was performed within the scientific program of the grant of the Ministry of Education and Science of the Republic of Kazakhstan on “New systemic therapy of malignant tumors of various origins and locations: a creation of a new paradigm” for 2012–2014, contract #395 of 31.08.2012.
References:
- 1.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30:1715–24. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 2.Health of population of the Republic of Kazakhstan and activity of health organizations in 2010–1012 years (statistical material) Vol. 2013. Astana: Medinform; p. 346. [Google Scholar]
- 3.Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–76. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361(9375):2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y-Y, Kwok AC, Jiang W, et al. High-Cost Imaging in Elderly Patients With Stage IV Cancer. J Natl Cancer Inst. 2012;104(15):1165–73. doi: 10.1093/jnci/djs286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitahara CM, Berndt SI, de González AB, et al. Prospective investigation of body mass index, colorectal adenoma, and colorectal cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J Clin Oncol. 2013;31(19):2450–59. doi: 10.1200/JCO.2012.48.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alibek K, Aituov B, Duisembekova A, Bulenova A. Pathogen-driven gastrointestinal cancers: Time for a change in treatment paradigm? Infect Agent Cancer. 2012;7(1):18. doi: 10.1186/1750-9378-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez-Lara K, Hernández D, Motola D, Green D. Association between over-weight, glucocorticoids and metabolic syndrome in cancer patients under chemotherapy. Nutr Hosp. 2013;28(1):182–87. doi: 10.3305/nh.2013.28.1.6177. [DOI] [PubMed] [Google Scholar]
- 9.Balogun N, Forbes A, Widschwendter M, Lanceley A. Noninvasive nutritional management of ovarian cancer patients: beyond intestinal obstruction. Int J Gynecol Cancer. 2012;22(6):1089–95. doi: 10.1097/IGC.0b013e318256e4d3. [DOI] [PubMed] [Google Scholar]
- 10.Extermann M. Metabolic syndrome and cancer: from bedside to bench and back. Interdiscip Top Gerontol. 2013;38:49–60. doi: 10.1159/000343621. [DOI] [PubMed] [Google Scholar]
- 11.The invention Patent #13868 of 16.07.2007. A method of emergency treatment of metabolic syndrome, including type 2 diabetes mellitus, arterial hypertension stage of 2–3, diabetic nephropathy and chronic renal failure stage of 1–2. Oshakbayev KP, Abylayuly Zh, Dzhusipov AK – bulletin 7. – Application #2002/0688.1 of 23.05.2002. – ÀÑ #38808
- 12.Oshakbayev KP, Abylayuly Zh.Clinical management of metabolic processes at metabolic syndrome, and the method of treatment «analimentary detoxification» (ANADET). /The state registration certificate of intellectual property of the Committee on Intellectual Property Rights of the Ministry of Justice of the Republic of Kazakhstan. – #206 of June 21, 2006– IP 01875 [Google Scholar]
- 13.Oshakbayev KP. In: Clinical management of metabolic syndrome: a practical guideline. Abylayuly Zh., editor. Vol. 2007. Àlmaty: Ziat Press; p. 326. [Google Scholar]
- 14.Priebe A, Tan L, Wahl H, et al. Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells. Gynecol Oncol. 2011;122:389–95. doi: 10.1016/j.ygyno.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Su Z, Graybill WS, Zhu Y. Detection and monitoring of ovarian cancer. Clin Chim Acta. 2013;415:341–45. doi: 10.1016/j.cca.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 16.Ulker V, Numanoglu C, Alpay V, et al. Characteristics and prognosis of ovarian metastatic tumors: review of a single-institution experience. Eur J Gynaecol Oncol. 2013;34(1):75–78. [PubMed] [Google Scholar]
- 17.Lim MC, Moon EK, Shin A, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999–2010. J Gynecol Oncol. 2013;24(4):298–302. doi: 10.3802/jgo.2013.24.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershenson DM. The life and times of low-grade serous carcinoma of the ovary. Am Soc Clin Oncol Educ Book. 2013:195–99. doi: 10.14694/EdBook_AM.2013.33.e195. [DOI] [PubMed] [Google Scholar]
- 19.Grann AF, Thomsen RW, Jacobsen JB, et al. Comorbidity and survival of Danish ovarian cancer patients from 2000–2011: a population-based cohort study. Clin Epidemiol. 2013;5(Suppl.1):57–63. doi: 10.2147/CLEP.S47205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres ML, Hartmann LC, Cliby WA, et al. Nutritional status, CT body composition measures and survival in ovarian cancer. Gynecol Oncol. 2013;129(3):548–53. doi: 10.1016/j.ygyno.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Kubota T. Update in polycystic ovary syndrome: new criteria of diagnosis and treatment in Japan. Reprod Med Biol. 2013;12(3):71–77. doi: 10.1007/s12522-013-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zabuliene L, Tutkuviene J. Body composition and polycystic ovary syndrome. Medicina (Kaunas) 2010;46(2):142–57. [PubMed] [Google Scholar]
- 23.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009;19(3):398–405. doi: 10.1016/s1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 24.Sousa RM, Chein MB, Silva DS, et al. Metabolic profile in women of different body mass indices with polycystic ovary syndrome. Rev Bras Ginecol Obstet. 2013;35(9):413–20. doi: 10.1590/s0100-72032013000900006. [DOI] [PubMed] [Google Scholar]
- 25.Ravn P, Haugen AG, Glintborg D. Overweight in polycystic ovary syndrome. An update on evidence based advice on diet, exercise and metformin use for weight loss. Minerva Endocrinol. 2013;38(1):59–76. [PubMed] [Google Scholar]
- 26.Panidis D, Tziomalos K, Papadakis E, et al. Lifestyle intervention and anti-obesity therapies in the polycystic ovary syndrome: impact on metabolism and fertility. Endocrine. 2013;44(3):583–90. doi: 10.1007/s12020-013-9971-5. [DOI] [PubMed] [Google Scholar]
- 27.Pothiwala P, Jain SK, Yaturu S. Metabolic syndrome and cancer. Metab Syndr Relat Disord. 2009;7(4):279–88. doi: 10.1089/met.2008.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves GK, Pirie K, Beral V, et al. Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett SV, Paul J, Hay A, et al. Scottish Gynaecological Cancer Trials Group. Does body mass index affect progression-free or overall survival in patients with ovarian cancer? Results from SCOTROC I trial. Ann Oncol. 2008;19(5):898–902. doi: 10.1093/annonc/mdm606. [DOI] [PubMed] [Google Scholar]
- 30.Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Review Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114(1):121–27. doi: 10.1016/j.ygyno.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Pavelka JC, Brown RS, Karlan BY, et al. Effect of obesity on survival in epithelial ovarian cancer. Cancer. 2006;107:1520–24. doi: 10.1002/cncr.22194. [DOI] [PubMed] [Google Scholar]
- 32.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 33.Matthews KS, Straughn JM, Jr, Kemper MK, et al. The effect of obesity on survival in patients with ovarian cancer. Gynecol Oncol. 2009;112:389–93. doi: 10.1016/j.ygyno.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Modesitt SC, van Nagell JR., Jr Review The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet Gynecol Surv. 2005;60(10):683–92. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Xie X, Lee AH, et al. Body mass index in relation to ovarian cancer survival. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1307–10. doi: 10.1158/1055-9965.EPI-04-0519. [DOI] [PubMed] [Google Scholar]
- 36.IARC Working Group on the Evaluation of Cancer-Preventive Agents . Weight Control and Physical Activity. Vol. 6. Lyon, France: IARC; 2002. IARC Handbooks of Cancer Prevention. [Google Scholar]
- 37.von Gruenigen VE, Courneya KS, Gibbons HE, et al. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecol Oncol. 2008;109(1):19–26. doi: 10.1016/j.ygyno.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 38.Gago-Dominguez M, Jiang X, Castelao JE. Review Lipid peroxidation, oxidative stress genes and dietary factors in breast cancer protection: a hypothesis. Breast Cancer Res. 2007;9(1):201. doi: 10.1186/bcr1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55(1):62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson R, Bryant S, Huntley AL. Green tea and green tea catechin extracts: an overview of the clinical evidence. Maturitas. 2012;73(4):280–87. doi: 10.1016/j.maturitas.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 42.Caan BJ, Kwan ML, Hartzell G, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19(10):1319–28. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 44.Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. A pilot safety and feasibility trial of a reduced carbohydrate diet in patients with advanced cancer. J Clin Oncol. 2011;29 (Suppl.; abstr e13573) [Google Scholar]
- 45.Doyle C, Kushi LH, Byers T, et al. Nutrition, Physical Activity and Cancer Survivorship Advisory Committee. Review Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. Cancer J Clin. 2006;56(6):323–53. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 46.Lemanne D, Cassileth B, Gubili J. The role of physical activity in cancer prevention, treatment, recovery, and survivorship. Oncology (Williston Park) 2013;27(6):580–85. [PubMed] [Google Scholar]
- 47.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–76. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 48.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, et al. Ketones and lactate «fuel» tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–14. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stattin P, Bjor O, Ferrari P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30:561–67. doi: 10.2337/dc06-0922. [DOI] [PubMed] [Google Scholar]
- 50.Ikeda F, Doi Y, Yonemoto K, et al. Hyperglycemia increases risk of gastric cancer posed by Helicobacter pylori infection: a populationbased cohort study. Gastroenterology. 2009;136:1234–41. doi: 10.1053/j.gastro.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 51.Young CD, Anderson SM. Sugar and fat – that’s where it’s at: metabolic changes in tumors. Breast Cancer Res. 2008;10:202. doi: 10.1186/bcr1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–16. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- 53.Priebe A, Tan L, Wahl H, et al. Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells. Gynecol Oncol. 2011;122:389–95. doi: 10.1016/j.ygyno.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 54.Kunkel M, Reichert TE, Benz P, et al. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–24. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 55.Yoshikawa T, Noguchi Y, Matsumoto A. Effects of tumor removal and body weight loss on insulin resistance in patients with cancer. Surgery. 1994;116:62–66. [PubMed] [Google Scholar]
- 56.Fine EJ, Segal-Isaacson CJ, Feinman R, Sparano J. Carbohydrate restriction in patients with advanced cancer: a protocol to assess safety and feasibility with an accompanying hypothesis. Commun Oncol. 2008;5:22–26. [Google Scholar]
- 57.Saleh AD, Simone BA, Palazzo J, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle. 2013;12:1955–63. doi: 10.4161/cc.25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simone BA, Champ CE, Rosenberg AL, et al. Selectively starving cancer cells through dietary manipulation: methods and clinical implications. Future Oncol. 2013;9:959–76. doi: 10.2217/fon.13.31. [DOI] [PubMed] [Google Scholar]
- 59.Klement RJ, Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond) 2011;8:75. doi: 10.1186/1743-7075-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caan BJ, Emond JA, Natarajan L, et al. Post-diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Treat. 2006;99(1):47–57. doi: 10.1007/s10549-006-9179-y. [DOI] [PubMed] [Google Scholar]
- 61.Camoriano JK, Loprinzi CL, Ingle JN, et al. Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol. 1990;8:1327–34. doi: 10.1200/JCO.1990.8.8.1327. [DOI] [PubMed] [Google Scholar]
- 62.Braun S, Bitton-Worms K, Leroith D. The Link between the Metabolic Syndrome and Cancer. Int J Biol Sci. 2011;7:1003–15. doi: 10.7150/ijbs.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou W, Mukherjee P, Kiebish MA, et al. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab. 2007;4:5. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Champa CE, Basergab R, Mishraa MV, et al. Nutrient Restriction and Radiation Therapy for Cancer Treatment: When Less Is More. Oncologist. 2013;18:97–103. doi: 10.1634/theoncologist.2012-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simone B, Champ CE, Rosenberg AL, et al. Selectively starving cancer cells through dietary manipulation: Methods and clinical implications. Future Oncol. 2013;9:959–76. doi: 10.2217/fon.13.31. [DOI] [PubMed] [Google Scholar]


