Abstract
Multiple sclerosis is one of the autoimmune diseases in the central nervous system. Multiple sclerosis occurs through multiple mechanisms, and it is also mediated in part by an apoptotic mechanism. Swimming exercise has been recommended for the prevention and treatment of chronic diseases. In the present study, we investigated the effects of swimming exercise on short-term memory in relation with apoptotic neuronal cell death in the hippocampus following induction of multiple sclerosis. For this study, step-down avoidance task, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, immunohistochemistry for caspase-3 were performed. The animal model of multiple sclerosis was made by bilateral intracerebral ventricle injection of ethidium bromide. The rats in the swimming exercise groups were forced to swim for 30 min once daily for 14 consecutive days, starting 3 days after induction of multiple sclerosis. In the present results, short-term memory was deteriorated in the multiple sclerosis-induced rats. The number of TUNEL-positive and caspase-3-positive cells in the hippocampal dentate gyrus was increased in the multiple sclerosis-induced rats. Swimming exercise alleviated multiple sclerosis-induced short-term memory impairment by suppressing apoptotic neuronal cell death in the hippocampus. These effects of swimming exercise may aid symptom relief in the incurable neurodegenerative diseases.
Keywords: Multiple sclerosis, Swimming, Apoptotic neuronal cell death, Short-term memory
INTRODUCTION
Multiple sclerosis (MS) is one of the autoimmune diseases in the central nervous system (CNS). This disease preferentially affects young adults between 20 and 40 yr-old, but sometimes also affects children and older adults. The exact cause of MS is still unknown, however, the hallmark of MS is demyelination in the CNS by genetic, environmental, and immunological factors (Huynh and Casaccia, 2013).
MS occurs through multiple mechanisms, and it is also mediated in part by an apoptotic mechanism (Artemiadis and Anagnostouli, 2010). Apoptosis, also known as the programmed cell death, is an ATP-dependent physiological process, and apoptosis is implicated in the tissue homeostasis and pathological conditions, such as infections and autoimmunity (Blank and Shiloh, 2007). The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay detects DNA fragmentation, which is one characteristic of apoptotic cell death (Gavrieli et al., 1992). Another important characteristic of apoptosis is caspases activation. Caspase -3 is one of the most widely studied caspases and caspase-3 is a key executor of apoptosis (Benchoua et al., 2001). Apoptotic neuronal cell death in MS is known to induce demyelination in CNS (Artemiadis and Anagnostouli, 2010).
MS deteriorates diverse body functions such as vision, balance, strength, sensation, coordination, and brain function. The symptoms of MS may be single or multiple, and the severity is mild or severe in intensity, and the duration is short or long. Among them, nearly two-thirds of patients with MS suffer cognitive impairment, and memory decline (Patti, 2012). Especially, cognitive impairment in MS may express difficulty in short-term memory, prospective memory (making and keeping plans), adjusting to changing circumstances, multi-tasking, name-face recognition, managing mathematical tasks, and functioning in multi-stimuli environments (Sumowski and Leavitt, 2013; Thornton and Raz, 1997).
Many studies have reported that exercise improves learning ability and memory function (Choi et al., 2013; Kim et al., 2013; Sim et al., 2005). Physical exercise attenuated and dendritic abnormalities of experimental autoimmune disease (Rossi et al., 2009).
In the present study, the effects of swimming exercise on the short-term memory in relation with apoptosis in the hippocampus were investigated using MS animal model. For this study, a step-down avoidance task, TUNEL assay, immunohistochemistry for caspase-3 were performed.
MATERIALS AND METHODS
Animals and grouping
Adult male Sprague-Dawley rats weighing 350±10 g (30 weeks old) were used for the experiments. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The rats were housed under controlled temperature (23±2°C) and lighting (08:00 h to 20:00 h) conditions with food and water available ad libitum. The rats were randomly divided into the following four groups (n=10 in each group): the sham-operation group, the sham-operation and swimming exercise group, the MS-induced group, and the MS-induced and swimming exercise group.
Induction of multiple sclerosis
For the induction of MS in the brain, the rats were anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France), and then placed in a stereotaxic frame. Burr holes were drilled in the skull on both sides over the lateral ventricles using the following coordinates: 0.8 mm posterior to bregma, 1.5 mm lateral to sagital suture, and 3.6 mm beneath the surface of brain. Through a hole drilled in the skull, a 26-gauge needle was lowered manually into each lateral ventricle. The lesioned groups received bilateral intracerebral ventricle (ICV) injection of ethidium bromide (EtBr; 0.01%, 7 μL in saline) with the same dosage according to the previously described method (Goudarzvand et al., 2010).
The rats in the sham-operation group underwent the same surgical procedures, but same volume of saline was injected instead of EtBr. After surgery, the rats were housed individually and had access to food and water freely.
Swimming exercise protocol
The rats in the swimming exercise groups were forced to swim for 30 min once daily for 14 consecutive days, starting 3 days after MS induction. The swimming apparatus consisted of a plastic tank 50 cm in height and 30 cm in diameter. The plastic tank was filled with water at 30°C to a depth of 40 cm.
Step-down avoidance task
The latency of the step-down avoidance task was determined to evaluate the short-term memory, according to the previously described method (Kim et al., 2014). The rats were trained in a step-down avoidance task 17 days after induction of the MS. Two hours after training, the latency (sec) was determined. The rats were placed on a 7×25 cm platform, which was 2.5 cm high. The platform faced a 42×25 cm grid of parallel 0.1 cm-caliber stainless steel bars spaced 1 cm apart. In training sessions, the animals received a 0.5 mA scramble foot shock for 2 sec immediately upon stepping down. The interval between the rats stepping down and placing all four paws on the grid was defined as the latency time. Latency over 300 sec was counted as 300 sec.
Tissue preparation
The animals were sacrificed immediately after determining the latency in the step-down avoidance task. The rats were anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). Brains were dissected, and storage overnight same fixative, then it was transferred to 30% sucrose for cryoprotection. For the immunohistochemistry, the slices were coronal sectioned at 40 μm thick using a cryostat (Leica, Nussloch, Germany). Ten slice sections on average in the dentate gyrus regions were collected from each rat. The sections of 2.5 mm to 2.7 mm posterior from the bregma were used for TUNEL staining and immunohistochemistry.
TUNEL assay
For visualizing DNA fragmentation, TUNEL staining was performed using the In Situ Cell Death Detection Kit® (Roche, Mannheim, Germany), according to the previously described method (Choi et al., 2013). The sections were post-fixed in ethanol-acetic acid (2:1) and rinsed. The sections were then incubated with proteinase K (100 μg/mL), rinsed, and incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.03% 3,3′-diaminobenzidine (DAB). Mayer’s hematoxylin (DAKO, Glostrup, Denmark) was used as a counter-stain, and the sections were mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature and dehydrated through a gradient of ethanol and covered with coverslips using Permount® (Fisher Scientific, New Jersey, NJ, USA).
Immunohistochemistry
To detect of apoptosis in the hippocampal dentate gyrus, caspase-3 immunohistochemistry was performed, according to the previously described method (Choi et al., 2013). The sections were drawn from each brain and incubated overnight with mouse anti-caspase-3 antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then for another 1 h with biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). Bound secondary antibody was then amplified with Vector Elite ABC kit® (1:100; Vector Laboratories). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.03% DAB and the sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific).
Data analysis
The numbers of TUNEL-positive and caspase-3-positive cells in the hippocampal dentate gyrus were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan), and they were expressed as the numbers of cells/mm2 of the hippocampal dentate gyrus. The area of the dentate gyrus region was measured by Image-Pro Plus image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA). Statistical analysis was performed using one-way ANOVA followed by Duncan’s post-hoc test, and the results are expressed as the mean±standard error of the mean (SEM). Significance was set as P<0.05.
RESULTS
Effects of swimming exercise on the short-term memory
The latencies of the step-down avoidance task are presented Fig. 1. The latency in the step-down avoidance task was 199.25±22.18 sec in the sham-operation group, 226.37±22.91 sec in the sham-operation and swimming exercise group, 103.87±17.07 sec in the MS-induced group, and 155.87±18.97 sec in the MS-induced and swimming exercise group.
Fig. 1.
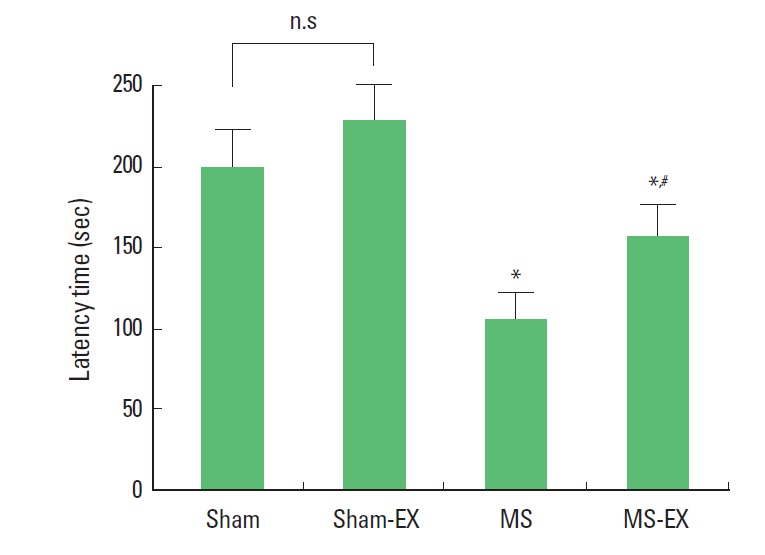
Effect of swimming exercise on the latency of step-down avoidance task. *Represents P< 0.05 compared with sham-operation group. #Represents P< 0.05 compared with MS-induced group. n.s, non-significant.
These results show that short-term memory was disturbed by induction of MS, whereas, swimming exercise alleviated MS-induced short-term memory impairment.
Effects of swimming exercise on the DNA fragmentation in the hippocampal dentate gyrus
Photomicrographs of TUNEL-positive cells in the hippocampal dentate gyrus are presented in Fig. 2. The number of TUNEL-positive cells was 28.40±6.95/mm2 in the sham-operation group, 27.80±5.71/mm2 in the sham-operation and swimming exercise group, 99.22±14.12/mm2 in the MS-induced group, and 64.40±2.88/mm2 in the MS-induced and swimming exercise group.
Fig. 2.
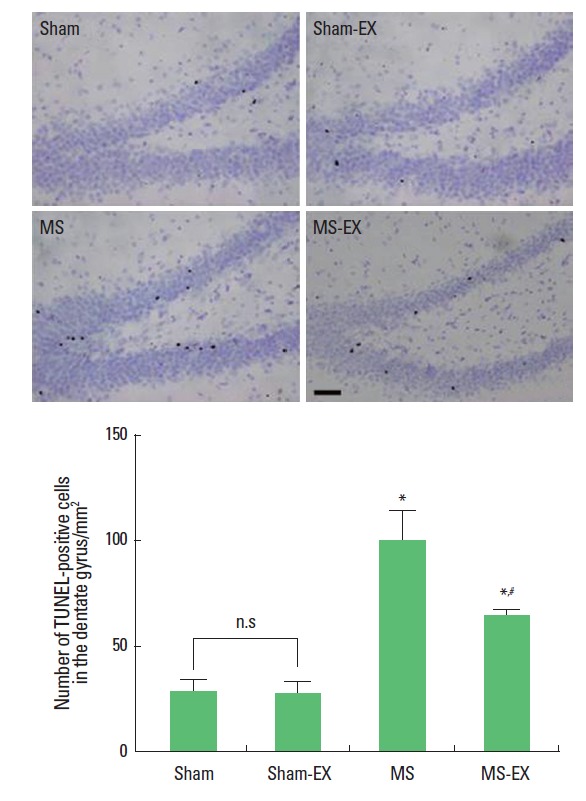
Effects of swimming exercise on DNA fragmentation in the hippocampal dentate gyrus. Upper: Photomicrographs of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells in the hippocampal dentate gyrus. The scale bar represents 150 μm. Lower: Number of TUNEL-positive cells in each group. *Represents P< 0.05 compared with sham-operation group. #Represents P< 0.05 compared with MS-induced group. n.s, non-significant.
These results show that induction of MS increased DNA fragmentation in the hippocampal dentate gyrus, whereas, swimming exercise reduced DNA fragmentation in the hippocampal dentate gyrus of MS-induced rats.
Effects of swimming exercise on the caspase-3 expression in the hippocampal dentate gyrus
Photomicrographs of caspase-3-positive cells in the hippocampal dentate gyrus are presented in Fig. 3. The number of caspase- 3-positive cells was 38.78±4.63/mm2 in the sham-operation group, 39.65±2.20/mm2 in the sham-operation and swimming exercise group, 103.84±8.91/mm2 in the MS-induced group, and 71.79±4.37/mm2 in the MS-induced and swimming exercise group.
Fig. 3.
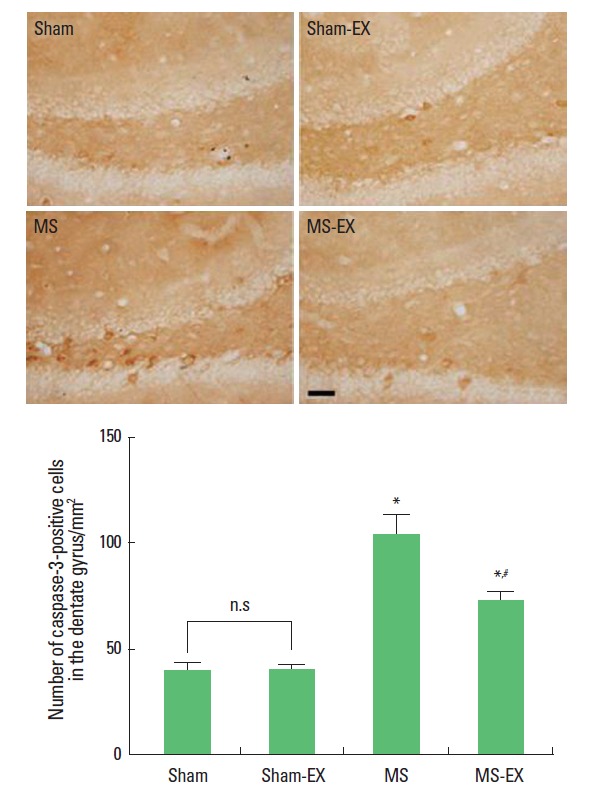
Effects of swimming exercise on caspase-3 expression in the hippo-campal dentate gyrus. Upper: Photomicrographs of caspase-3-positive cells in the hippocampal dentate gyrus. The scale bar represents 150 μm. Lower: Number of caspase-3-positive cells in each group. *Represents P< 0.05 compared with sham-operation group. #Represents P< 0.05 compared with MS-induced group. n.s, non-significant.
These results show that induction of MS increased caspase-3 expression in the hippocampal dentate gyrus, whereas, swimming exercise reduced caspase-3 expression in the hippocampal dentate gyrus of MS-induced rats.
DISCUSSION
Among the experimental MS models, autoimmune encephalomyelitis is the oldest and most widely used MS model (Goverman and Brabb, 1996). However, this model has limitation in the use because of motor dysfunction. Moreover, analysis of various physical functions including memory and sensory functions are difficult (Mix et al., 2008). EtBr is a gliotoxic drug that induces demyelination (Nassar et al., 2009), and EtBr has extensively been used as the demyelinating rat model to assess endogenous remyelination (Blakemore and Franklin, 2008).
Thornton and Raz (1997) revealed significant impairment across all memory domains and failed to support a retrieval-based account of long-term memory dysfunction in MS patients. MS patients with more severe disease burden (lesion load and cerebral atrophy visualized with magnetic resonance imaging) are at increased risk for cognitive impairment (Sumowski and Leavitt, 2013).
In the present study, the latency of the step-down avoidance task was shortened by injection of EtBr. The present results represent that short-term memory was deteriorated by induction of MS.
Apoptotic neuronal cell death is one of the hallmarks of MS animal models (Carlson et al., 2006; Zindler and Zipp, 2010). The morphological characteristics of apoptosis include cell shrinkage, chromatin condensation, membrane blebbing, internucleosomal DNA fragmentation, and the formation of apoptotic bodies (Li et al., 1995). Furthermore, caspase-3 is up-regulated and activated in the early stages of apoptosis following MS (Carlson et al., 2006).
In the present study, the numbers of TUNEL-positive and caspase-3 positive cells in the hippocampal dentate gyrus were significantly increased following injection of EtBr. The present results indicate that apoptotic neuronal cell death in the hippocampal dentate gyrus was enhanced by induction of MS.
Physical exercise enhances neurogenesis and increases long-term potentiation (Kim et al., 2010; van Praag et al., 1999). Under neurodegenerative status, treadmill exercise suppressed ischemia-induced increase in DNA fragmentation and caspase-3 expression in the hippocampus and facilitated recovery of short-term memory (Choi et al., 2013; Kim et al., 2014; Sim et al., 2005).
In the present study, swimming exercise suppressed the MS-induced increase in DNA fragmentation and caspase-3 expressions in the MS-induced rats. Swimming exercise also alleviated short-term memory disturbance in the MS-induced rats. These results show that swimming exercise inhibited MS-induced apoptosis in the hippocampus, resulted in the improvement of short-term memory.
Here in this study, swimming exercise ameliorated MS-induced short-term memory disturbance through suppressing apoptosis. These effects of swimming exercise may aid symptom relief in the incurable neurodegenerative diseases.
Acknowledgments
This study was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2011-327-G00128).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Artemiadis AK, Anagnostouli MC. Apoptosis of oligodendrocytes and post-translational modifications of myelin basic protein in multiple sclerosis: possible role for the early stages of multiple sclerosis. Eur Neurol. 2010;63:65–72. doi: 10.1159/000272940. [DOI] [PubMed] [Google Scholar]
- Benchoua A, Guégan C, Couriaud C, Hosseini H, Sampaïo N, Morin D, Onténiente B. Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci. 2001;21:7127–7134. doi: 10.1523/JNEUROSCI.21-18-07127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF, Franklin RJ. Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol. 2008;318:193–212. doi: 10.1007/978-3-540-73677-6_8. [DOI] [PubMed] [Google Scholar]
- Blank M, Shiloh Y. Programs for cell death: apoptosis is only one way to go. Cell Cycle. 2007;6:686–695. doi: 10.4161/cc.6.6.3990. [DOI] [PubMed] [Google Scholar]
- Carlson NG, Hill KE, Tsunoda I, Fujinami RS, Rose JW. The pathologic role for COX-2 in apoptotic oligodendrocytes in virus induced demyelinating disease: implications for multiple sclerosis. J Neuroimmunol. 2006;174:21–31. doi: 10.1016/j.jneuroim.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Choi JH, Kim TS, Park JK, Sim YJ, Kim K, Lee SJ. Short-term treadmill exercise preserves sensory-motor function through inhibiting apoptosis in the hippocampus of hypoxic ischemia injury rat pups. J Exerc Rehabil. 2013;9:457–462. doi: 10.12965/jer.130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol. 2010;30:289–299. doi: 10.1007/s10571-009-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J, Brabb T. Rodent models of experimental allergic encephalomyelitis applied to the study of multiple sclerosis. Lab Anim Sci. 1996;46:482–492. [PubMed] [Google Scholar]
- Huynh JL, Casaccia P. Epigenetic mechanisms in multiple sclerosis: implications for pathogenesis and treatment. Lancet Neurol. 2013;12:195–206. doi: 10.1016/S1474-4422(12)70309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BK, Shin MS, Kim CJ, Baek SB, Ko YC, Kim YP. Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats. J Exerc Rehabil. 2014;10:2–8. doi: 10.12965/jer.140086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Shin MS, Kim CJ, Jin BK, Hong HP, Jee YS. Treadmill exercise and wheel exercise enhance expressions of neutrophic factors in the hippocampus of lipopolysaccharide-injected rats. Neurosci Lett. 2013;538:54–59. doi: 10.1016/j.neulet.2013.01.039. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M, Jiang N, Zhang ZG, Zaloga C. Induction of DNA fragmentation after 10 to 120 minutes of focal cerebral ischemia in rats. Stroke. 1995;26:1252–1257. doi: 10.1161/01.str.26.7.1252. [DOI] [PubMed] [Google Scholar]
- Mix E, Meyer-Rienecker H, Zettl UK. Animal models of multiple sclerosis for the development and validation of novel therapies-potential and limitations. J Neurol. 2008;255:7–14. doi: 10.1007/s00415-008-6003-0. [DOI] [PubMed] [Google Scholar]
- Nassar CC, Bondan EF, Alouche SR. Effects of aquatic exercises in a rat model of brainstem demyelination with ethidium bromide on the beam walking test. Arq Neuropsiquiatr. 2009;67:652–656. doi: 10.1590/s0004-282x2009000400014. [DOI] [PubMed] [Google Scholar]
- Patti F. Treatment of cognitive impairment in patients with multiple sclerosis. Expert Opin Investig Drugs. 2012;21:1679–1699. doi: 10.1517/13543784.2012.716036. [DOI] [PubMed] [Google Scholar]
- Rossi S, Furlan R, De Chiara V, Musella A, Lo Giudice T, Mataluni G, Cavasinni F, Cantarella C, Bernardi G, Muzio L, Martorana A, Martino G, Centonze D. Exercise attenuates the clinical, synaptic and dendritic abnormalities of experimental autoimmune encephalomyelitis. Neurobiol Dis. 2009;36:51–59. doi: 10.1016/j.nbd.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Sim YJ, Kim H, Kim JY, Yoon SJ, Kim SS, Chang HK, Lee TH, Lee HH, Shin MC, Shin MS, Kim CJ. Long-term treadmill exercise overcomes ischemia-induced apoptotic neuronal cell death in gerbils. Physiol Behav. 2005;84:733–738. doi: 10.1016/j.physbeh.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Leavitt VM. Cognitive reserve in multiple sclerosis. Mult Scler. 2013;19:1122–1127. doi: 10.1177/1352458513498834. [DOI] [PubMed] [Google Scholar]
- Thornton AE, Raz N. Memory impairment in multiple sclerosis: a quantitative review. Neuropsychology. 1997;11:357–366. doi: 10.1037//0894-4105.11.3.357. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running, enhances neurogenesis learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindler E, Zipp F. Neuronal injury in chronic CNS inflammation. Best Pract Res Clin Anaesthesiol. 2010;24:551–562. doi: 10.1016/j.bpa.2010.11.001. [DOI] [PubMed] [Google Scholar]


