Abstract
Aims: To investigate the role of endogenous hydrogen sulfide (H2S) in the control of aging and healthspan of Caenorhabditis elegans. Results: We show that the model organism, C. elegans, synthesizes H2S. Three H2S-synthesizing enzymes are present in C. elegans, namely cystathionine γ lyase (CSE), cystathionine β synthetase, and 3-mercaptopyruvate transferase (MPST or 3-MST). Genetic deficiency of mpst-1 (3-MST orthologue 1), but not cth-2 (CSE orthologue), reduced the lifespan of C. elegans. This effect was reversed by a pharmacological H2S donor (GYY4137). GYY4137 also reduced detrimental age-dependent changes in a range of physiological indices, including pharyngeal contraction and defecation. Treatment of C. elegans with GYY4137 increased the expression of several age-related, stress response, and antioxidant genes, whereas MitoSOX Red fluorescence, indicative of reactive oxygen species generation, was increased in mpst-1 knockouts and decreased by GYY4137 treatment. GYY4137 additionally increased the lifespan in short-lived mev-1 mutants with elevated oxidative stress and protected wild-type C. elegans against paraquat poisoning. The lifespan-prolonging and health-promoting effects of H2S in C. elegans are likely due to the antioxidant action of this highly cell-permeable gas. Innovation: The possibility that novel pharmacological agents based on the principle of H2S donation may be able to retard the onset of age-related disease by slowing the aging process warrants further study. Conclusion: Our results show that H2S is an endogenous regulator of oxidative damage, metabolism, and aging in C. elegans and provide new insight into the mechanisms, which control aging in this model organism. Antioxid. Redox Signal. 20, 2621–2630.
Introduction
Hydrogen sulfide (H2S) affects cell metabolism notably by inhibiting cytochrome c oxidase and thereby reducing mitochondrial adenosine triphosphate (ATP) production (4). It was originally thought that H2S would kill cells at low concentrations (by starving the cell of ATP), but it is now becoming increasingly clear that physiologically relevant concentrations are not toxic and that H2S actually exhibits cytoprotective effects in a wide range of cells and tissues (11). For example, H2S protects the heart (12) against damage due to ischemia reperfusion.
Innovation.
While exogenous hydrogen sulfide (H2S) reportedly protects Caenorhabditis elegans against stress and increases longevity, the role of endogenous H2S, if any, is unknown. Here, we report that (i) C. elegans contains cystathionine γ lyase, cystathionine β synthetase, and 3-mercaptopyruvate transferase enzyme orthologues and synthesizes H2S, (ii) genetic deficiency of mpst-1 reduces endogenous H2S production and negatively affects health and lifespan, and (iii) GYY4137, which releases low concentrations of H2S over an extended time period, prolongs the lifespan and retards age-dependent deterioration in physiological functions. GYY4137 seems well suited as a prototype for the development of a new class of drugs with beneficial effects on healthy aging.
Many of the cytoprotective effects of H2S are likely due to its ability to quench, or modulate, the formation of damaging free radical species. It has been known for many years that reactive oxygen species (ROS) trigger accumulating damage in aging cells thus causing loss of function or even death and that they may also act as signaling molecules (1). The fact that exogenous H2S gas prolongs the lifespan of Caenorhabditis elegans (18) suggests a link between this gas and aging. However, whether C. elegans synthesizes H2S naturally and whether endogenous H2S affects the lifespan in this organism perhaps by an antioxidant action is not known.
In the present work, we have characterized endogenous H2S biosynthesis in C. elegans and investigated its effect and mechanism of action on lifespan and other markers of health in this organism.
Results
Endogenous H2S synthesis in C. elegans
Mammals synthesize H2S using a combination of the following enzymes: cystathionine γ lyase (CSE), cystathionine β synthetase (CBS), and 3-mercaptopyruvate transferase (MPST; usually abbreviated in the literature to 3-MST). CSE and CBS convert l-cysteine to H2S in the presence of pyridoxal 5′ phosphate (PPP), whereas 3-MST utilizes 3-mercaptopyruvate (3-MP) as substrate. All three of these enzymes are evolutionally conserved in lower species with two CSE orthologues (cth-1, cth-2), two CBS orthologues (cbs-1, cbs-2), and seven 3-MST orthologues (mpst1-7) predicted in C. elegans (16).
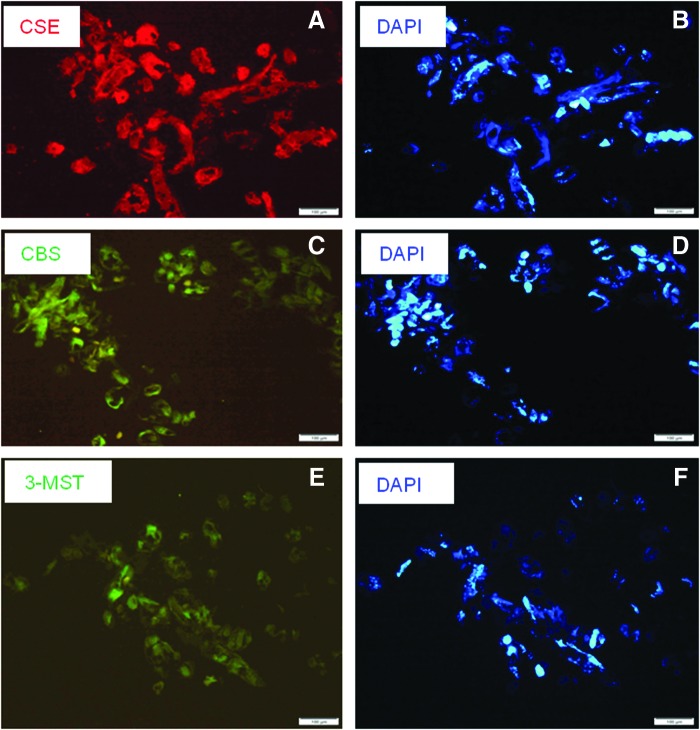
In preliminary experiments, we demonstrated the presence of CSE, CBS, and 3-MST in adult C. elegans using immunofluorescence (Fig. 1A–F) and diaminobenzidine staining (Supplementary Fig. S1A–C; Supplementary Data are available online at www.liebertpub.com/ars). By quantitative polymerase chain reaction (qPCR), we also confirmed the presence and relative expression levels of all the named genes predicted to encode for CSE, CBS, and 3-MST proteins (Supplementary Fig. S2). In separate experiments, we assessed the formation of H2S from exogenous l-cysteine (to assay the CSE and CBS activity) and 3-MP (to assay the 3-MST activity) in homogenates of adult C. elegans and found significant biosynthesis after incubation with either l-cysteine (1.49±0.05 nmol/mg protein/min, n=12) or 3-MP (1.09±0.05 nmol/mg/min, n=3). Finally, we used a sensitive rp-HPLC assay to identify endogenous l-cysteine (2.03±0.47 μM, n=6), 3-MP (0.13±0.03 μM, n=6), and H2S itself (3.97±0.87 μM, n=6) in homogenates of adult C. elegans. From these results, we conclude that H2S, its synthesizing enzymes, and substrates thereof occur naturally in C. elegans.
FIG. 1.
Expression of hydrogen sulfide (H2S)-synthesizing enzymes in Caenorhabditis elegans. Transverse sections showing immunoreactive staining for cystathionine γ lyase (CSE) (A), cystathionine β synthetase (CBS) (C), and 3-mercaptopyruvate transferase (3-MST) (E) in C. elegans. 4′,6-diamidino-2-phenylindole (DAPI) staining of nuclei is shown in (B, D, and F). Magnification is×20. Horizontal bar shows scale (100 μm).
3-MST regulates the lifespan and health of C. elegans
Since exposure of C. elegans to H2S gas (50 ppm) prolongs the lifespan in this organism (18), we wondered whether naturally produced (i.e., endogenous) H2S could exhibit a similar lifespan-prolonging effect in this organism.
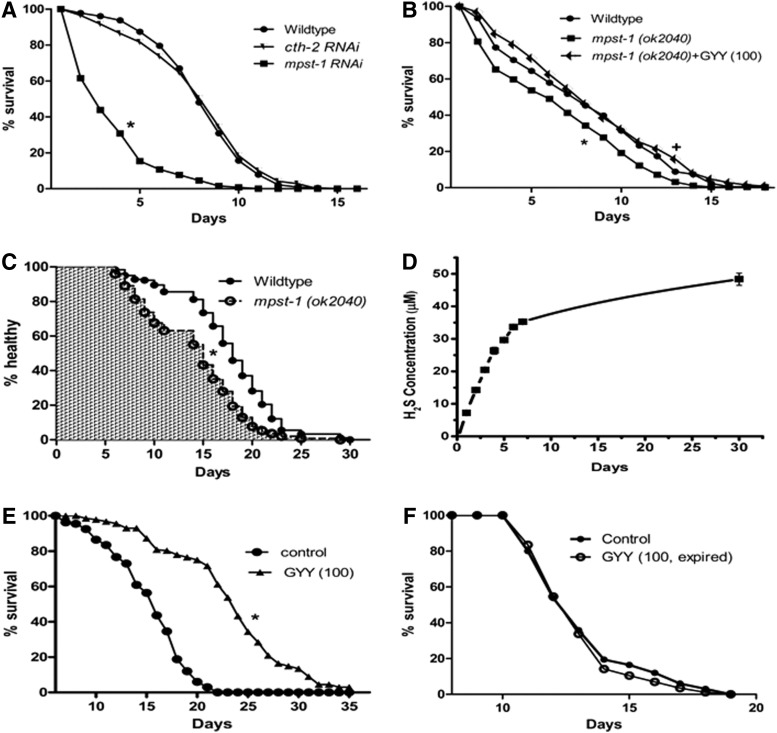
To test this possibility, we examined the effect of genetic (RNAi) knockdown of CSE or 3-MST on lifespan. CBS was excluded from this part of the study since RNAi-mediated knockdown of the CBS orthologue reportedly impairs the development (time to adulthood >9 days) and caused abnormalities in the gut and pharynx (30). There are no previous descriptions of CSE or 3-MST knockdown in C. elegans. The knockdown efficiency of cth-2 (ZK1127.10) and mpst-1 (D2023) as determined by qPCR was similar, that is, 66.5%±0.6% and 64.2%±0.6%, respectively (n=3). While lifespan was unchanged in cth-2 RNAi animals (mean lifespan: 12.15±0.16 days compared to (cf) 12.23±0.16 days, p>0.05), it was substantially reduced (36.5%) in mpst-1 RNAi animals (mean lifespan: 7.76±0.18 days, p<0.05) (Fig. 2A), implying a possible role for 3-MST, but probably not CSE, in regulating the lifespan in this organism.
FIG. 2.
Response of C. elegans to 3-MST deficiency and effect of GYY4137. (A) Effect of cth-2 and mpst-1 RNAi knockdown on lifespan, n=130–288 animals per group, *p<0.05 compared to wild type. (B) Comparison of lifespan in wild-type and mpst-1 (ok2040) null animals and rescue of mpst-1 null animals by GYY4137 (GYY; 100 μM), n=340–516 animals per group, *p<0.05, mpst-1 null versus wild type, +p<0.05 mpst-1 null versus mpst-1 null+GYY4137. (C) Healthspan comparison in wild-type and mpst-1 knockout animals as assessed over 30 days, n=108–149 animals per group, *p<0.05. (D) Spontaneous release of H2S from GYY4137 (100 μM) incubated for up to 30 days at 20°C. Results are mean±SE mean, n=6–9 (where not shown standard error bars lie within dimensions of symbol). (E) Effect of GYY4137 (100 μM) on lifespan of wild-type C. elegans, n=114–123, *p<0.05. (F) Effect of time-expired GYY4137 (100 μM, 10 days at room temperature) on lifespan of C. elegans, n=67–86 animals per group, p>0.05.
Further experiments were then carried out using C. elegans carrying an mpst-1 null deletion allele (ok2040). Initial characterization of the mpst-1 knockout phenotype revealed a reduction in brood size (4 days: 41.8±2.7 c.f. 206.9±4.9 animals, p<0.05) but no significant difference in the rate of development to the adult stage between mpst-1 knockouts and wild-type controls (Supplementary Fig. S3A). Loss of mpst-1 and 3-MST protein was confirmed in these animals by qPCR (Supplementary Fig. S3B) and immunohistochemically (Supplementary Fig. S3C–F). The former also revealed significant, presumably compensatory, upregulation of five of the other six 3-MST orthologues (i.e., mpst 3-7) in these mpst-1 deletion mutants. Subsequent lifespan experiments confirmed the short-lived (15.4% reduced compared to wild-type controls) phenotype of mpst-1 knockouts (Fig. 2B). Interestingly, partial knockdown of mpst-1 was more effective than complete deletion of this gene in terms of shortening the lifespan in these animals. Again, this may be due to the compensatory upregulation of other 3-MST orthologues in mpst-1 knockouts as noted above.
Since lifespan was detrimentally affected in mpst-1 knockouts, we wondered whether there was a corresponding decline in the general health status of these animals. To assess this possibility, we compared the healthspan in mpst-1 knockout and wild-type C. elegans. While healthspan deteriorated over time in both wild types and mutants, the rate of loss of normal healthy behavior was significantly accelerated in the mpst-1 knockouts (Fig. 2C).
Finally, we attempted to correlate the changes in lifespan and healthspan found in mpst-1 knockouts with the ability of these animals to synthesize H2S. The amount of H2S detected in homogenates of synchronized adult (day 5), mpst-1 knockout C. elegans (38.99±3.32 nmol/mg, n=8), was ∼57% less than that found in wild types (92.25±19.80 nmol/mg, n=8, p<0.05), which shows that the deletion of mpst-1 significantly reduces H2S production in these animals. The fact that H2S production was not completely abolished in mpst-1 knockouts is probably due to compensatory upregulation of other 3-MST orthologues coupled with the continued presence in these animals of additional H2S-synthesizing enzymes, such as CSE and CBS.
Mimicking the effect of endogenous H2S using the slow-releasing donor, GYY4137
Since genetic deficiency of mpst-1 reduced lifespan and decreased H2S levels in C. elegans, we decided to investigate whether replenishing H2S in mpst-1 knockout animals was able to reverse the lifespan deficit.
We have previously reported that GYY4137 is a slow-releasing H2S donor (15). In preliminary experiments, GYY4137 (100 μM) was incubated at 20°C in aqueous vehicle for up to 30 days to mimic, as far as possible, the treatment of C. elegans during a complete lifespan experiment. Under these conditions, the hydrolysis of GYY4137 was slow with increasing concentrations of H2S (∼5–7 μM) generated each day up to a plateau at day 7 thereafter increasing only very slowly (<0.5 μM per day) up to day 30 (Fig. 2D). The maximum daily exposure of C. elegans to H2S during GYY4137 treatment in the first 7 days can therefore not be >50% higher than the concentration found naturally in homogenates (i.e., 3.97 μM). Indeed, the true exposure of C. elegans to H2S gas released from GYY4137 in this way is likely to be considerably less as some H2S generated will not be absorbed and H2S release from GYY4137 declines after day 7. Previous researchers have demonstrated the lifespan-enhancing effect of H2S in C. elegans following exposure to H2S gas (50 ppm) (18). This is equivalent to a dissolved H2S concentration of 1.47 mM, which is more than 370 times greater than the presumed endogenous concentration of this gas in C. elegans. For this reason, we chose not to use H2S gas in the present experiments, but GYY4137, which most likely better mimics the natural exposure rate of C. elegans to this gas.
Treatment of mpst-1 knockout animals with GYY4137 (Fig. 2B) completely restored, without overshooting, the shortened lifespan of mpst-1 knockouts (Fig. 2B). This result suggests that replenishment with H2S, under these conditions, rescued the mpst-1 knockout phenotype and, importantly, also pinpoints H2S as a key regulator of lifespan under this perturbation. Intriguingly, GYY4137 also prolonged lifespan in wild-type C. elegans (Fig. 2E) an effect not seen when time-expired (released >85% of its available H2S) compound was used (Fig. 2F). This effect of GYY4137 was similar to, or even greater than the lifespan-enhancing response reported for rapamycin (100 μM, 19% increase; 26) or resveratrol (1 mM, 19% increase; 29) or those typically seen with antioxidant interventions (24) but less than that in C. elegans exposed to H2S gas (50 ppm) in which lifespan was increased by 74% (18). The apparent discrepancy between our results using GYY4137 to donate H2S and those of other researchers using H2S gas may be explained by the very different concentrations of H2S to which these animals are exposed by the two treatment regimens.
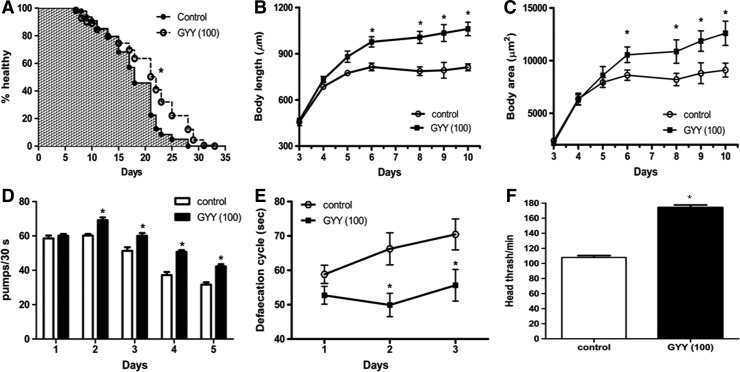
Since loss of endogenous H2S following mpst-1 knockout reduced healthspan in C. elegans, we also determined whether GYY4137 treatment had the reverse effect, that is, to improve the healthspan in wild-type animals. GYY4137 not only significantly increased overall healthspan (Fig. 3A) but also, at least partially, reversed a number of other age-dependent physiological/behavioral changes, including the decline in body length and area (Fig. 3B, C) and the reduction in pharyngeal pumping rate (Fig. 3D), defecation (Fig. 3E), and head thrashing (Fig. 3F).
FIG. 3.
Effect of GYY4137 on health and behavior of C. elegans. Time course (days of adulthood, i.e., post L4) on the effect of GYY4137 (GYY; numbers in parenthesis show concentration in μM) on (A) healthspan, n=76–92, (B) body length, n=20, (C) body area, n=20, (D) pharyngeal pumping rate, n=10, (E) defecation rate, n=10, and (F) head shake frequency studied on day 1 of adulthood of C. elegans, n=20. Results show mean±SEM, *p<0.05.
Thus, the treatment of C. elegans with continuous low concentrations of exogenous H2S, released from GYY4137, (i) rescued the phenotype (i.e., normalized the lifespan) of mpst-1 knockouts, (ii) increased the lifespan of wild-type C. elegans, and (iii) improved overall health, that is, reduced the age-related deterioration of physiological parameters in this organism.
Mechanism of action of H2S
Since knockout of mpst-1 is likely to affect the expression of a wide range of genes due to compensatory changes and many of these are presumably secondary to altered health and/or lifespan, we decided, as a first step to understanding the role of H2S in these processes, to examine the effect of GYY4137 on the transcriptome profile of wild-type C. elegans. To gauge intra-assay variation and to justify statistical analysis, three separate groups of animals were treated with either GYY4137 (100 μM) or vehicle (water). Microarray analysis showed a good degree of conformity between individual batches with significant (p<0.05) changes in the expression of 1045 genes between GYY4137- and vehicle-treated groups (Supplementary Fig. S4). Gene ontology analysis revealed significant differences in the expression of 18 aging- and 31 stress-related genes (Supplementary Tables S1 and S2). Notable age-related genes upregulated by GYY4137 include dod-6 (1.4×), dod-17 (1.6×), dod-21 (8.7×), dod-22 (4.6×), dod-23 (1.8×), and dod-24 (1.9×) as well as ins-7 (insulin-related, 2.4×). Daf-16 is a key gene involved in aging in C. elegans and its activation triggers numerous downstream genes collectively referred to as downstream of daf-16 or dod genes (9). Stress response and antioxidant genes upregulated by GYY4137 included hsp-17 (heat shock protein, 1.6×), catalase (1.4×), and glutathione S-transferase (1.15–1.2×).
The role of mitochondrial ROS, ROS-mediated damage, endogenous antioxidant systems, and small-molecule antioxidants in the control of lifespan in C. elegans is the subject of much active debate and research (1, 5). H2S is a powerful, highly membrane permeable reducing agent and metabolic modulator, and the possibility that its effect on health and lifespan may be mediated through an antioxidant mechanism is intriguing. We therefore decided to investigate this possibility in greater depth.
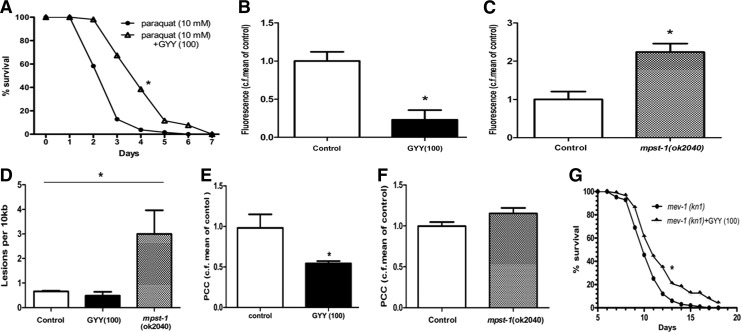
First, we showed that GYY4137 exhibited pronounced antioxidant activity in two standard biochemical assays in vitro (Supplementary Fig. S5A, B). In addition, GYY4137 increased the survival of paraquat-exposed wild-type C. elegans (Fig. 4A). Paraquat kills C. elegans by promoting ROS generation, an effect associated with the upregulation of the stress response gene, pqm-1 (1.5×). In further experiments, we used three separate assays to monitor ROS production and associated damage in adult C. elegans. In the first assay, mitochondrial ROS generation, as determined using the fluorescent dye MitoSOX Red, was reduced in C. elegans treated with GYY4137 (Fig. 4B) but was increased (cf, wild type) in mpst-1 null animals (Fig. 4C). In the second assay, mtDNA lesions due to oxidative damage were significantly higher in mpst-1 knockouts than in untreated and GYY4137-treated wild-type controls (Fig. 4D). Using this assay, we also noted a trend toward lower mtDNA damage levels in GYY4137-treated wild types, although this effect did not reach statistical significance (p=0.07, Fig. 4D). However, mtDNA damage in wild-type controls is extremely low, in the order of one lesion per mtDNA molecule and hence it is not surprising that damage levels are not suppressed even further by GYY4137 treatment. In the third assay, the protein carbonyl (PC) content, another marker of oxidative damage, was significantly reduced by GYY4137 in wild-type animals (Fig. 4E), while a nonsignificant (p=0.08) trend to increased PC content was noted in mpst-1 null animals (Fig. 4F). Thus, each of these biochemical assays supports the hypothesis that the degree of oxidative stress/ROS generation is correlated with H2S concentration, that is, reduced by GYY4137 treatment and increased in mpst-1 knockouts with diminished endogenous H2S-synthesizing capacity. In addition, we used the dichlorofluorescein diacetate (DCF-DA) fluorescence assay to assess ROS production from C. elegans in these experiments. We note that the validity of this assay using lysed worms has been challenged since this causes the release of transition metal ions, such as iron, which can generate ROS by redox cycling. However, in the present experiments, we used whole animals that had not been disrupted and in which iron therefore remained sequestered. We are aware of the controversy regarding the actual ROS responsible for DCF-DA fluorescence and, more generally regarding the meaning of this fluorescence. However, we believe that when used in whole C. elegans and interpreted with the appropriate caveats and especially when combined with other assays, DCF-DA fluorescence can be a useful indicator of global ROS flux. Indeed, we have previously utilized this assay to show a clear age-dependent increase in this marker of global ROS flux (5). Using DCF-DA, we noted increased ROS production in mpst-1 RNAi C. elegans, which was reduced by GYY4137 treatment both in these mutants and in wild-type controls (Supplementary Fig. S6).
FIG. 4.
H2S and markers of oxidative stress in C. elegans. (A) Effect of GYY4137 (GYY; 100 μM) on paraquat-evoked (10 mM) toxicity. Data show median survival assessed daily over a 7-day period, n=154–187, *p<0.05. (B) Effect of GYY4137 (100 μM) treatment on reactive oxygen species (ROS) production determined by MitoSOX Red fluorescence, data show mean±SEM of three independent experiments, *p<0.05. (C) MitoSOX Red fluorescence indicative of ROS production in wild-type (control) and mpst-1 knockout C. elegans, data show mean±SEM of three independent experiments, *p<0.05. (D) Measurement of mtDNA damage in wild type (control), wild type treated with GYY4137 (100 μM), and mpst-1 knockout C. elegans, data show mean±SEM of three independent experiments, *p<0.05. Measurement of protein carbonyl content (PCC) in GYY4137-treated (GYY; 100 μM) wild-type controls (E) and in mpst-1 knockout C. elegans (F), data show mean±SEM, n=10–13, *p<0.05. (G) Effect of GYY4137 (GYY; numbers in parenthesis show concentration in μM) on survival of mev-1 (kn1) C. elegans mutants, n=52–58, *p<0.05.
Finally, we used the C. elegans mutant, mev-1 (kn1), which is short lived due to its overproduction of ROS. GYY4137 significantly increased the lifespan in mev-1 C. elegans again pointing to an antioxidant effect as a likely mechanism of action (Fig. 4G).
Taken together, these results strongly suggest that both endogenous and exogenous H2S prolongs the lifespan and healthspan in C. elegans by acting as antioxidant and/or modulator of ROS production.
Discussion
Exposing C. elegans to H2S in air increases their lifespan, improves their tolerance to elevated temperature (18), and increases their resistance to killing by pseudomonas (2). While pharmacological treatment with H2S gas in this way clearly protects C. elegans against some forms of stress and increases their longevity, it is not known whether endogenous H2S, made by C. elegans, has similar activity. This is important as it would imply a hitherto unknown physiological role of this molecule and is therefore the focus of the present work. We describe herein four important findings, (i) C. elegans contains functional CSE, CBS, and 3-MST enzyme orthologues and substrates thereof and synthesize H2S, (ii) genetic deficiency of mpst-1 negatively affects health and lifespan and reduces endogenous H2S production, (iii) GYY4137, which releases low, physiologically relevant, concentrations of H2S over a period of several weeks, prolongs lifespan, and retards age-dependent deterioration in physiological functions, and (iv) the effect of H2S deficiency (in mpst-1 knockouts) and excess (after GYY4137 treatment) are likely due to the modulation of ROS-mediated damage by H2S.
To date, a total of 11 orthologues of CSE, CBS, and 3-MST have been predicted to occur in the C. elegans genome (Wormbase; http://wormbase.org/). These enzymes play important roles in regulating sulfur metabolism in this organism as they do in mammals. Whether these enzymes, like their mammalian counterparts, also synthesize H2S, has not previously been shown. CSE and CBS have been extensively studied and the biological roles of H2S generated by their activity have been well characterized in mammals. In contrast, the biological relevance of 3-MST-derived H2S is not so clear. 3-MST catalyzes the transulfuration of 3-MP to pyruvate during degradation of l-cysteine and, in this way, can also increase the intracellular concentration of thioredoxin and reduced glutathione (GSH), both of which contribute to cellular redox homeostasis (19). The fact that MitoSOX Red and mtDNA (marker of general ROS production/activity) were elevated in mpst-1 knockouts suggests that these animals were in a state of oxidative stress. 3-MST also synthesizes H2S in mammalian liver, kidney, and brain in vitro (32), and since H2S is not only a strong reducing agent and scavenger of free radicals in mammalian cells (31) but also increases intracellular GSH levels (10), it seems likely that at least part of the protective effect of 3-MST in oxidative stress in C. elegans is due to its ability to generate H2S. We therefore propose that (i) 3-MST deficiency reduces the lifespan in C. elegans because of diminished formation of endogenous antioxidant H2S, (ii) GYY4137 restores normal biological aging to these mutants by replenishing the lost H2S gas, and (iii) GYY4137 prolongs the lifespan in wild-type C. elegans by a similar mechanism.
Several lines of experimental evidence support these proposals. Alongside changes in free radical generation in mpst-1 mutants, which have been noted above, we also report here that GYY4137 (i) has a potent antioxidant activity in biochemical assays in vitro, (ii) alters the expression of a range of genes associated with both aging and oxidation and reduction, (iii) reduces markers of oxidative stress including MitoSOX Red and DCF-DA fluorescence, mtDNA damage, and PCs, (iv) decreases paraquat lethality, which is believed to be secondary to ROS generation (8), and (v) enhances the lifespan of mev-1 mutants, which exhibit premature death due to overproduction of superoxide anions due to depleted complex II enzyme succinate-CoQ oxidoreductase activity (6). Taken together, these data suggest that both endogenous and exogenous H2S oppose oxidative stress through both direct and indirect mechanisms in C. elegans and that this likely forms the basis for the effect of this gas on lifespan and health status in this organism.
In conclusion, small amounts of endogenous H2S, generated either from 3-MP by 3-MST enzyme activity or by spontaneous decomposition of GYY4137, prolongs the lifespan and delays the adverse health consequences of aging in C. elegans by an antioxidant effect. Whether endogenous H2S also prolongs the lifespan in mammals is a key question, which has yet to be directly addressed. However, circumstantial evidence, such as the identification of increased aortic CSE and CBS expression in aged rats (23) and reduced plasma H2S concentration in humans older than 50 years (3), suggests that H2S may play a part in physiological changes in aging mammals as well. Moreover, while the treatment with H2S gas is clearly not a viable H2S delivery strategy in the clinic, the water-soluble H2S-releasing drug, GYY4137, seems well suited, at least as a prototype, for the development of a new class of drugs with beneficial effects on healthy aging.
Materials and Methods
Use of C. elegans
All C. elegans strains were obtained from the Caenorhabditis Genetic Centre (University of Minnesota) and cultured on nematode growth medium (NGM, 3 g/L NaCl, 17 g/L bacto-agar, 2.5 g/L bacto-peptone, 5 mg/L cholesterol, 25 mM KPO4 [pH 6], 1 mM MgSO4, and 1 mM CaCl2) seeded with Escherichia coli OP50 (OD595 3.4–3.6) at 20°C as described elsewhere (7). C. elegans strains used in this study were Bristol N2 (wild type), mev-1(kn1), and mpst-1 (ok2040). The mpst-1 mutant strain was backcrossed to the wild type four times and single worm genotyping carried out using standard procedures. Only homozygous mutant strains were used. For some experiments, RNAi-mediated silencing via double-stranded RNA (dsRNA) was used to knock down C. elegans genes that are orthologous to the human H2S-synthesizing enzymes, CSE and 3-MST. Genes targeted (and their enzyme product) were cth-2 ZK1127.10 (CSE) and mpst-1 D2023.5 (3-MST). E. coli strain HT115 (DE3) expressing dsRNA of the gene of interest (cth-2 or mpst-1 cloned into pPD129.36) was fed to C. elegans maintained on NGM plates containing isopropyl β-D-1-thiogalactopyranoside and ampicillin. RNAi controls were fed HT115 (DE3) bacteria containing the empty pPD129.36 vector.
C. elegans synchronization and drug treatment
Adult wild-type and mutant animals were washed from NGM plates with M9 buffer (22 mM KH2PO4, 42.2 mM Na2HPO4, 85.5 mM NaCl, and 1 mM MgSO4·7H2O) and centrifuged (2500g, 2 min). The resulting pellets were washed with M9 buffer and digested with hypochlorite solution (1.0%–1.3% w/v sodium hypochlorite, 500 mM sodium hydroxide). Samples were mixed by vigorous shaking (2.5 min), centrifuged (2500g, 1 min), and the supernatant was discarded. Thereafter, the pellets were resuspended in M9 buffer, centrifuged (2500g, 1 min), and the wash procedure was repeated four times after which 10 volumes of M9 buffer was added to the final egg pellets, and the resulting suspension gently rotated overnight at room temperature to allow hatching into the first larval stage (L1) animals. Synchronized L1 animals were cultured (48 h) in sealed tubes in the medium containing M9 buffer and E. coli OP50 (1:2 v/v) in the presence or absence of GYY4137 (100 μM) or water (vehicle control) at room temperature. During this time, animals developed to the fourth larval stage (L4).
Determination of the effect of drugs on aging and life traits of C. elegans
The effect of drugs on lifespan of C. elegans was assessed at 20°C using L4 worms as detailed previously (7). Lifespan was monitored daily, and death was scored by failure of the animal to move in response to gentle prodding with a platinum wire. Drug-induced changes in body size (length and area) of C. elegans were observed using a digital camera (×1.5 magnification) attached to a microscope (Nikon UK Ltd.). The volumetric area within the perimeter of the nematodes was determined using Image-Pro Express v5.1 software (Media Cybernetics). Additional age-related parameters, including head thrashing, pharyngeal pumping, and defecation rates, were assessed by manual counting using a fully apochromatic corrected stereomicroscope (Leica M205 with FusionOptics™ with a zoom capacity of 20.5:1 and a resolution of 1050 lp/mm).
A further index of age-related deterioration in physiological capacity (i.e., healthspan), based on measurement of the progressive decline in locomotor function with aging was used (20, 27). Briefly, C. elegans (up to 200 animals) were observed for locomotor activity at daily intervals and subdivided into three groups: class A animals were healthy, showed spontaneous movements, and were highly mobile; class B animals showed movement only after prodding; and class C nematode movement was restricted to the head and/or tail upon prodding with a platinum wire. Only A type nematodes were counted as healthy. Healthspan curves were determined by scoring the percentage of worms in the synchronized aging cohort that still remain in the healthy category (A-type) as a function of age. Data are expressed as% healthy (i.e., category A).
Survival assay in the presence of paraquat
Paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride) resistance was determined by transferring L4 worms previously treated (48 h) with either GYY4137 (100 μM) or water (vehicle) to plates containing paraquat (10 mM) with/without GYY4137 (100 μM) and scored as described above for lifespan determination.
Quantitative real-time polymerase chain reaction
C. elegans were homogenized in the presence of 0.5-mm glass beads to maximize tissue/cell breakage and total RNA extracted according to the manufacturer's instructions using Tri-reagent (Sigma). Total RNA (0.5–1 μg) was reverse-transcribed to cDNA using M-MLV reverse transcriptase enzyme (Promega), and real-time qPCR was performed with primers specific for each enzyme. A list of primers used is provided in Supplementary Table S3.
Microarray analysis
C. elegans (minimum, 5000 animals) were incubated with GYY4137 (100 μM) or water vehicle from L1 to L4. Three separate batches of animals were prepared for each treatment. C. elegans were homogenized, and total RNA (∼20 μg) was extracted according to the manufacturer's instructions using Trizol reagent (Invitrogen) and purified using an RNeasy kit followed by a DNase digestion kit (Qiagen). RNA was not amplified before labeling. Synthesis of double-stranded cDNA and biotin-labeled cRNA was performed according to the manufacturer's instruction (Affymetrix). Fragmented cRNA preparations were hybridized to C. elegans genome oligonucleotide arrays using Affymetrix hybridization Oven 640, washed, and scanned on a GeneChip Scanner 3000 (Affymetrix). Initial data extraction and normalization within each array was performed using Affymetrix GCOS software. Data intensities were log transformed and normalized within, and between, arrays using the quantile normalization method, and two-tail, pair-wise analysis or a two-way analysis of variance was employed using Spotfire Decision Site software package 7.2 v10.0 (Spotfire, Inc.) to extract the statistically significant data from each group of animals. Each treatment replicate was analyzed twice (technical repeats). Thereafter, the log2 values (GYY4137-treated net intensity/normalized control net intensity) from each replicate were averaged. A gene was considered for analysis if the hybridization signal for the corresponding probe was deemed to be present on the array (significantly greater than background level, p<0.05) in at least two of three relevant arrays in the drug-treated experiment. A gene was considered induced/repressed if the difference in expression levels was significant (p<0.05) compared to the control using Student's t test and Significance Analysis of Microarrays analysis. Gene enrichment analysis was performed using DAVID 6.7 to obtain a ranking of functional categories based on co-occurrence with sets of genes in the gene list (p<0.05). Genes were annotated using Wormbase (http://wormbase.org/). The MIAME accession number for the microarray data reported in this article is GSE16975.
Assay of antioxidant effect of GYY4137
The antioxidant effect of GYY4137 (and NaHS for comparison) in vitro was assessed using two standard assays. The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assay was carried out as described elsewhere (25), and data are expressed as the Trolox equivalent antioxidant capacity. The reducing power of GYY4137 and NaHS was determined using the potassium ferricyanide–ferric chloride method (21). ROS formation in C. elegans was measured using MitoSOX Red mitochondrial superoxide indicator (Molecular Probes) as described elsewhere (13) with modifications. For this assay, ∼150 wild-type animals were treated with GYY4137 (100 μM) or vehicle in agar plates for 24 h before being manually transferred into each well of a black 96-well microtiter plate containing M9 buffer (100 μl). Similar experiments were carried out using mpst-1 knockouts. MitoSOX red reagent (100 μl of 20 μM solution) was added to each well, and superoxide-associated fluorescence was assessed kinetically (every 2 min for 5 h, room temperature) with a Synergy H1 multimode microplate reader (BioTek Instruments) using excitation and emission frequencies of 510 and 580 nm, respectively. All samples were normalized to bacterial control in separate wells on the same plate. In some experiments, ROS formation was also determined using DCF-DA as described previously (5). To measure sequence-specific mitochondrial DNA (mtDNA) damage, a minimum of 10,000 animals were washed three times on ice with S basal buffer (100.1 mM NaCl, 5.7 mM K2HPO4, 44.1 mM KH2PO4, 0.01 mM cholesterol) and then twice with isolation buffer (210 mM mannitol, 70 mM sucrose, 0.1 mM EDTA, 5 mM Tris-HCl, pH 7.4). Animals were collected by centrifugation and the resulting C. elegans pellets were homogenized in isolation buffer using a Teflon-glass homogenizer. Debris and nuclei were removed from the homogenate by centrifugation (600g, 4°C, 10 min), and the supernatant containing mitochondria was further centrifuged (7200g, 4°C, 10 min) to obtain a mitochondrial pellet, which was then resuspended in Tris-EDTA buffer (50 mM Tris-HCl, 0.1 mM EDTA, pH 7.4). DNA from crude mitochondria was purified using Prepman Ultra Sample Preparation Reagent (Applied Biosystems), according to the manufacturer's protocol. Quantitative PCR was performed using the GeneAMP XL PCR kit (Applied Biosystems) based on a protocol described elsewhere (17). mtDNA damage was assessed by comparing amplification efficiency in a 6.3 kb region of the mitochondrial genome (17) to a 71 bp region (forward primer: 5′-GAGCGTCATTTATTGGGAAGAAGA-3′ and reverse primer: 5′-TGTGCTAATCCCATAAATGTAACCTT-3′). PC content was measured as described elsewhere (27). Briefly, about 200 wild-type or mpst-1 knockout worms exposed to GYY4137 (100 μM) on agar plates for 24 h were collected, washed to remove residual bacteria, and resuspended in phosphate-buffered saline containing tween 20 (0.1% v/v) and phenylmethylsulfonyl fluoride (1 mM). Samples were then sonicated, and protein concentration of the lysates was determined by the Dc-Protein Assay (Bio-Rad). Worm lysates (5 μl, 2 μg) were mixed with 12% v/v SDS (5 μl) and 2,4-dinitrophenylhydrazine (DNPH, 20 mM, 10 μl) for 15 min at room temperature and neutralized with Tris-Cl buffer (2 mM containing 30% v/v glycerol, 75 μl). Slot blot was conducted (1 h, room temperature) using anti-DNPH antibody (1:150; Chemicon International) with secondary detection using horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (1:300). All chemicals and antibodies for this assay were from the Oxyblot Protein Oxidation Detection Kit (Chemicon International). Antibody-bound proteins were detected by chemiluminescence using a Chemidoc XRS imaging system (Bio-Rad).
Immunohistochemical detection of H2S-synthesizing enzymes
C. elegans were fixed with 4% v/v paraformaldehyde in phosphate buffer at 4°C and washed with PBS. Whole mount or cryo-sections (10 μm) of the worms were then washed with PBS and blocked in 5% v/v normal rabbit or mouse serum for 1 h at room temperature. Sections were then incubated overnight with the following primary antibodies: rabbit anti-CBS (1: 200; Abcam), rabbit anti-3-MST (1:200; Abcam), and mouse anti-CSE (1:250; Abcam) in PBS containing 0.1% v/v Triton X-100 (PBS-TX). Sections were washed in PBS and incubated in anti-rabbit IgG conjugated to FITC (1:200; Sigma) or anti-mouse IgG conjugated to Cy3 (1:200; Sigma) both diluted in PBS-TX for 1 h at room temperature and then counterstained with 4′,6-diamidino-2-phenylindole (DAPI). 3-MST was also stained in mpst-1 mutants. Control sections were also incubated as described above but without the primary antibodies and showed no significant staining. Photoimages were captured in a fluorescence microscope equipped with a digital camera (Olympus BX51; Olympus).
Measurement of H2S release from GYY4137 and H2S-synthesizing activity
GYY4137 (100 μM) was incubated in buffer solution in capped glass vials and incubated at 20°C. Measurements were conducted on days 0–7 and 30. The concentration of H2S was determined using the fluorescent probe, dansyl azide (0.6 mM) (22), with excitation and emission wavelengths of 344 and 533 nm, respectively. Enzymatic H2S synthesis by C. elegans homogenate was measured as described (14) with the following modifications: 430 μl supernatant derived from a homogenate of at least 4000 adult (day 5) worms was incubated with PPP (2 mM) and substrate l-cysteine (10 mM) and sealed with a double parafilm layer to avoid leakage of H2S gas generated after incubation at 37°C for 30 min. In experiments to measure the 3-MST activity, homogenates were incubated with 3-MP (1 mM) instead of l-cysteine and PPP was omitted. Baseline controls contained trichloroacetic acid (TCA; 10% w/v, 250 μl) prepared in parallel. At the end of the incubation period, zinc acetate (1% w/v, 250 μl) was injected to trap the H2S followed by TCA (10% w/v, 250 μl) to terminate the reaction. Subsequently, N,N-dimethyl-p-phenylenediamine sulfate dye (NNDPD; 20 mM) in 7.2 M HCl was added, followed by the addition of FeCl3 (30 mM) in 1.2 M HCl. After centrifugation (14,000 rpm, 4 min, 4°C), absorbance (670 nm) of the resulting methylene blue in the supernatant was measured using a 96-well microplate reader (Tecan Systems) and compared against a standard curve of NaHS. Results are expressed as nmol H2S formed per mg protein per min. Protein concentration in each homogenate was estimated according to the manufacturer's instructions using the Bradford assay (Bio-Rad). The presence of H2S, cysteine, and 3-MP in homogenates of C. elegans was determined after derivatizing with monobromobimane, using a reverse-phase high-pressure liquid chromatography with fluorescence detection as described elsewhere (28). In brief, C. elegans (∼1000 animals) were homogenized and 15 μl homogenate incubated with Tris-HCl buffer (70 μl, 100 mM, pH 9.5, 0.1 mM diethylene triamine pentaacetic acid) and monobromobimane solution (50 μl, 10 mM in acetonitrile). The reaction was stopped after 30 min by adding ice-cold sulfosalicylic acid (50 μl, 200 mM), and samples were vortexed and placed on ice before rp-hplc as described elsewhere (28). Detection of thiols was assessed by measurement of the fluorescent sulfide-dibimane derivatives (excitation at 390 nm/emission at 475 nm). The retention times of authentic cysteine, 3-MP, and H2S were 4.1±0.02, 14.5±0.01, and 24.4±0.01 min (all n=3), respectively.
Statistical analysis
Data show mean±SEM. Multiple comparisons were made using one-way ANOVA followed by post hoc Tukey test. The difference between lifespan and healthspan survival curves was calculated using the log-rank (Mantel-Cox) test. Statistical significance was set at p<0.05.
Supplementary Material
Abbreviations Used
- 3-MP
3-mercaptopyruvate
- 3-MST
3-mercaptopyruvate transferase
- CBS
cystathionine β synthetase
- CSE
cystathionine γ lyase
- DAB
diaminobenzidine
- DCF
2,7-dichlorofluorescein
- DCF-DA
2,7-dichlorofluorescein diacetate
- GO
gene ontology
- GSH
glutathione
- H2S
hydrogen sulfide
- NGM
nematode growth medium
- PPP
pyridoxal 5′ phosphate
- ROS
reactive oxygen species
- TCA
trichloroacetic acid
Acknowledgments
This work was supported by the King's College London (S.R.S.), the National University of Singapore (P.K.M.), and a grant (MOE2012-T2-2-003) from the Ministry of Education, Singapore (P.K.M., J.G., B.H.). B.Q. was supported by a State of Kuwait funded studentship.
Authors' Contribution
SRS., P.K.M., J.G., B.H., and L.L. designed the research; B.Q. and L.L. also performed research, analyzed data, and contributed to the writing of the article; M.T.P., L.F.N., S.D.K., P.R., and F.W. performed research; C-H.T. and B.W.D. contributed new reagents/analytic tools; SRS and SCS analyzed microarray data; J.G., SRS and P.K.M. analyzed data and wrote the article.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Back P, Braeckman BP, and Matthijssens F. ROS in aging Caenorhabditis elegans: damage or signaling? Oxid Med Cell Longev, 2012. [Epub ahead of print]; DOI: 10.1155/2012/608478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budde MW. and Roth MB. The response of Caenorhabditis elegans to hydrogen sulfide and hydrogen cyanide. Genetics 189: 521–532, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen YH, Yao WZ, Geng B, Ding YL, Lu M, Zhao MW, and Tang CS. Endogenous hydrogen sulfide in patients with COPD. Chest 128: 3205–3211, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Cooper CE. and Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 40: 533–539, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Gruber J, Ng LF, Fong S, Wong YT, Koh SA, Chen CB, Shui G, Gheong FW, Schaffe S, Wenk MR, and Halliwell B. Mitochondrial changes in ageing caenorhabditis elegans—what do we learn from superoxide dismutase knockouts? PLoS One 6: e19444, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosokawa H, Ishii N, Ishida H, Ichimori K, Nakazawa H, and Suzuki K. Rapid accumulation of fluorescent material with aging in an oxygen-sensitive mutant mev-1 of Caenorhabditis elegans. Mech Ageing Dev 74: 161–170, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Hughes S. and Stürzenbaum SR. Single and double metallothionein knockout in the nematode C. elegans reveals cadmium dependent and independent toxic effects on life history traits. Environ Pollut 145: 395–340, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Kearney M, Matthijssens F, Sharpe M, Vanfleteren J, and Gems D. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic Biol Med 37: 239–250, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Kenyon C. A pathway that links reproductive status to lifespan in Caenorhabditis elegans. Ann N Y Acad Sci. 1204: 156–162, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Kimura Y, Goto Y, and Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 12: 1–13, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Kimura H, Shibuya N, and Kimura Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal 17: 45–57, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King AL. and Lefer DJ. Cytoprotective actions of hydrogen sulfide in ischaemia-reperfusion injury. Exp Physiol 96: 840–846, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Kulich SM, Horbinski C, Patel M, and Chu CT. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic Biol Med 43: 372–383, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, and Moore PK. Hydrogen sulfide is a novel mediator of endotoxic shock. FASEB J 19: 1196–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, and Moore PK. Characterisation of a novel, water soluble hydrogen sulfide releasing molecule (GYY4137): new insights into the biology of hydrogen sulphide. Circulation 117: 2351–2360, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Mathew ND, Schlipalius DI, and Ebert PR. Sulfurous gases as biological messengers and toxins: comparative genetics of their metabolism in model organisms. J Toxicol 2011: 394970, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melov S, Lithgow GJ, Fischer DR, Tedesco PM, and Johnson TE. Increased frequency of deletions in the mitochondrial genome with age of Caenorhabditis elegans. Nucleic Acids Res 23: 1419–1425, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller DL. and Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A 104: 20618–206122, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagahara N. and Katayama A. Post-translational regulation of mercaptopyruvate sulfurtransferase via a low redox potential cysteine sulfenate in the maintenance of redox homeostasis. J Biol Chem 280: 34569–34576, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Onken B. and Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One 5: e8758, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jap J Nutr 44: 307–315, 1986 [Google Scholar]
- 22.Peng H, Cheng Y, Dai C, King AL, Predmore BL, Lefer DJ, and Wang B. A fluorescent probe for fast and quantitative detection of hydrogen sulfide in blood. Angew Chem Int Ed Engl 50: 9672–9675, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Predmore BL, Alendy MJ, Ahmed KI, Leeuwenburgh C, and Julian D. The hydrogen sulfide signaling system: changes during aging and the benefits of caloric restriction. Age (Dordr) 32: 467–481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pun PB, Gruber J, Tang SY, Schaffer S, Ong RL, Fong S, Ng LF, Cheah I, and Halliwell B. Ageing in nematodes: do antioxidants extend lifespan in Caenorhabditis elegans? Biogerontology 11: 17–30, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, and Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231–1237, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, and Blackwell TK. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab 15: 713–724, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaffer S, Gruber J, Ng LF, Fong S, Wong YT, Tang SY, and Halliwell B. The effect of dichloroacetate on health- and lifespan in C. elegans. Biogenterology 12: 195–209, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, and Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 50: 1021–1031, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viswanathan M, Kim SK, Berdichevsky A, and Guarente L. A role for SIR2.1. Regulation of ER stress response genes in determining C. elegans life span. Dev Cell 9: 605–615, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Vozdek R, Hnízda A, Krijt J, Kostrouchová M, and Kožich V. Novel structural arrangement of nematode cystathionine β-synthases: characterization of Caenorhabditis elegans CBS-1. Biochem J 443: 535–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, and Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem 90: 765–768, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Wróbel M, Jurkowska H, Sliwa L, and Srebro Z. Sulfurtransferases and cyanide detoxification in mouse liver, kidney, and brain. Toxicol Mech Methods 14: 331–337, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.