Abstract
Purpose of review
Long-term survival of liver transplant recipients is threatened by increased rates of de-novo malignancy and recurrence of hepatocellular carcinoma (HCC), both events tightly related to immunosuppression.
Recent findings
There is accumulating evidence linking increased exposure to immunosuppressants and carcinogenesis, particularly concerning calcineurin inhibitors (CNIs), azathioprine and antilymphocyte agents. A recent study including 219 HCC transplanted patients showed that HCC recurrence rates were halved if a minimization of CNIs was applied within the first month after liver transplant. With mammalian target of rapamycin (mTOR) inhibitors as approved immunosuppressants for liver transplant patients, pooled data from several retrospective studies have suggested their possible benefit for reducing HCC recurrence.
Summary
Randomized controlled trials with sufficiently long follow-up are needed to evaluate the influence of different immunosuppression protocols in preventing malignancy after LT. Currently, early minimization of CNIs with or without mTOR inhibitors or mycophenolate seems a rational strategy for patients with risk factors for de-novo malignancy or recurrence of HCC after liver transplant. A deeper understanding of the immunological pathways of rejection and cancer would allow for designing more specific and safer drugs, and thus to prevent cancer after liver transplant.
Keywords: cancer, hepatocellular carcinoma, immunosuppression, liver transplantation, malignancy
INTRODUCTION
The improvement in surgical techniques and medical care has prolonged survival after liver transplantation, leading to a parallel increase of long-term complications such as de-novo malignancy, which is becoming a major source of morbidity and mortality [1▪]. Several population-based studies worldwide have reported a two- to three-fold increased cancer rates in liver transplant patients, when compared with age and sex-matched populations [2–12]. Moreover, in patients transplanted with hepatocellular carcinoma (HCC), tumour recurrence affects 15–20% of patients despite a careful selection of candidates based on the Milan criteria [13], and therapeutic options are very limited in this situation. In a recent analysis of 93 634 patients from the European Liver Transplant Registry (1968–2009), 21% of deaths occurred because of de-novo tumours or recurrence of HCC, demonstrating the critical importance of these complications in the current liver transplantation scenario [14].
The link between immunosuppression and oncogenesis is well established, as the integrity of the immune system is one of the defenses against cancer [15]. In the initial stages of carcinogenesis, several components of the immune system are able to locate and destroy cancer cells, delay tumour progression and prevent vascular invasion and metastasis. The immune system also allows for control of viral infections related to cancer. Animal models with defective function of natural killer cells and/or T cells (CD8+ cytotoxic or CD4+ T helper) have increased risk and aggressiveness of tumours, suggesting a cumulative cancer promoting effect, when both the innate and the adaptive immune pathways are impaired [16]. Conversely, cancer cells from highly aggressive tumours are able to paralyze infiltrating immune cells by secreting immunosuppressive molecules such as transforming growth factor (TGF)-β and CCL21 [17,18]. Indeed the types of cancer with the highest standardized incidence ratio after liver transplantation are related to infections (Kaposi sarcoma, nasopharyngeal carcinoma, cervical and vulvar cancer) [19], have an origin in the immune system (lymphoproliferative disorders particularly Burkitt lymphoma [20]), or are located in exposed areas (skin cancer, head and neck cancer) (Table 1) [2,3,5–8,10–12]. Thus, the increased risk of overall malignancy after liver transplantation is partly related to these otherwise less frequent tumours, leading to a specific ‘cancer pattern’ related to immunosuppression. It is not surprising that this ‘cancer pattern’ is reproduced in AIDS wherein effective antiretroviral therapies have prolonged survival [21], and establish a chronic immunosuppressive status [22,23]. New therapies that enhance the immune system are becoming a reality in the management of several types of cancer.
Table 1.
Studies reporting types of cancer and their standardized incidence ratio after liver transplantation published in the last decade. Only selective data on liver transplant recipients are shown. The marked standardized incidence ratio values (∗) indicate statistical significance at P < 0.05
| Authors | Year | Country | Type | Period | n | Overall | Lymphoma | Skin | Head-neck | Renal | Others |
| Krynitz et al. [11] | 2013 | Sweden | National Survey | 1970–2008 | 10 476 | 3.4* | 9.6* | 16* | 4.6* | 1.9 | Colon: 2.2; Breast: 1; Prostate: 0.5; Lung: 1.8. |
| Chatrath et al. [10] | 2013 | United States | Single centre | 1997–2004 | 534 | 3.1* | 7.1* | – | – | – | – |
| Schrem et al. [6] | 2013 | Germany | Single centre | 1983–2010 | 2000 | 1.94* | 10.9* | – | Oral: 1.7; Larynx: 2.3. | 2.6* | Colorectal:1.41*; Breast: 0.83; Vulvar: 23.8*; Prostate: 0.62; Lung: 1.85*. |
| Engels et al. [8] | 2011 | United States | National survey | 1987–2008 | 37 888 | – | Non-Hodgkin: 7.77* | – | — | 1.8* | Lung: 1.95*; Liver: 43.8*. |
| Baccarani et al. [3] | 2010 | Italy | Two centres | 1991–2005 | 417 | 2.6* | 13.8* | – | 7* | – | Colon: 1.4; Lung: 1.6; Breast: 0.6. |
| Jiang et al. [5] | 2008 | Canada | National survey | 1983–1998 | 2034 | 2.5* | Non-Hodgkin: 20.8* | – | 2.5 | 3.1 | Colorectal: 2.6*; Breast: 0.6; Prostate: 1. |
| Aberg et al. [2] | 2008 | Finland | National survey | 1982–2005 | 540 | 2.59* | Non-Hodgkin: 13.9*; Hodgkin: 14.7 | 38.5* | Lip: 21.3; Mouth: 14.8. | 4.17 | Colorectal: 1.59; Breast: 0.26; Prostate:1.24; Stomach: 4.97. |
| Collet et al. [7] | 2010 | United Kingdom | National survey | 1980–2007 | 6846 | 2.2* | Non-Hodgkin: 13.3*; Hodgkin: 8.9*. | 6.6* | Lip: 20*; Oral: 10*. | 1.8 | Colorectal: 2.3*; Breast: 0.8; Lung: 1.6*. |
| Oo et al. [12] | 2005 | United Kingdom | Single centre | 1982–2004 | 1778 | 2.07* | 10.3* | 5.8* | – | – | Colon: 4.9*; Breast: 0.97; Lung: 1.96*. |
Box 1.
no caption available
However, there are few studies evaluating immunosuppression protocols to prevent or reduce malignancy after liver transplantation, and they have a poor level of evidence (Fig. 1). There are no randomized controlled trials powered to detect differences in de-novo tumours or recurrence of HCC, mainly because of the heterogeneity in the biology of different types of cancer, and the prolonged follow-up required. The available evidence comes from observational studies, and thus results should be interpreted with caution because of the great variability in clinical practice between liver transplant institutions, which increases the risk of bias when analyzing multicentre pooled data. Despite this situation, the general behaviour among clinicians is to minimize the exposure to immunosuppressants as much as possible after liver transplantation. With these background caveats, we present a comprehensive review of the available evidence about the relationship between the immunosuppressive drugs most frequently used in liver transplant patients and the risk of malignancy. We conclude with some recommendations to prevent de-novo malignancy and recurrence of HCC after liver transplantation.
FIGURE 1.
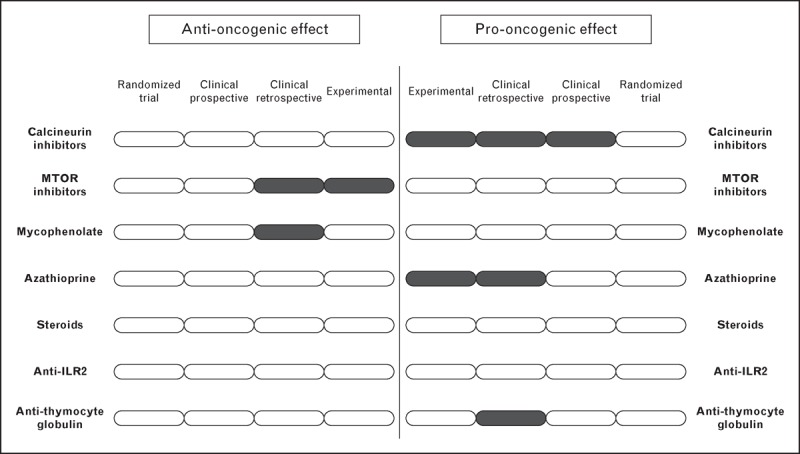
Summary of the level of evidence regarding the influence of the most frequently used immunosuppressive drugs after liver transplantation and the risk of malignancy (including lymphoproliferative disorders, any type of solid malignancy and recurrence of hepatocellular carcinoma). The figure points out the scarce number of studies available and the reduced level of evidence.
CALCINEURIN INHIBITORS
Calcineurin inhibitors (CNIs) are the mainstay of immunosuppression after liver transplantation. Tacrolimus is the most frequently used because of reduced acute rejection rates and better graft and patient survival, compared with cyclosporine [24]. In-vitro studies and animal models have shown that both tacrolimus and cyclosporine are able to activate proto-oncogenes and pathways of cancer such as TGF-β in a dose-dependent fashion, thus promoting tumour proliferation, resistance to apoptosis and metastasis [25–27].
The risk of malignancy with tacrolimus or cyclosporine should be similar in clinical practice. To our knowledge, only one single-centre study has described an increased risk of malignancy in liver transplant patients treated with cyclosporine compared with tacrolimus [28]. However, the authors admit that a different monitoring was used for cyclosporine, and lower rejection rates were detected in this group, suggesting higher immunosuppressive potency with cyclosporine than with tacrolimus in this series. The only randomized controlled trial aiming to detect differences in de-novo malignancy compared two regimens with cyclosporine in kidney transplant recipients: conventional trough levels (i.e. 150–250 ng/ml) vs. reduced trough levels (i.e. 75–125 ng/ml). The group with conventional exposure had increased rates of de-novo tumours (32 vs. 19%; P = 0.03) [29]. Thus, the risk of malignancy related to CNI in clinical practice may come from the dosage rather than the type of CNI used.
Several retrospective studies have shown a dose-dependent relationship between CNI and recurrence of HCC after liver transplantation [30,31,32▪▪]. The over-exposure to CNI may be particularly relevant early after liver transplantation when the immune system should be able to detect and destroy remaining HCC cells in the bloodstream. Indeed, an observational study with 219 patients transplanted with HCC showed that those patients having mean trough concentrations more than 10 ng/ml for tacrolimus or more than 300 ng/ml for cyclosporine within the first 30 days after liver transplantation had nearly a three-fold increased risk of HCC recurrence, after controlling for possible confounding factors [32▪▪]. Moreover, tacrolimus trough concentrations 7–10 ng/ml within the first 30 days after liver transplantation result in similar rejection rates [33▪], halved renal impairment [33▪] and longer graft survival [34], when compared with trough concentrations more than 10 ng/ml, which is the ‘conventional’ exposure in randomized trials [35]. Therefore, tacrolimus trough concentrations 7–10 ng/ml should be the standard of care within the first month after liver transplantation, and future randomized trials should implement reduced tacrolimus trough concentrations in their design.
MTOR INHIBITORS
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase involved in cellular growth, proliferation, metabolism and angiogenesis, and it comprises two signalling pathways: mTOR complex 1, responsible for cellular growth and proliferation in response to immune regulatory signals, and mTOR complex 2 involved in aspects of cell metabolism [36▪]. There are two mTOR inhibitors available for liver transplant patients. Sirolimus is a non-selective inhibitor of mTOR complexes 1 and 2, and everolimus has a restricted effect on mTOR complex 1. Both drugs have shown immunosuppressive and anticancer properties in animal models including HCC [37–40].
In liver transplantation, the mTOR inhibitors are used mainly as renal sparing agents, owing to a potent immunosuppressive effect, which allows reduction of CNI exposure, or even for a conversion from a CNI based to a mTOR inhibitor-based regimen [41,42]. There are no published randomized controlled trials evaluating the effect of mTOR inhibitors in preventing de-novo malignancy or recurrence of HCC after LT. The available evidence is based on clinical reports and retrospective studies, thus making it difficult to extract solid conclusions. Despite this, many transplant centres frequently add or convert to a mTOR inhibitor when there are risk factors for malignancy after liver transplantation, or even when a tumour has been diagnosed. There are several reports of improved outcome of lymphoproliferative disorders and Kaposi sarcoma after switching to a mTOR inhibitor [43].
Regarding prevention of HCC recurrence, there are five retrospective studies evaluating the role of sirolimus-based immunosuppression after liver transplantation [44–48], the results of which have been summarized in two meta-analyses [49▪,50▪]. The conclusions from both are similar suggesting a protective effect of sirolimus against HCC recurrence with an odds ratio (OR) ranging from 0.30 [(95% confidence interval (CI) 0.16–0.55 to 0.42 (95% CI 0.21–0.83), and also a benefit in 5-year overall survival with OR from 0.35 (95% CI 0.20–0.61) to 0.40 (95% CI 0.28–0.58). However an analysis of 26 414 patients from the Scientific Registry of Transplant Recipients Database (USA) showed an increased risk of all-cause mortality in patients with hepatitis C treated with sirolimus (hazard ratio = 1.29; 95% CI 1.08–1.55) [51]. The limitations of these studies are evident: observational and retrospective design, some based on registries originally designed for a different purpose, and a high risk of reporting bias. The ongoing SiLVER study, which is a multicentre randomized controlled trial evaluating the role of sirolimus in HCC transplanted patients, may determine the true effect of mTOR inhibitors in preventing tumour recurrence, but the results will not be available before 2016 [52].
With respect to everolimus, there are no clinical studies suggesting a significant protective effect against HCC recurrence or de-novo malignancy after liver transplantation. As everolimus has a selective inhibition on mTOR complex 1, a more specific antitumour effect would be expected [37], and an improved metabolic profile has been hypothesized [53]. However, the activation of feedback signals makes prediction of the consequences difficult in clinical practice. The selective blocking of mTOR complex 1 with everolimus on the severity of hepatitis C recurrence and graft loss also deserves further investigation.
ANTIMETABOLITES
The antimetabolites interfere with de novo synthesis of nucleotides, and herein have a potent cytostatic effect on lymphocytes, which is more pronounced than in other cell types. Mycophenolate is the most widely used after liver transplantation, mainly in combination with CNI as a renal sparing agent [54,55]. To date there is no evidence suggesting a link between the use of mycophenolate and de novo malignancy after liver transplantation, even in the long term. A large observational study included 6751 renal transplant patients who received mycophenolate from the United Network for Organ Sharing (UNOS) and the collaborative transplant study registries. There was no significant increase in either lymphoma or any malignancy when compared with a matched cohort without mycophenolate. In the subgroup from the collaborative transplant study, a longer time to malignancy was found for mycophenolate-treated patients (log rank 0.026) [56]. In 3895 heart transplant patients, the use of mycophenolate had a protective effect against de-novo malignancy (relative risk = 0.75; 95% CI 0.56–0.95) [57]. The studies evaluating the role of immunosuppression on HCC recurrence showed no influence of mycophenolate [31,32▪▪].
Azathioprine used as 1 mg/kg/day is preferred in some institutions for patients transplanted with hepatitis C because of a slower fibrosis progression and reduced risk of decompensation of severe recurrent disease, as recently shown in a randomized controlled trial with a median follow-up of 8 years and with protocol biopsies [58]. The prolonged use of azathioprine has been associated with an increased risk of nonmelanoma skin cancer, which may be explained by a dual mechanism, including an increased DNA damage powered by the interaction between ultraviolet-A radiation and 6-thioguanine, and an impaired DNA repair system [59,60]. The actual implication of nonmelanoma skin cancer, which usually is diagnosed at early stages, on prognosis is a matter of debate [61,62]. In inflammatory bowel disease patients treated with azathioprine, there is a four- to five-fold increased risk of lymphoproliferative disorders, especially in elderly patients, but still lymphoma is a very uncommon malignancy [63]. However, these data are hardly transferred to the liver transplant population, as doses of azathioprine are at least twice in patients with inflammatory bowel disease. The risk of Burkitt lymphoma is increased in liver transplantation patients compared with renal recipients according to a large registry from the USA, and azathioprine appeared as an independent risk factor in this cohort [20]. In liver transplantation patients, Benlloch et al.[64] retrospectively analyzed a single-institution experience (n = 772) on the development of non-skin malignancy and the risk factors involved. They found azathioprine as an independent predictor of any tumour development after liver transplantation (OR = 3.8; 95% CI 1.7–8.6; P = 0.004).
OTHER IMMUNOSUPPRESSIVES
Corticosteroids form part of most immunosuppression protocols within the first months after liver transplantation. The heterogeneity in dosage and tapering schedule between institutions makes it difficult to obtain a reliable analysis of pooled data [65], and there is no evidence suggesting that the use of corticosteroids influences de novo malignancy after liver transplantation. In a single registry-based study, the use of corticosteroids was protective against Burkitt lymphoma [20].
The interleukin-2 receptor blockers (basiliximab and daclizumab – the latter has been withdrawn from the market) are frequently used immediately after liver transplantation as induction immunosuppression agents in order to delay the introduction of CNI as part of a renal-sparing strategy [66]. A large observational study with 929 renal transplant patients was unable to find any relationship between the use of anti-interleukin-2 receptors and de-novo malignancy. However, induction with anti-thymocyte globulins, which are polyclonal antibodies directed against multiple T- and B-cell antigens responsible for a depletion of peripheral lymphocytes, is associated with an increased risk for lymphoma after renal transplantation (0.67 vs. 0.43% with no induction; P = 0.002), whereas basiliximab was not (0.38 vs. 0.43% with no induction; P = 0.33) [67]. However, studies in liver transplant population are lacking.
FUTURE PERSPECTIVES
The reduced clinical significance of acute cellular rejection after liver transplantation, which progresses to chronic rejection and graft loss in less than 5% of patients, together with the increasing risk of renal impairment, infections and malignancy, demonstrates that liver transplant patients have been, and are still, over-immunosuppressed in many transplant centres [33▪,68]. There are still recent randomized trials using quadruple immunosuppression as a standard of care for liver transplantation patients [69], leading to unnecessarily reduced rejection rates, a more severe recurrence of hepatitis C, more frequent infections and new-onset diabetes [70]. A certain grade of acute rejection early after liver transplantation provides a benefit in terms of long-term survival [34,71], and it has been hypothesized that a complete suppression of acute rejection may prevent operational tolerance, and therefore it is neither necessary nor appropriate [71,72].
The current immunosuppression protocols in liver transplantation interfere with the immune system at many different levels simultaneously, impairing both the innate and the adaptative immune responses. A better understanding of the mechanisms underlying rejection is needed to develop more specific drugs, able to target selective rejection effectors, while keeping the ability to fight cancer cells, infections and to develop tolerance. In the future, a tailored ‘cocktail’ of monoclonal antibodies against specific cytokines, receptors and growth factors depending on individual patient's immunological features will form the standard of care to prevent chronic rejection and graft loss. The therapeutic paradigm will change from immunosuppression to immunomodulation [73]. With the available immunosuppressants, the best approach to prevent malignancy after liver transplantation nowadays would be to decrease the number and the dosage of the immunosuppressive drugs to the minimum, starting as soon as possible after liver transplantation. A certain grade of immune response is desirable to prevent cancer, as it is to facilitate long-term graft tolerance. A single episode of acute rejection early after liver transplantation is easily managed and derived mortality is zero, whereas a single cancer forms a life-threatening condition with very limited therapeutic options.
CONCLUSION
The studies linking immunosuppression and cancer after liver transplantation have serious biases, which weaken the evidence: heterogeneity of immunosuppression protocols between centres, variable biologic tumour behaviour and inadequate follow-up. Future studies if these biases are removed will have a problem of increased follow-up costs. However, the evidence taken as a whole suggests that the immunosuppression regimen plays a central role in de-novo malignancy and in the recurrence of HCC after liver transplantation. The minimization of CNI should be universally applied, beginning as soon as possible after liver transplantation. In selected patients requiring a more potent immunosuppression, the addition of either mTOR inhibitors or mycophenolate would be the most rational approach. Prospective studies and randomized controlled trials with a large number of patients and sufficiently long follow-up are urgently needed to support the recommendations given, and to explore the best ‘anti-tumour’ immunosuppression regimen. The next steps should be to identify the critical nodes in the physiopathology of rejection, and to develop more specific drugs with a better safety profile including reduced pro-oncogenic effects.
Acknowledgements
Dr Manuel Rodríguez-Perálvarez and Professor Manuel de la Mata would like to acknowledge the career of Professor Andrew Kenneth Burroughs (who sadly past away on the 15th March 2014 aged 60) for his great contribution to the fields of hepatology and liver transplantation.
Conflicts of interest
The authors of the present manuscript do not have any conflict of interest to disclose.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18:1277–1289 [DOI] [PubMed] [Google Scholar]; This excellent review summarizes the impact of cancer on the prognosis after liver transplantation and highlights the most important risk factors.
- 2.Aberg F, Pukkala E, Hockerstedt K, et al. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl 2008; 14:1428–1436 [DOI] [PubMed] [Google Scholar]
- 3.Baccarani U, Piselli P, Serraino D, et al. Comparison of de novo tumours after liver transplantation with incidence rates from Italian cancer registries. Dig Liver Dis 2010; 42:55–60 [DOI] [PubMed] [Google Scholar]
- 4.Herrero JI, Lorenzo M, Quiroga J, et al. De Novo neoplasia after liver transplantation: an analysis of risk factors and influence on survival. Liver Transpl 2005; 11:89–97 [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Villeneuve PJ, Fenton SS, et al. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl 2008; 14:1588–1597 [DOI] [PubMed] [Google Scholar]
- 6.Schrem H, Kurok M, Kaltenborn A, et al. Incidence and long-term risk of de novo malignancies after liver transplantation with implications for prevention and detection. Liver Transpl 2013; In press [DOI] [PubMed] [Google Scholar]
- 7.Collett D, Mumford L, Banner NR, et al. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant 2010; 10:1889–1896 [DOI] [PubMed] [Google Scholar]
- 8.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011; 306:1891–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haagsma EB, Hagens VE, Schaapveld M, et al. Increased cancer risk after liver transplantation: a population-based study. J Hepatol 2001; 34:84–91 [DOI] [PubMed] [Google Scholar]
- 10.Chatrath H, Berman K, Vuppalanchi R, et al. De novo malignancy postliver transplantation: a single center, population controlled study. Clin Transplant 2013; 27:582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krynitz B, Edgren G, Lindelof B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008–a Swedish population-based study. Int J Cancer 2013; 132:1429–1438 [DOI] [PubMed] [Google Scholar]
- 12.Oo YH, Gunson BK, Lancashire RJ, et al. Incidence of cancers following orthotopic liver transplantation in a single center: comparison with national cancer incidence rates for England and Wales. Transplantation 2005; 80:759–764 [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208–1236 [DOI] [PubMed] [Google Scholar]
- 14.Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol 2012; 57:675–688 [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674 [DOI] [PubMed] [Google Scholar]
- 16.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007; 121:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields JD, Kourtis IC, Tomei AA, et al. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010; 328:749–752 [DOI] [PubMed] [Google Scholar]
- 19.Schulz TF. Cancer and viral infections in immunocompromised individuals. Int J Cancer 2009; 125:1755–1763 [DOI] [PubMed] [Google Scholar]
- 20.Mbulaiteye SM, Clarke CA, Morton LM, et al. Burkitt lymphoma risk in U.S. solid organ transplant recipients. Am J Hematol 2013; 88:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008; 123:187–194 [DOI] [PubMed] [Google Scholar]
- 23.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370:59–67 [DOI] [PubMed] [Google Scholar]
- 24.McAlister VC, Haddad E, Renouf E, et al. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant 2006; 6:1578–1585 [DOI] [PubMed] [Google Scholar]
- 25.Maluccio M, Sharma V, Lagman M, et al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation 2003; 76:597–602 [DOI] [PubMed] [Google Scholar]
- 26.Suthanthiran M, Hojo M, Maluccio M, et al. Posttransplantation malignancy: a cell autonomous mechanism with implications for therapy. Trans Am Clin Climatol Assoc 2009; 120:369–388 [PMC free article] [PubMed] [Google Scholar]
- 27.Datta D, Contreras AG, Basu A, et al. Calcineurin inhibitors activate the proto-oncogene Ras and promote protumorigenic signals in renal cancer cells. Cancer Res 2009; 69:8902–8909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tjon AS, Sint Nicolaas J, Kwekkeboom J, et al. Increased incidence of early de novo cancer in liver graft recipients treated with cyclosporine: an association with C2 monitoring and recipient age. Liver Transpl 2010; 16:837–846 [DOI] [PubMed] [Google Scholar]
- 29.Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet 1998; 351:623–628 [DOI] [PubMed] [Google Scholar]
- 30.Vivarelli M, Cucchetti A, Piscaglia F, et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl 2005; 11:497–503 [DOI] [PubMed] [Google Scholar]
- 31.Vivarelli M, Cucchetti A, La Barba G, et al. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg 2008; 248:857–862 [DOI] [PubMed] [Google Scholar]
- 32▪▪.Rodriguez-Peralvarez M, Tsochatzis E, Naveas MC, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol 2013; 59:1193–1199 [DOI] [PubMed] [Google Scholar]; This article analyzes 219 consecutive patients with hepatocellular carcinoma receiving a liver transplantation. The reduced exposure to calcineurin inhibitors within the first month after liver transplantation (mean tacrolimus trough concentrations <10 ng/ml or cyclosporine <300 ng/ml) dramatically decreased the risk of tumour recurrence.
- 33▪.Rodriguez-Peralvarez M, Germani G, Darius T, et al. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant 2012; 12:2797–2814 [DOI] [PubMed] [Google Scholar]; This meta-analysis shows that the use of immunosuppression protocols with reduced tacrolimus early after liver transplantation (mean trough concentrations <10 ng/ml) is well tolerated in terms of acute rejection, whereas renal impairment rates are minimized among other benefits.
- 34.Rodriguez-Peralvarez M, Germani G, Papastergiou V, et al. Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. J Hepatol 2013; 58:262–270 [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Peralvarez M, Germani G, Darius T, et al. Tacrolimus exposure after liver transplantation in randomized controlled trials: too much for too long. Am J Transplant 2013; 13:1371–1372 [DOI] [PubMed] [Google Scholar]
- 36▪.Xu X, Ye L, Araki K. Ahmed R. mTOR, linking metabolism and immunity. Semin Immunol 2012; 24:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]; This review describes the mechanisms underlying mTOR inhibitors and their potential benefits for clinical practice.
- 37.Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 2008; 135:1972–19831983 e1971-1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaumann A, Schlitt HJ, Geissler EK. Immunosuppression and tumor development in organ transplant recipients: the emerging dualistic role of rapamycin. Transpl Int 2008; 21:207–217 [DOI] [PubMed] [Google Scholar]
- 39.Schumacher G, Oidtmann M, Rueggeberg A, et al. Sirolimus inhibits growth of human hepatoma cells alone or combined with tacrolimus, while tacrolimus promotes cell growth. World J Gastroenterol 2005; 11:1420–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piguet AC, Semela D, Keogh A, et al. Inhibition of mTOR in combination with doxorubicin in an experimental model of hepatocellular carcinoma. J Hepatol 2008; 49:78–87 [DOI] [PubMed] [Google Scholar]
- 41.De Simone P, Nevens F, De Carlis L, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant 2012; 12:3008–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson CJ, Gimson AE, Alexander GJ, et al. A randomized controlled trial of late conversion from calcineurin inhibitor (CNI)-based to sirolimus-based immunosuppression in liver transplant recipients with impaired renal function. Liver Transpl 2007; 13:1694–1702 [DOI] [PubMed] [Google Scholar]
- 43.Lebbe C, Euvrard S, Barrou B, et al. Sirolimus conversion for patients with posttransplant Kaposi's sarcoma. Am J Transplant 2006; 6:2164–2168 [DOI] [PubMed] [Google Scholar]
- 44.Zhou J, Wang Z, Wu ZQ, et al. Sirolimus-based immunosuppression therapy in liver transplantation for patients with hepatocellular carcinoma exceeding the Milan criteria. Transplant Proc 2008; 40:3548–3553 [DOI] [PubMed] [Google Scholar]
- 45.Vivarelli M, Dazzi A, Zanello M, et al. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma. Transplantation 2010; 89:227–231 [DOI] [PubMed] [Google Scholar]
- 46.Zimmerman MA, Trotter JF, Wachs M, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl 2008; 14:633–638 [DOI] [PubMed] [Google Scholar]
- 47.Chinnakotla S, Davis GL, Vasani S, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl 2009; 15:1834–1842 [DOI] [PubMed] [Google Scholar]
- 48.Toso C, Merani S, Bigam DL, et al. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology 2010; 51:1237–1243 [DOI] [PubMed] [Google Scholar]
- 49▪.Menon KV, Hakeem AR, Heaton ND. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2012; 37:411–419 [DOI] [PubMed] [Google Scholar]; This meta-analysis of observational studies points out a possible benefit of the mTOR inhibitor sirolimus in preventing the recurrence of hepatocellular carcinoma after liver transplantation.
- 50▪.Liang W, Wang D, Ling X, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl 2012; 18:62–69 [DOI] [PubMed] [Google Scholar]; This paper describes another meta-analysis of observational studies with the same conclusion as the previous one. The limited quality of the available evidence is discussed.
- 51.Watt KD, Dierkhising R, Heimbach JK, Charlton MR. Impact of sirolimus and tacrolimus on mortality and graft loss in liver transplant recipients with or without hepatitis C virus: an analysis of the Scientific Registry of Transplant Recipients Database. Liver Transpl 2012; 18:1029–1036 [DOI] [PubMed] [Google Scholar]
- 52.Schnitzbauer AA, Zuelke C, Graeb C, et al. A prospective randomised, open-labeled, trial comparing sirolimus-containing versus mTOR-inhibitor-free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Cancer 2010; 10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boudjema K, Camus C, Saliba F, et al. Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. Am J Transplant 2011; 11:965–976 [DOI] [PubMed] [Google Scholar]
- 55.Neuberger JM, Mamelok RD, Neuhaus P, et al. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the ’ReSpECT’ study. Am J Transplant 2009; 9:327–336 [DOI] [PubMed] [Google Scholar]
- 56.Robson R, Cecka JM, Opelz G, et al. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant 2005; 5:2954–2960 [DOI] [PubMed] [Google Scholar]
- 57.O’Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006; 25:1186–1191 [DOI] [PubMed] [Google Scholar]
- 58.Manousou P, Cholongitas E, Samonakis D. Reduced fibrosis in recurrent HCV with tacrolimus, azathioprine and steroids versus tacrolimus: randomised trial long term outcomes. Gut 2013; In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brem R, Karran P. Multiple forms of DNA damage caused by UVA photoactivation of DNA 6-thioguanine. Photochem Photobiol 2012; 88:5–13 [DOI] [PubMed] [Google Scholar]
- 60.Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer 2008; 8:24–36 [DOI] [PubMed] [Google Scholar]
- 61.Christenson LJ, Cherikh WS, Otley CC, et al. Allograft and overall survival of patients with posttransplant skin cancer. Dermatol Surg 2011; 37:183–191 [DOI] [PubMed] [Google Scholar]
- 62.Zavos G, Karidis NP, Tsourouflis G, et al. Nonmelanoma skin cancer after renal transplantation: a single-center experience in 1736 transplantations. Int J Dermatol 2011; 50:1496–1500 [DOI] [PubMed] [Google Scholar]
- 63.Smith MA, Irving PM, Marinaki AM, Sanderson JD. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther 2010; 32:119–130 [DOI] [PubMed] [Google Scholar]
- 64.Benlloch S, Berenguer M, Prieto M, et al. De novo internal neoplasms after liver transplantation: increased risk and aggressive behavior in recent years? Am J Transplant 2004; 4:596–604 [DOI] [PubMed] [Google Scholar]
- 65.Sgourakis G, Radtke A, Fouzas I, et al. Corticosteroid-free immunosuppression in liver transplantation: a meta-analysis and meta-regression of outcomes. Transpl Int 2009; 22:892–905 [DOI] [PubMed] [Google Scholar]
- 66.Turner AP, Knechtle SJ. Induction immunosuppression in liver transplantation: a review. Transpl Int 2013; 26:673–683 [DOI] [PubMed] [Google Scholar]
- 67.Kirk AD, Cherikh WS, Ring M, et al. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant 2007; 7:2619–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiesner RH, Fung JJ. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transpl 2011; 17 Suppl 3:S1–S9 [DOI] [PubMed] [Google Scholar]
- 69.Ramirez CB, Doria C, Frank AM, et al. Completely steroid-free immunosuppression in liver transplantation: a randomized study. Clin Transplant 2013; 27:463–471 [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Peralvarez M, Manousou P, Lerut J, et al. How much immunosuppression is needed after liver transplantation? Clin Transplant 2013; In press [DOI] [PubMed] [Google Scholar]
- 71.Wiesner RH, Demetris AJ, Belle SH, et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology 1998; 28:638–645 [DOI] [PubMed] [Google Scholar]
- 72.Starzl TE. Immunosuppressive therapy and tolerance of organ allografts. N Engl J Med 2008; 358:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kovarik J. From immunosuppression to immunomodulation: current principles and future strategies. Pathobiology 2013; 80:275–281 [DOI] [PubMed] [Google Scholar]


