Supplemental Digital Content is available in the text.
Keywords: newborn, infant, meningitis, drug resistance, microbial, Africa
Abstract
Background:
Neonatal meningitis is an important cause of morbidity in sub-Saharan Africa and requires urgent empiric treatment with parenteral administered antibiotics. Here we describe the etiology, antimicrobial susceptibility and suitability of the World Health Organization first-line recommended antibiotics (penicillin and gentamicin) for bacterial meningitis in young infants in Malawi.
Methods:
We reviewed all cerebrospinal fluid samples received from infants ≤2 months of age with clinically suspected meningitis between January 1, 2002, and December 31, 2008, at the Queen Elizabeth Central Hospital in Blantyre, Malawi.
Results:
We identified 259 culture-positive isolates from 259 infants ≤2 months of age. Sixty isolates were from neonates ≤7 days old, in whom the most common pathogens were Group B Streptococcus (27/60; 45.0%), Streptococcus pneumoniae (13/60; 21.7%) and nontyphoidal Salmonella enterica (7/60; 11.7%). One hundred and ninety one isolates were from young infants who were >7 days and ≤2 months of age. In this group, the most common isolates were S. pneumoniae (80/191; 41.9%), Group B Streptococcus (38/191; 19.9%) and nontyphoidal Salmonella enterica (34/191; 17.8%). More isolates were susceptible to ceftriaxone than to the combination of penicillin and gentamicin (218/220; 99.1% vs. 202/220; 91.8%, Fisher’s exact test P = 0.006). In particular, Gram-negative isolates were significantly more susceptible to ceftriaxone than to gentamicin (72/74; 97.3% vs. 63/74; 85.1%, Fisher’s exact test P = 0.020). Penicillin and gentamicin provided less coverage for Gram-negative than Gram-positive isolates (74/86; 86.0% vs. 155/163; 95.1%, χ2 = 6.24, P = 0.012).
Conclusions:
In view of these results, the World Health Organization recommendations for empiric penicillin and gentamicin for suspected neonatal meningitis should be reevaluated.
An estimated 3.6 million neonatal deaths occurred worldwide in 2008, one-third of which were in Africa.1 Of these deaths, 17% were associated with sepsis.1 Although the exact burden of neonatal bacterial meningitis in Africa remains unknown, it represents an important cause of morbidity and mortality. A recent Kenyan study reported that 3–6% of all hospital admissions <59 days old were due to meningitis, with case fatality ratios of 26% in the first week of life and 18% between 7 and 59 days of age.2
Studies into neonatal meningitis in sub-Saharan Africa have reported a wide range of pathogens that vary both between and within countries. Streptococcus pneumoniae, Group B Streptococcus (GBS), Escherichia coli and nontyphoidal Salmonella species appear to predominate in East Africa.3–10 In West Africa, S. pneumoniae, E. coli and Salmonella species remain common, but a greater role is also seen for Staphylococcus aureus.11–15 In Southern Africa, GBS, E. coli and Salmonella species predominate, with a lesser role for S. pneumoniae.16–20
The range of pathogens within sub-Saharan Africa provides a major challenge to global antibiotic guidelines, particularly as treatment is required urgently. The World Health Organization (WHO) has outlined recommendations for empirical antimicrobial treatment for meningitis in neonates and young infants <2 months of age (summarized in Supplemental Digital Content 1, http://links.lww.com/INF/B777).21–28
The WHO Pocket Book of Hospital Care for Children provides the most comprehensive guidance and is intended for use in secondary level health care. It recommends either benzylpenicillin/ampicillin with gentamicin or ceftriaxone/cefotaxime if available as first-line treatment of meningitis in infants <2 months of age.25 Other WHO guidance suggests a third generation cephalosporin if there is no improvement after 48 hours.22,23 The country-specific appropriateness for these guidelines can only be judged with knowledge of local etiologic organisms and their antimicrobial susceptibility patterns.
Previous etiologic studies of neonatal meningitis have included populations of differing ages. Some have included neonates <30 days,4,8–10,12,13,15,16,18 young infants <60 days7 or <90 days3,5,11 of age and others have described their population simply as neonates.6,14,17 To ensure our results were as relevant as possible to WHO guidance, we elected to include all young infants ≤60 days in an audit of bacterial meningitis in the Queen Elizabeth Central Hospital (QECH) in Blantyre from 2002 to 2008 to determine the etiology and pathogen resistance patterns and therefore evaluate the suitability of WHO recommendations for first-line antibiotic therapy.
MATERIALS AND METHODS
Setting
QECH in Blantyre, Malawi is a large teaching, referral and district general hospital serving a population of approximately 1 million and admitting around 25,000 children per year. Sick or premature babies born in hospital are cared for in a separate ward from those born outside the hospital. There is no provision for mechanical ventilation, total parenteral nutrition or central venous lines. During the study, benzyl penicillin (50,000 units or 30 mg/kg 3 or 4 times daily) and gentamicin (6 mg/kg/d) were first-line antibiotic therapy for meningitis in young infants ≤2 months of age in accordance with WHO guidelines.25 Antibiotic therapy may be changed in light of cerebrospinal fluid sample (CSF) culture and susceptibility results, which typically become available after 48 hours. It should be remembered that QECH is fortunate to benefit from bacterial culture facilities that are not available in many other hospitals in the region.
Identification of Patients
All CSF from young infants ≤60 days of age with suspected meningitis received between January 1, 2002, and December 31, 2008, were reviewed. Demographic data were retrieved from laboratory and ward admission books. No infants in the inborn hospital nursery were >60 days of age. Outcome data and HIV status were not available.
Organism Identification and Speciation
All diagnostic testing and quality control was performed in the Malawi Liverpool Wellcome Trust Programme laboratories as part of routine clinical surveillance.29,30 CSF (1–2 mL) was analyzed by Gram’s stain if the white blood cell count was >10/mm3. All samples were cultured on sheep blood and chocolate agar for 48 hours under aerobic and microaerophilic (candle jar) conditions. Bacteria were identified using standard methods as previously described.31 The laboratory participates in internationally recognized quality control programs.
Susceptibility Testing
Antibiotic susceptibilities of the bacterial isolates were determined on all isolates by disc testing (Oxoid, Hampshire, United Kingdom) and zone size measurement using the British Society for Antimicrobial Chemotherapy sensitivity method for direct cultures.32
Duplicated Samples
When repeat isolates were obtained from an infant within 28 days of age, these were considered part of the same meningitis episode and were excluded. If >28 days had passed, this was considered a separate episode.
Organisms Excluded
The following organisms were considered contaminants and excluded from analysis: Bacillus spp., Diptheroids, Micrococcus spp. and any Streptococcus spp. (other than S. pneumoniae, Group A, B or D streptococci). Although Staphylococcus epidermidis/coagulase-negative staphylococci may represent pathogens in well-resourced neonatal intensive care settings, these were considered to be contaminants in our setting because care at QECH does not include central venous access or other invasive procedures. Isolates that were recorded only as “Coliform spp.” were also excluded, because we were unable to identify them to a species level.
Analysis
Data were collected and coded in Excel 2007 (Microsoft Corporation, Redmond, WA). Double data entry was performed. Statistical analyses were performed using STATA SE/11.0 (StataCorp LP, College Station, TX). χ2 test was used where at least 80% of cells had an expected frequency of 5 or more, otherwise Fisher’s exact test was used.
Ethics
Ethical approval was not required as this was a retrospective audit of unlinked laboratory data.
RESULTS
Between January 1, 2002, and December 31, 2008 (inclusive), 259 pathogens were isolated from 259 young infants. Of these infants, 60 (23.2%) were ≤7 days (mean: 5.4 days, median: 6.5 days, interquartile range: 4–7 days) and 191 (73.7%) were >7 days and ≤2 months (mean: 28.4 days, median: 21 days, interquartile range: 14–30 days) of age. Eight (3.1%) were admitted as “neonates” to the dedicated neonatal ward but their exact ages were not recorded. Isolates from this last group were included in the overall analysis of pathogens and susceptibility patterns, but excluded from age-specific analyses (Table 1).
TABLE 1.
Frequency of CSF Isolates by Age Group N (%).
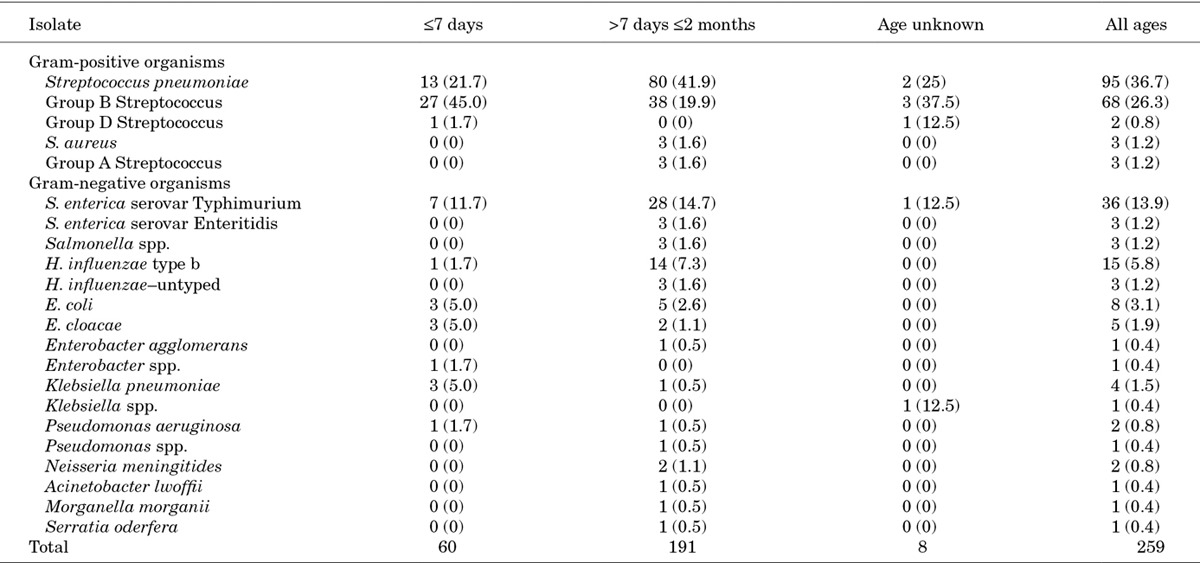
Across all age groups, 171/259 (66.0%) of isolates were Gram-positive and 88/259 (34.0 %) were Gram-negative. Overall, the commonest pathogens were S. pneumoniae in 95/259 (36.7%), GBS in 68/259 (26.3%), nontyphoidal Salmonella enterica (NTS) in 42/259 (16.2%) and Haemophilus influenzae type b (Hib) in 15/259 (5.8%, Table 1).
In neonates ≤7 days, GBS was the most common organism found (27/60; 45.0%), followed by S. pneumoniae (13/60; 21.7%) and NTS (7/60; 11.7%). For young infants >7 days to ≤2 months of age, the most frequent isolate was S. pneumoniae (80/191; 41.9%), followed by GBS (38/191; 19.9%) and NTS in 34 of 191 (17.8%, Table 1).
Antimicrobial Susceptibility
In vitro isolate susceptibility to 9 antibiotics (penicillin, erythromycin, ampicillin, chloramphenicol, gentamicin, cotrimoxazole, tetracycline, ciprofloxacin and ceftriaxone) is summarized in Table 2 (full data in Supplemental Digital Content 2, http://links.lww.com/INF/B778). Not all isolates were tested against all antibiotics, in particular, 10 isolates were not tested against either penicillin or gentamicin and 33 were not tested against ceftriaxone.
TABLE 2.
In Vitro Antibiotic Susceptibility by CSF Isolate

Overall, 229/249 (92.0%) of isolates tested showed in vitro susceptibility to the first-line combination of penicillin and gentamicin (Table 2). Gram-positive isolates were significantly more likely to be susceptible than Gram-negative isolates (155/163; 95.1% vs. 74/86; 86.0%, χ2 = 6.24, P = 0.012). Of the 226 isolates tested against ceftriaxone, 224 (99.1%) were susceptible (Table 2). A subset of 220 isolates from the 226 was tested against both a first-line antibiotic and ceftriaxone. These isolates were significantly more susceptible to ceftriaxone than to the combination of penicillin and gentamicin (n = 218/220; 99.1% vs. n = 202/220; 91.8%, Fisher’s exact test P = 0.006). Gram-positive isolates were all susceptible in vitro to ceftriaxone (n = 151); however, of the 162 tested against penicillin, 153 (94.4%) were susceptible (Table 2). A subset of 145 Gram-positive isolates were tested against both penicillin and ceftriaxone, with 139 of 145 (95.9%) susceptible to penicillin and all 145 (100%) to ceftriaxone. Of 92 S. pneumoniae isolates tested against penicillin, 6 (6.5%) had intermediate resistance (detected by disc testing only), whereas all 92 (100%) of the isolates tested against ceftriaxone were susceptible (Table 2).
Gram-negative isolates were also significantly more susceptible to ceftriaxone than to first-line antibiotics. Eighty six Gram-negative isolates were tested against gentamicin, of which 74 (86.0%) were susceptible, compared with 73 of 75 (97.3%) which were susceptible to ceftriaxone (Table 2). A subset of 74 Gram-negative isolates were tested against both gentamicin and ceftriaxone, and 63 of 74 (85.1%) were susceptible to gentamicin while 72 of 74 (97.3%) were susceptible to ceftriaxone (Fisher’s exact test P = 0.020).
All NTS isolates were tested against gentamicin and 37 of 42 (88.1%) were susceptible. Of these isolates, 33 were tested against ceftriaxone and showed universal susceptibility (Table 2). Among those 33 isolates susceptible to ceftriaxone, 5 were resistant to gentamicin.
Only 2 isolates were resistant to ceftriaxone: Klebsiella pneumoniae and Enterobacter cloacae, isolated from 4- and 5-day-old neonates, respectively. These 2 isolates were also resistant to gentamicin, but showed in vitro susceptibility to ciprofloxacin. Both isolates were identified in 2007, towards the end of the study period.
Although numbers were small, no significant trend in annual resistance patterns was found amongst isolates to the combination of penicillin and gentamicin (χ2 for trend, P = 0.291, Supplemental Digital Content 3, http://links.lww.com/INF/B779).
DISCUSSION
CSF Isolates
Our findings are similar to those of a previous study of neonatal sepsis ≤30 days of age in the same unit in Blantyre (1996–2001).9 This earlier study examined neonatal meningitis (n = 202) as a subset of neonatal sepsis (n = 784), but did not analyze CSF isolates further by age group. The authors reported GBS as the most common CSF isolate ≤30 days of age (60/202; 29.7%), followed by S. pneumoniae (47/202; 23.3%) and NTS (33/202; 16.3%) but used a cut off of 30 days (favoring GBS) as opposed to 60 days in our study. Analysis of our data by age group revealed GBS was the commonest pathogen in neonates ≤7 days and S. pneumoniae in those >7 days but ≤2 months of age. Therefore, our results may not represent a true change in isolate frequency, but are more likely due to the inclusion of older infants in our study.
S. pneumoniae
S. pneumoniae was the major CSF pathogen in 36.7% of infants ≤2 months of age in this study, which is consistent with reports from East Africa3,5,7–10 and West Africa.11,12 S. pneumoniae resistance to penicillin is therefore of particular interest. In a recent review of invasive pneumococcal disease across all age groups (>3 months, n = 4445) penicillin resistance was reported to be stable at ≈10% over a decade.33 In our study, resistance to penicillin was 6.5% (6/92).
S. pneumoniae isolates in our study were universally susceptible to ceftriaxone. No ceftriaxone resistance amongst S. pneumoniae isolates has been reported in this setting since the antibiotic’s introduction.33 However, a recent study from our setting did report an increase in minimum inhibitory concentration (from 0.0016 to 0.125 mg/mL), suggesting S. pneumoniae isolates are becoming less susceptible to ceftriaxone.33
S. pneumoniae resistance to ceftriaxone may hasten with increasing use of this antibiotic. However, although penicillin use has been widespread in our setting over a long period, resistance has been fairly static over the past decade.33 Ongoing surveillance is therefore critical to identify resistance if it emerges. Molecular tools that may predict this emergence are currently under development in this and other laboratories.
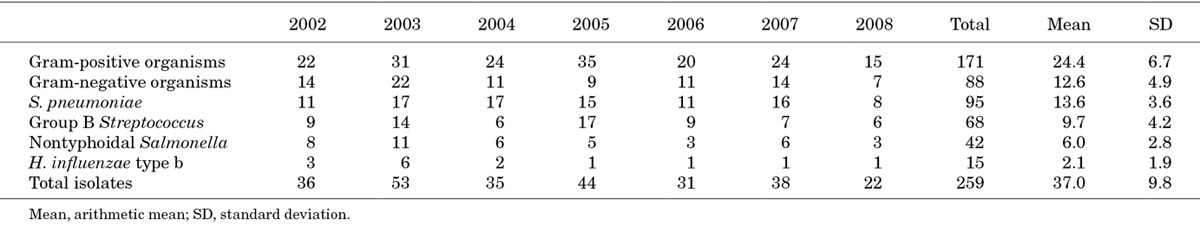
S. pneumoniae was the most common CSF isolate year on year (Table 3), underlining the importance of pneumococcal immunization. The 13-valent pneumococcal conjugate vaccine was introduced into the Malawian extended program for immunization in November 2011. It is hoped that herd immunity will protect the unimmunized population of young infants ≤2 months of age. The impact of pneumococcal vaccination on neonatal meningitis will be an important topic for surveillance in the coming years.
TABLE 3.
Frequency of CSF Isolates by Year
Group B Streptococcus
GBS was the leading cause of meningitis (45%) in neonates ≤7 days and is emerging as an important cause of neonatal meningitis in sub-Saharan Africa.6,8–10,16–18,34 GBS infections may be under-represented in community-born infants as the disease can be rapidly fatal and infants may die before reaching a health center.19
As GBS is vertically acquired from the maternal genital tract, maternal vaccination against the pathogen is attractive. However, as serotypes differ between countries, efforts to produce a globally effective vaccine are necessary. The successful genomic sequencing of GBS may have provided global candidate antigens35 and a phase II vaccine study has just been completed in Malawi and another is in progress in South Africa (National Clinical Trial numbers NCT01412801and NCT01193920, respectively).
Nontyphoidal Salmonella
NTS was the most common Gram-negative organism responsible for meningitis and NTS neonatal meningitis has been reported in Malawi,8,9 Kenya5,7 and Niger.12 Fecal-oral transmission may put neonates at particularly high risk of invasive NTS infections due to relative achlorhydria and a reduction in gastric acids by frequent milk feeds.36 Clinical implications are serious, with a reported case fatality rate of 64–89%.8,9
Among the NTS isolates, 5/42 (11.9%) were resistant to gentamicin but there was universal susceptibility to ceftriaxone. Encouragingly, gentamicin resistance at QECH has decreased from 55% (1996–1998)37 to 47% (1996–2001)9 and now 11.9%. However, doubts remain as to the suitability of gentamicin to treat NTS meningitis due to poor intracellular and blood-brain barrier penetration.38
H. influenzae Type b
In our study, Hib was responsible for only 5.8% of meningitis in neonates and young infants, which may be a consequence of recent vaccination introduction. Hib conjugate vaccine was introduced into the Malawian immunization schedule in February 2002 (first dose at 2 months). A subsequent review of Hib meningitis between 2 months and 15 years in QECH showed a mean of 53.2 annual cases between 1997 and 2002, which decreased to 9.7 between 2003 and 2009.39 In our study, Hib annual incidence fell from 6 cases in 2003 to 1 case per year between 2005 and 2008 (Table 3), which suggests a herd immunity effect amongst unvaccinated infants ≤2 months of age.
Antimicrobial Susceptibility
First-line Antibiotic Susceptibility—Penicillin and Gentamicin
In this study, 92.0% (229/249 tested) of all isolates showed in vitro susceptibility to either penicillin or gentamicin. Therefore, in this setting, WHO first-line antibiotics provide adequate empirical treatment for cases of suspected neonatal meningitis. However, these antibiotics provided significantly lower coverage for Gram-negative than Gram-positive isolates (74/86; 86.0% vs. 155/163; 95.1%, χ2 = 6.24, P = 0.012). Although WHO first-line antibiotics appear appropriate for meningitis in young infants in Blantyre where Gram-positive isolates predominate, they may not provide sufficient cover in settings where Gram-negative isolates have a larger role.
The combination of penicillin and gentamicin is cheap and widely available but requires 4 injections a day for at least 14 days; a minimum of 56 injections. In addition, gentamicin has a narrow therapeutic index and without the ability to measure therapeutic drug levels, the risks of ototoxicity and nephrotoxicity may be increased and/or therapeutic levels may not be attained.
A study in Kilifi, Kenya investigated the suitability of once daily gentamicin dosing in neonates in a setting similar to ours where routine therapeutic drug monitoring was not possible.40 The authors reported that 72-hour peak gentamicin levels were below the target of 4 μg/mL in 12% of neonates (potentially subtherapeutic) and 96-hour trough levels were above the target of 1 μg/mL in 24% of patients (potentially toxic). This study also reported possible gentamicin-related nephrotoxicity in 1% of patients.
First-line Antibiotic Susceptibility—Ceftriaxone
Ceftriaxone has been available in the pediatric unit at QECH since 2001, although not for neonatal use. Its use has become more widespread since 2007 and in 2009, it was introduced as the second-line antibiotic for neonatal meningitis. Ceftriaxone has good CSF penetrance41 and is given once daily, reducing pressure on nursing staff. These factors are of particular importance in resource-poor settings.
In vitro susceptibility to ceftriaxone was 99.1% (224/226) and was significantly higher than for penicillin and gentamicin. The difference in susceptibility was particularly marked for Gram-negative isolates. Our data shows only 2 cases of ceftriaxone resistance in neonatal meningitis involving a Klebsiella pneumoniae and an E. cloacae isolate. Emerging resistance to third generation cephalosporins is of particular concern in resource limited settings. For example, only 72% of Gram-negative blood culture isolates showed susceptibility to cefotaxime in Kenyan infants of the same age group.7 A recent study of Enterobacteriaceae in blood cultures from all age groups in QECH (n = 1191) identified 19 ceftriaxone-resistant isolates, 10 of which showed extended-spectrum β-lactamases (ESBL) phenotyopes.42 These were predominantly isolated from pediatric patients and the authors advised that increased use of cephalosporins was likely to result in a rapid ESBL expansion.42 Although ESBL genotyping was not available for our study, the presence of ceftriaxone-resistant Gram-negative organisms in the absence of widely available alternative antibiotics is cause for considerable concern.
Questions remain regarding the safety of ceftriaxone use in neonates, including precipitation with calcium containing compounds,43 biliary sludging44 and the displacement of albumin causing kernicterus.45 In vitro susceptibilities to antibiotics may not translate into good outcomes. A prospective randomized controlled trial to investigate the outcome of neonatal meningitis when treated with penicillin and gentamicin compared with a third generation cephalosporin and the safety of ceftriaxone in neonates is currently underway in Malawi (NCT01247909).
As a single center study, generalization of these results must be made with caution. This study was hospital based and may underrepresent early neonatal meningitis if babies born at home who died before attending the hospital. There was also no reliable way of distinguishing between community-acquired and hospital-acquired infections.
CONCLUSION
Ceftriaxone provides significantly better in vitro coverage than the WHO-recommended combination of penicillin and gentamicin in meningitis in young infants in Blantyre, Malawi where Gram positive isolates predominate. This gap in susceptibility may be even higher in settings where Gram-negative isolates have a larger role. Ceftriaxone’s once daily dosing schedule also reduces pressure on nursing staff but resistance is emerging among Gram-negative isolates and the safety profile of ceftriaxone in neonates is yet to be fully confirmed. In view of these results, the WHO recommendations of empirical penicillin and gentamicin for suspected neonatal meningitis should be reevaluated. This is particularly important for those settings where the prevalence of gentamicin resistant Gram-negative meningitis is high.
ACKNOWLEDGMENTS
The authors would like to thank laboratory manager Mike Moore for his advice and Amanda Gwee for her assistance with data collection. We would also like to extend our gratitude to the staff, patients and their families at QECH.
Footnotes
The study was performed at Department of Paediatrics, University of Malawi College of Medicine, Blantyre, Malawi.
The microbiology surveillance service at QECH is provided by the Malawi-Liverpool-Wellcome Clinical Research Programme which is funded by a core grant from the Wellcome Trust. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the article.
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Black RE, Cousens S, Johnson HL, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Mwaniki MK, Talbert AW, Njuguna P, et al. Clinical indicators of bacterial meningitis among neonates and young infants in rural Kenya. BMC Infect Dis. 2011;11:301. doi: 10.1186/1471-2334-11-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muhe L, Tilahun M, Lulseged S, et al. Etiology of pneumonia, sepsis and meningitis in infants younger than three months of age in Ethiopia. Pediatr Infect Dis J. 1999;18(10 suppl):S56–S61. doi: 10.1097/00006454-199910001-00010. [DOI] [PubMed] [Google Scholar]
- 4.Gebremariam A. Neonatal meningitis in Addis Ababa: a 10-year review. Ann Trop Paediatr. 1998;18:279–283. doi: 10.1080/02724936.1998.11747960. [DOI] [PubMed] [Google Scholar]
- 5.English M, Ngama M, Musumba C, et al. Causes and outcome of young infant admissions to a Kenyan district hospital. Arch Dis Child. 2003;88:438–443. doi: 10.1136/adc.88.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laving AM, Musoke RN, Wasunna AO, et al. Neonatal bacterial meningitis at the newborn unit of Kenyatta National Hospital. East Afr Med J. 2003;80:456–462. doi: 10.4314/eamj.v80i9.8742. [DOI] [PubMed] [Google Scholar]
- 7.Talbert AW, Mwaniki M, Mwarumba S, et al. Invasive bacterial infections in neonates and young infants born outside hospital admitted to a rural hospital in Kenya. Pediatr Infect Dis J. 2010;29:945–949. doi: 10.1097/INF.0b013e3181dfca8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molyneux E, Walsh A, Phiri A, et al. Acute bacterial meningitis in children admitted to the Queen Elizabeth Central Hospital, Blantyre, Malawi in 1996-97. Trop Med Int Health. 1998;3:610–618. doi: 10.1046/j.1365-3156.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- 9.Milledge J, Calis JC, Graham SM, et al. Aetiology of neonatal sepsis in Blantyre, Malawi: 1996-2001. Ann Trop Paediatr. 2005;25:101–110. doi: 10.1179/146532805X45692. [DOI] [PubMed] [Google Scholar]
- 10.Nathoo KJ, Pazvakavamba I, Chidede OS, et al. Neonatal meningitis in Harare, Zimbabwe: a 2-year review. Ann Trop Paediatr. 1991;11:11–15. doi: 10.1080/02724936.1991.11747472. [DOI] [PubMed] [Google Scholar]
- 11.Mulholland EK, Ogunlesi OO, Adegbola RA, et al. Etiology of serious infections in young Gambian infants. Pediatr Infect Dis J. 1999;18(10 suppl):S35–S41. doi: 10.1097/00006454-199910001-00007. [DOI] [PubMed] [Google Scholar]
- 12.Campagne G, Schuchat A, Djibo S, et al. Epidemiology of bacterial meningitis in Niamey, Niger, 1981-96. Bull World Health Organ. 1999;77:499–508. [PMC free article] [PubMed] [Google Scholar]
- 13.Longe AC, Omene JA, Okolo AA. Neonatal meningitis in Nigerian infants. Acta Paediatr Scand. 1984;73:477–481. doi: 10.1111/j.1651-2227.1984.tb09958.x. [DOI] [PubMed] [Google Scholar]
- 14.Airede AI. Neonatal bacterial meningitis in the middle belt of Nigeria. Dev Med Child Neurol. 1993;35:424–430. doi: 10.1111/j.1469-8749.1993.tb11664.x. [DOI] [PubMed] [Google Scholar]
- 15.Airede KI, Adeyemi O, Ibrahim T. Neonatal bacterial meningitis and dexamethasone adjunctive usage in Nigeria. Niger J Clin Pract. 2008;11:235–245. [PubMed] [Google Scholar]
- 16.Coovadia YM, Mayosi B, Adhikari M, et al. Hospital-acquired neonatal bacterial meningitis: the impacts of cefotaxime usage on mortality and of amikacin usage on incidence. Ann Trop Paediatr. 1989;9:233–239. doi: 10.1080/02724936.1989.11748638. [DOI] [PubMed] [Google Scholar]
- 17.Adhikari M, Coovadia YM, Singh D. A 4-year study of neonatal meningitis: clinical and microbiological findings. J Trop Pediatr. 1995;41:81–85. doi: 10.1093/tropej/41.2.81. [DOI] [PubMed] [Google Scholar]
- 18.Nel E. Neonatal meningitis: mortality, cerebrospinal fluid, and microbiological findings. J Trop Pediatr. 2000;46:237–239. doi: 10.1093/tropej/46.4.237. [DOI] [PubMed] [Google Scholar]
- 19.Dagnew AF, Cunnington MC, Dube Q, et al. Variation in reported neonatal group B streptococcal disease incidence in developing countries. Clin Infect Dis. 2012;55:91–102. doi: 10.1093/cid/cis395. [DOI] [PubMed] [Google Scholar]
- 20.Edmond KM, Kortsalioudaki C, Scott S, et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012;379:547–556. doi: 10.1016/S0140-6736(11)61651-6. [DOI] [PubMed] [Google Scholar]
- 21.The World Health Organization Young Infants Study Group. Conclusions. from the WHO multicenter study of serious infections in young infants. Pediatr Infect Dis J. 1999;18:S32–S34. doi: 10.1097/00006454-199910001-00006. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization, UNICEF. Management of the Child With a Serious Infection or Severe Malnutrition: Guidelines for Care at the First-referral Level in Developing Countries. Geneva: World Health Organization; 2000. [Google Scholar]
- 23.World Health Organization, UNAIDS. Managing Newborn Problems a Guide for Doctors, Nurses and Midwives. Geneva: World Health Organization; 2003. [Google Scholar]
- 24.World Health Organization, UNICEF. Handbook: IMCI Integrated Management of Childhood Illness. Geneva: World Health Organization; 2005. [Google Scholar]
- 25.World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illness With Limited Resources. Geneva: World Health Organization; 2005. [Google Scholar]
- 26.World Health Organization, UNICEF. Integrated Management of Childhood Illness (Chart Booklet) Geneva: World Health Organization; 2008. [Google Scholar]
- 27.World Health Organisation, UNICEF. Integrated Management of Childhood Illness for High HIV Settings (Chart Booklet) Geneva: World Health Organization; 2008. [PubMed] [Google Scholar]
- 28.World Health Organization, UNICEF. Integrated Management of Childhood Illness. ICMI Adaptation Guide—Part 2c—Technical Basis for Adapting the Clinical Guidelines, Feeding Recommendations, and Local Terms. Geneva: World Health Organization; 2002. [Google Scholar]
- 29.Gordon MA, Graham SM, Walsh AL, et al. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica Serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46:963–969. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 30.Bronzan RN, Taylor TE, Mwenechanya J, et al. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 31.Barrow G, Feltham R. Cowan and Steel’s Manual for the Identification of Medical Bacteria. Third edition. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 32.Andrews JM. The development of the BSAC standardized method of disc diffusion testing. J Antimicrob Chemother. 2001;48(suppl 1):29–42. doi: 10.1093/jac/48.suppl_1.29. [DOI] [PubMed] [Google Scholar]
- 33.Everett DB, Mukaka M, Denis B, et al. Ten years of surveillance for invasive Streptococcus pneumoniae during the era of antiretroviral scale-up and cotrimoxazole prophylaxis in Malawi. PLoS One. 2011;6:e17765. doi: 10.1371/journal.pone.0017765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haffejee IE, Bhana RH, Coovadia YM, et al. Neonatal group B streptococcal infections in Indian (Asian) babies in South Africa. J Infect. 1991;22:225–231. doi: 10.1016/s0163-4453(05)80003-9. [DOI] [PubMed] [Google Scholar]
- 35.Johri AK, Paoletti LC, Glaser P, et al. Group B Streptococcus: global incidence and vaccine development. Nat Rev Microbiol. 2006;4:932–942. doi: 10.1038/nrmicro1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham SM, Molyneux EM, Walsh AL, et al. Nontyphoidal Salmonella infections of children in tropical Africa. Pediatr Infect Dis J. 2000;19:1189–1196. doi: 10.1097/00006454-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Graham SM, Walsh AL, Molyneux EM, et al. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg. 2000;94:310–314. doi: 10.1016/s0035-9203(00)90337-7. [DOI] [PubMed] [Google Scholar]
- 38.Price EH, de Louvois J, Workman MR. Antibiotics for Salmonella meningitis in children. J Antimicrob Chemother. 2000;46:653–655. doi: 10.1093/jac/46.5.653. [DOI] [PubMed] [Google Scholar]
- 39.McCormick DW, Molyneux EM. Bacterial meningitis and Haemophilus influenzae type b conjugate vaccine, Malawi. Emerg Infect Dis. 2011;17:688–690. doi: 10.3201/eid1704.101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.English M, Mohammed S, Ross A, et al. A randomised, controlled trial of once daily and multi-dose daily gentamicin in young Kenyan infants. Arch Dis Child. 2004;89:665–669. doi: 10.1136/adc.2003.032284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherubin CE, Eng RH, Norrby R, et al. Penetration of newer cephalosporins into cerebrospinal fluid. Rev Infect Dis. 1989;11:526–548. doi: 10.1093/clinids/11.4.526. [DOI] [PubMed] [Google Scholar]
- 42.Gray KJ, Wilson LK, Phiri A, et al. Identification and characterization of ceftriaxone resistance and extended-spectrum beta-lactamases in Malawian bacteraemic Enterobacteriaceae. J Antimicrob Chemother. 2006;57:661–665. doi: 10.1093/jac/dkl037. [DOI] [PubMed] [Google Scholar]
- 43.Bradley JS, Wassel RT, Lee L, et al. Intravenous ceftriaxone and calcium in the neonate: assessing the risk for cardiopulmonary adverse events. Pediatrics. 2009;123:e609–e613. doi: 10.1542/peds.2008-3080. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez-Coca González J, Cebrero García M, Vecilla Rivelles MC, et al. [Transient biliary lithiasis associated with the use of ceftriaxone]. An Esp Pediatr. 2000;53:366–368. [PubMed] [Google Scholar]
- 45.Martin E, Fanconi S, Kälin P, et al. Ceftriaxone–bilirubin-albumin interactions in the neonate: an in vivo study. Eur J Pediatr. 1993;152:530–534. doi: 10.1007/BF01955067. [DOI] [PubMed] [Google Scholar]


