Supplemental Digital Content is available in the text.
Keywords: preterm infant, respiratory syncytial virus, palivizumab
Abstract
Background:
The Respiratory Syncytial Virus (RSV) Respiratory Events Among Preterm Infants Outcomes and Risk Tracking (REPORT) study evaluated RSV disease burden in US preterm infants 32–35 weeks gestational age (wGA) not receiving RSV prophylaxis.
Methods:
Preterm infants <6 months of age as of November 1st were followed prospectively at 188 clinics from September to May 2009–2010 or 2010–2011. Nasal and pharyngeal swabs were collected for medically attended acute respiratory illnesses (MAARI) and tested for RSV by qRT-polymerase chain reaction. Risk factors were assessed using multivariate Cox proportional hazard model adjusted for seasonality.
Results:
Of 1642 evaluable infants, 287 experienced RSV MAARI. Rates of RSV-related MAARI, outpatient lower respiratory tract illness, emergency department visits and hospitalization (RSVH) during November to March were 25.4, 13.7, 5.9 and 4.9 per 100 infant-seasons, respectively. Preschool-aged, nonmultiple-birth siblings and daycare attendance were consistently associated with increased risk of RSV. RSVH rates were highest in infants 32–34 and 35 wGA who were <6 months of age during November to March with daycare attendance or nonmultiple-birth, preschool-aged siblings (8.9 and 9.3 per 100 infant-seasons, respectively, versus 3.5 for all other infants, P<0.001). Chronologic age <3 months was associated with a higher RSVH rate for infants 35 wGA but not for infants 32–34 wGA.
Conclusions:
In US preterm infants who were 32–35 wGA, <6 months on November 1st and not receiving RSV prophylaxis, the burden of RSV MAARI was 25 per 100 infant-seasons. The highest RSVH rates occurred among those with daycare attendance or nonmultiple-birth, preschool-aged siblings while they were <6 months of age during the RSV season.
Respiratory syncytial virus (RSV) is the most important cause of lower respiratory tract illness (LRI) in infants and young children.1 In the United States, the RSV season generally occurs between November and March, with considerable regional and local variability.1–4 Conditions that increase the risk of severe RSV disease in young children include chronic lung disease of prematurity, hemodynamically significant congenital heart disease and preterm birth ≤35 weeks gestational age (wGA).5–8 In 2010, based on US birth statistics using the wGA recorded on birth certificates,9,10 infants ≤35 wGA comprised 6.0% of US births (n = 241,520).11
A monoclonal antibody, palivizumab, is approved for prevention of serious lower respiratory tract RSV disease in certain high risk children as a monthly injection administered before and throughout the RSV season.12 Among preterm infants, RSV prophylaxis is recommended by the American Academy of Pediatrics (AAP) for infants ≤28 wGA who are <12 months at RSV season start and those 29–31 wGA <6 months at RSV season start.13,14 AAP recommendations regarding RSV prophylaxis for infants 32–35 wGA have evolved. Current recommendations are based on a desire to provide prophylaxis to those infants at greatest risk of RSV hospitalization and concerns regarding the cost of prophylaxis if given to all infants ≤35 wGA.13,14 Since 2009, the AAP has not recommended RSV prophylaxis for otherwise healthy preterm infants 35 wGA.13 In 2009, the AAP also recommended monthly RSV prophylaxis for infants 32–34 wGA who attend day care or have preschool-aged siblings only while the infants are <90 days chronologic age, because it has been thought that in infants ≥90 days of age the risk of hospitalization attributable to RSV is reduced.15 In 2012, the AAP recommendations clarified that multiple births <1 year of age should not qualify as preschool-aged siblings.13
Although 73% of US infants ≤35 wGA are born at 32–35 wGA,11 there have been no large, prospective studies of the burden of medically attended, laboratory-confirmed RSV disease in the United States in this population. Prospective studies conducted in Canada, Spain and the Netherlands have shown that infants 32–35 wGA with environmental exposure factors such as day care attendance and siblings at home are at higher risk of RSV hospitalization.16–19 Additionally, although the effect of chronologic age on the risk of RSV hospitalization has been described for all US infants,20–25 prospective data are needed regarding the burden of RSV disease in US infants 32–35 wGA overall and as a function of chronologic age and gestational age. This prospective cohort study of US infants 32–35 wGA, <6 months on November 1st and not receiving RSV prophylaxis was conducted to determine the incidence of laboratory-confirmed, medically attended illness and associated risk factors for RSV disease.
MATERIALS AND METHODS
Study Design and Conduct
A prospective observational study, the RSV Respiratory Events Among Preterm Infants Outcomes and Risk Tracking (REPORT) study (ClinicalTrials.gov Identifier: NCT00983606), was conducted over 2 RSV seasons (2009–2010 and 2010–2011) at 188 US outpatient clinics in 38 states and the District of Columbia. Criteria for study entry included preterm birth between 32 weeks 0 days and 35 weeks 6 days GA, birth in May through February and chronologic age ≤6 months at enrollment. Infants were excluded from the study if they had chronic lung disease of prematurity, hemodynamically significant congenital heart disease, a life expectancy of <6 months or received or were being considered for RSV prophylaxis. Subjects were enrolled in the outpatient setting at any time they met inclusion criteria between September 1 and February 28; those born in May through August could only be enrolled through November. All eligible subjects were to be approached by sites; enrollment materials were available in English and Spanish. All subjects were followed prospectively through May 31. The study protocol and informed consent were approved by independent institutional review boards before study initiation. Written informed consent was obtained from each subject’s parent(s) or legal representative.
If a subject presented for outpatient care with acute respiratory illness during the study, nasal and pharyngeal swabs were collected following a standard procedure.22 Swabs were combined and stored at −20°C or colder at each site and shipped to a central laboratory for RSV testing using a quantitative real-time reverse transcriptase polymerase chain reaction (Gen-Probe/Prodesse, Waukesha, WI).26 Parents sought medical care based on usual practices; the only required visit was a study termination visit in June. Demographic information, environmental risk factors, medical history, family history of asthma/eczema and respiratory symptoms were collected at entry and updated at all study visits. For visits to the emergency department (ED) or hospital for respiratory illness, the investigator extracted the date, diagnosis, disease course, disposition and any RSV testing from medical records. For respiratory ED visits and hospitalizations without RSV testing, the event was considered RSV-related if a sample collected by study personnel within 7 days of the ED visit or hospitalization identified RSV.
RSV-related, medically attended acute respiratory illness (MAARI) was defined as any laboratory-documented RSV illness associated with an outpatient visit (upper or lower respiratory tract illness), ED visit or hospitalization. LRI was defined as pneumonia, bronchiolitis or wheezing. The quality of study data was assured through monitoring of all investigational sites.
Statistical Analysis
Because the observation period for each infant varied, incidence rates of RSV events were calculated based on the cumulative months of observation for the subjects enrolled. Rates were calculated per 100 infant-years for the entire study period and for September to October, November to March and April to May. To enable comparison with other studies19,22,27 and help inform policy regarding RSV prophylaxis, which is only administered during the peak months of RSV activity, event rates per 100 infant-seasons were also calculated with a season defined as November to March; only events and person-time contributed during November to March were included. Available data from subjects who did not complete the study were included. A single illness in a subject could contribute to multiple event types (MAARI, outpatient upper respiratory tract illness (URI), outpatient LRI, ED visit, hospitalization); however, to be conservative, subjects with multiple events within a type were only counted once (eg, a subject with 2 RSV hospitalizations was counted once). To provide demographic information on the reference population of US infants 32–35 wGA without reported congenital heart disease or life-threatening conditions, 2010 US Centers for Disease Control and Prevention natality statistics were analyzed based on the recorded GA.9 Given the size of the Centers for Disease Control and Prevention cohort, even trivial differences between study population and Centers for Disease Control and Prevention cohort demographics were statistically significant; as a result, comparisons were qualitative.
Risk factors for RSV events were identified using a Cox proportional hazard model, with calendar time input to adjust for seasonality; the model also adjusted for subject differences in exposure time. Each event type was analyzed in a separate model. Fixed and time-varying risk factors are identified in Table, Supplemental Digital Content 1, http://links.lww.com/INF/B739.45 For time-varying factors other than chronologic age, dates of status changes were imputed as the midpoint between visits with discordant results. Risk factors with P < 0.15 were identified and the model was then fit iteratively by removing the factor with the highest P value until each of the remaining risk factors had P < 0.05. Multiple-birth sibling pairs (n = 227 pairs) were included as separate individuals; sensitivity analyses examining pairs as separate or single individuals yielded similar results. Exploratory models were constructed to examine relationships between RSV event incidence and chronologic age for infants 32−34 and 35 wGA.
To help inform RSV immunoprophylaxis policy, a descriptive analysis was conducted of RSV event rates per 100 infant-seasons (November to March) by wGA (32–34 vs. 35), chronologic age on November 1st (<3 months and 3 to <6 months), presence of 2012 AAP environmental risk factors at enrollment (day care attendance or having nonmultiple-birth preschool-aged siblings) and chronologic age during November to March (<3 months, 3 to <6 months and ≥6 months). For chronologic age during November to March, subjects contributed events and exposure time only while they were within the specific age ranges.
RESULTS
Of the 3317 infants screened for enrollment, 1646 (50%) were enrolled (n = 407 in season 1 and n = 1239 in season 2). The 65 sites that enrolled subjects in year 1 were increased to 176 sites in year 2 due to low enrollment. At each of the 188 total sites, 1–44 subjects were enrolled. Subjects were enrolled in 38 states and the District of Columbia; California had the highest enrollment with 7.2% of subjects enrolled. Enrollment by month was similar, with 14–19% of subjects enrolled each month from September through February. Chronologic age and wGA of subjects enrolled were similar across enrollment months. Common reasons for nonenrollment were past or planned receipt of RSV prophylaxis and family’s unwillingness to participate. Study completion rates were similar for seasons 1 and 2 (81.6% and 84.5%, respectively). Principal reasons for noncompletion were loss to follow-up (6.4%) and receipt of RSV prophylaxis (3.8%; see Figure, Supplemental Digital Content 2, http://links.lww.com/INF/B740, which shows patient disposition). Four subjects born in April were enrolled in the study but were excluded from analysis due to violation of entry criteria. Demographic and baseline characteristics of study subjects are shown in Table, Supplemental Digital Content 1, http://links.lww.com/INF/B739. Compared with the US population of infants 32–35 wGA, the study population was similar with regard to age, sex, maternal age at birth, multiple births and history of mechanical ventilation after birth (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B739). Major differences included fewer infants 32–34 wGA, more low birthweight infants, fewer Hispanic infants and more highly educated mothers (≥16 years of education) in the study population.
During the 2 seasons, 1032 subjects developed 3023 episodes of outpatient MAARI, of which 72% (n = 2163) had samples collected for RSV testing. Of subjects with ED visits and hospitalizations for LRI, 50% (57 of 113) and 46% (38 of 82) lacked an RSV test in the medical record, respectively. With inclusion of study polymerase chain reaction tests performed within 7 days (median, 2 days from admission), 21% (24 of 113) of LRI ED visits and 5% (4 of 82) of LRI hospitalizations lacked an RSV test.
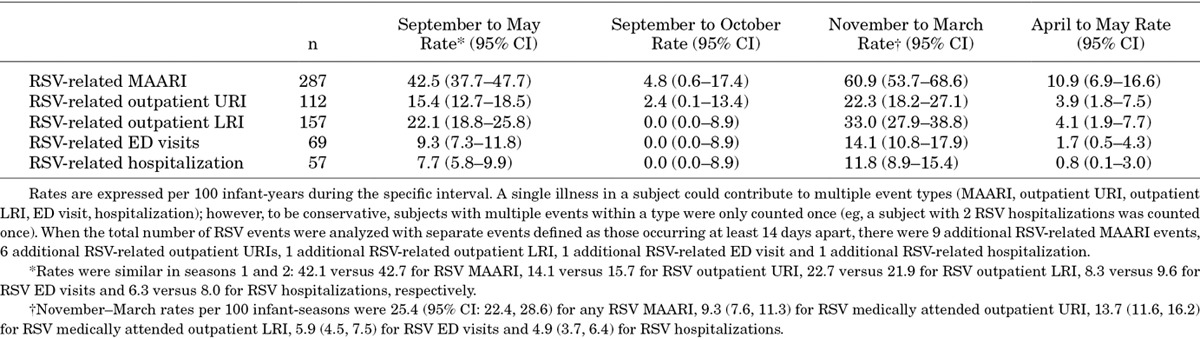
During the study, 287 subjects developed a laboratory-confirmed RSV MAARI (Fig. 1). Among these 287 subjects, 112 experienced RSV outpatient URI visits, 157 outpatient LRI visits, 69 ED visits and 57 hospitalizations. Rates of RSV-related MAARI, outpatient URI, outpatient LRI, ED visits and hospitalizations per 100 infant-years are outlined in Table 1. This incidence resulted in November to March rates per 100 infant-seasons of 25.4 [95% confidence interval (CI): 22.4, 28.6] for any RSV MAARI, 9.3 (7.6, 11.3) for RSV medically attended outpatient URI, 13.7 (11.6, 16.2) for RSV medically attended outpatient LRI, 5.9 (4.5, 7.5) for RSV ED visits and 4.9 (3.7, 6.4) for RSV hospitalizations. Nineteen subjects had an RSV MAARI at enrollment (1.2%); these events were included in the analysis. The care patterns of infants with RSV MAARI are included in Figure 1. No RSV hospitalization was recorded for 2 subjects with RSV whose ED discharge disposition was hospital admission [1 with intensive care unit (ICU) admission], because hospitalization records could not be obtained. Among the 57 subjects with RSV hospitalizations, the median duration was 4.0 days (range, 2–18 days); 61% had a chest radiograph, 39% antibiotics, 16% ICU care and 11% mechanical ventilation. The proportion with ICU admission was similar among infants 32–34 wGA (4 of 21) and 35 wGA (5 of 36).
FIGURE 1.
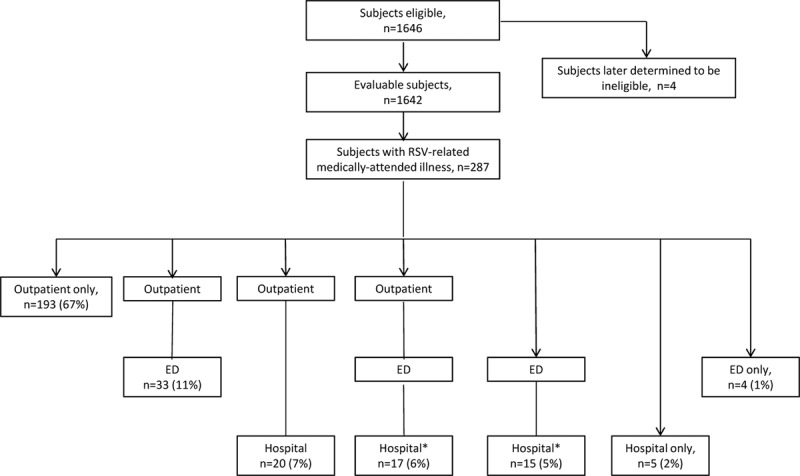
Study subject disposition and care patterns among infants with RSV-related MAARI. *Of the 32 patients with an ED and hospital visit, 9 were discharged home but hospitalized 0–8 days later, and 23 were admitted immediately to the hospital. No RSV hospitalization was recorded for 2 subjects with RSV whose ED discharge disposition was hospital admission (1 with ICU admission), because hospitalization records could not be obtained.
TABLE 1.
Incidence Rates Per 100 Infant-years for Medically Attended RSV Illness by Interval
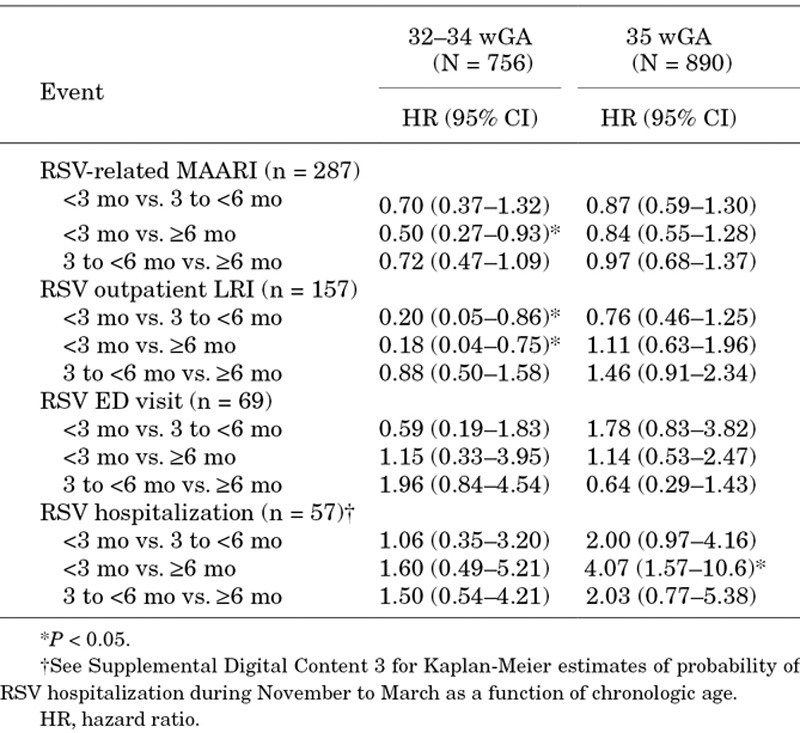
Risk factors that were statistically significant independent predictors of RSV MAARI are shown in Figure 2. Consistent risk factors were day care attendance and preschool-aged nonmultiple-birth siblings. Although day care attendance was not associated with a statistically significant increased risk for RSV hospitalization in the multivariate model, it did appear to contribute to RSV hospitalization risk among those without preschool-aged nonmultiple-birth siblings (odds ratio 2.1, P = 0.17). Controlling for other factors, chronologic age <3 months was associated with a lower risk of RSV-related MAARI in infants 32–34 wGA (Table 2). Among 35 wGA infants, chronologic age <3 months was associated with a higher risk of RSV hospitalization. In infants 32–34 wGA, there was no evidence of a difference in RSV hospitalization risk through 6 months (P = 0.9), with a hazard ratio of 1.06 for <3 versus 3 to <6 months (Table 2 and Figure, Supplemental Digital Content 3, http://links.lww.com/INF/B741).
FIGURE 2.
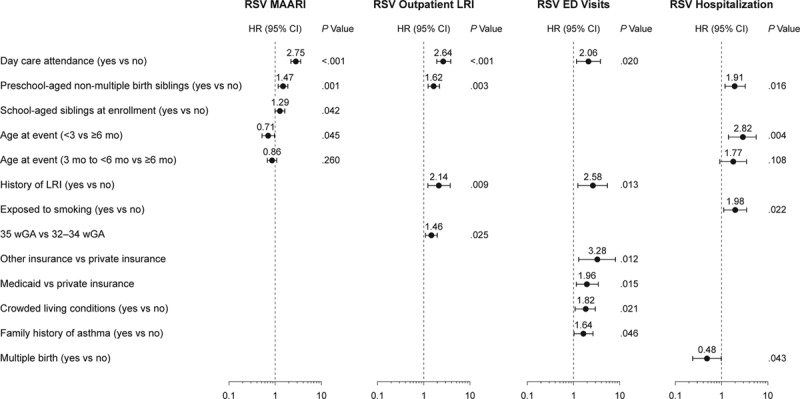
Multivariate risk factor analysis. HR, hazard ratio; wGA, weeks gestational age. Variables that were not statistically significant predictors for the specific outcome evaluated and thus were not retained in the multivariate model were not presented.
TABLE 2.
Effect of Chronologic Age in Multivariate Models on Risk for Preterm Infants 32–34 wGA and 35 wGA
RSV-related event rates by wGA, chronologic age and 2012 AAP environmental risk factors are shown in Figure 3 and Supplemental Digital Content 4, http://links.lww.com/INF/B742. The RSV hospitalization rates among infants 35 wGA at <3 months and 3 to <6 months of age were similar to RSV hospitalization rates in those 32–34 weeks. Consistent with multivariate models, the population at highest risk for RSV hospitalization during November to March were infants 32–35 wGA with 2012 AAP risk factors while <6 months of age. The RSV hospitalization rate among this population was 9.2 (95% CI: 6–13.4) per 100 infant-seasons (8.9 and 9.3 for 32–34 and 35 wGA, respectively) compared with 3.5 (95% CI: 2.3–4.5) for all other infants enrolled (P < 0.001). Those with 2012 AAP risk factors while <6 months of age also accounted for 75% of RSV ICU admissions.
FIGURE 3.
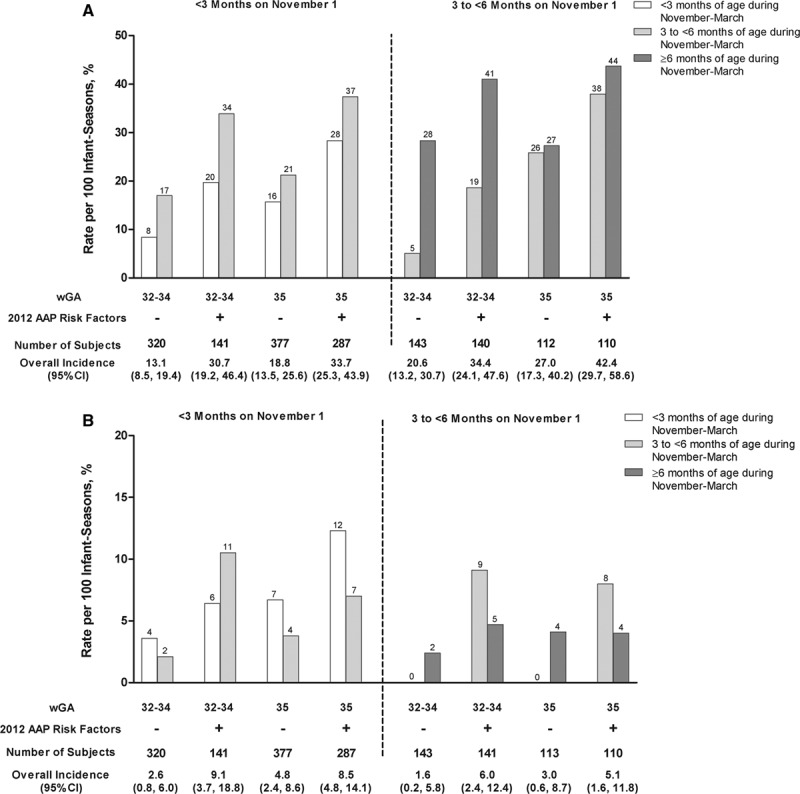
Risk of RSV disease during November to March by GA, chronologic age on November 1st, chronologic age during November to March and 2012 AAP environmental risk factors (A) RSV-related MAARI; (B) RSV Hospitalizations. GA, gestational age. Rates are per 100 infant-seasons. For chronologic age during November to March, subjects contributed events and exposure time only while they were within specific age ranges. Overall incidence rates are for November to March regardless of chronologic age during November to March. The plus (+) and minus (−) symbols represent the presence or absence of 2012 AAP environmental risk factors, respectively. AAP risk factors were associated with increased rates of RSV-related events (P < 0.02 for all event types). Older chronologic age was associated with an increased risk of RSV-related MAARI (P < 0.01 for both <3 vs. ≥3 months and <6 vs. ≥6 months). There was no evidence of a decrease in RSV hospitalization rates in infants 32–34 wGA at 3 to <6 months versus <3 months of age (P = 0.9).
Among enrolled infants, 216 (13%) were eligible for RSV prophylaxis at season start based on the 2009 AAP guidelines (32–34 wGA <3 months of age with preschool-aged siblings or day care attendance),14 the policy in effect during the study. Beyond expected differences in chronologic age and preschool-aged children at home, eligible infants were more likely to be African American (26% vs. 20%) and Hispanic (17% vs. 10%; P = 0.003) and of birthweight >1500 g (89% vs. 81%; P = 0.009) compared with ineligible infants 32–34 wGA.
DISCUSSION
To our knowledge, this is the first prospective study to evaluate the burden of laboratory-confirmed RSV in preterm infants 32–35 wGA in the United States. Few studies of RSV in preterm infants in any country have included prospective surveillance and active testing for RSV. In US preterm infants 32–35 wGA <6 months on November 1st and not receiving RSV prophylaxis, RSV hospitalizations occurred in approximately 1 in 20 infants per season, RSV ED visits in 1 in 17, and RSV-related MAARI was observed in 1 in 4. The observed RSV hospitalization rate is similar to rates observed in other studies conducted outside the United States of infants 32–35 wGA that employed active surveillance for laboratory-confirmed RSV.19,28 ICU and mechanical ventilation rates among those hospitalized with RSV are also consistent with previous observations.17,18,29,30 Recent data suggest that the elevated risk of severe RSV disease in these infants may be related to persistent lung function impairment31–34 and lower levels of maternal antibody.35
The observed rates of RSV hospitalization and MAARI in this study were approximately 3- and 2-fold greater, respectively, than those reported in the general population of US infants <6 months of age.22 However, the rate of RSV ED visits was similar. One explanation may be that more direct hospitalizations from the outpatient setting occur among preterm infants.
Overall, RSV disease risk was highest in children with day care attendance and preschool-aged siblings, consistent with previous observations and current AAP guidelines.8,16,36 Tobacco smoke exposure increased the risk of RSV hospitalization, which has also been previously demonstrated.19,30 Infants from a multiple birth were at decreased risk of RSV hospitalization. This association has been observed previously37 and may be due to differences in provider or parental behavior. Other earlier studies associated multiple birth with a higher risk of RSV pneumonia38 or RSV hospitalization39,40 in infants 24–36 wGA. In a study by Simoes et al,38 all members of a multiple birth set often developed RSV illness concurrently, which was not observed in the current study. Overall, these results indicate that the 2012 AAP environmental risk factors can identify the infants 32–35 wGA with an elevated risk for severe RSV disease.
Although previous studies of RSV hospitalization in preterm infants have examined the relationship between risk and birth month or chronologic age at RSV season start,16–19,30,36,41 only 3 examined the relationship between RSV hospitalization risk and chronologic age during the RSV season.42–44 All 3 demonstrated an elevated risk of RSV hospitalization in preterm infants 32–36 wGA through 4.5–6 months of age. In the current study, the risk of RSV ED visits and hospitalizations by infant-time exposure was highest through 6 months chronologic age in infants 32–34 wGA and 35 wGA with risk factors. In infants 32–34 wGA, there was no evidence of a difference in the incidence of severe RSV at <3 months and 3 to <6 months chronologic age, which appeared related to a lower risk of acquiring medically attended RSV illness in younger 32–34 wGA infants but a higher probability of severe disease once illness was acquired. Because rates were based on exposure time following enrollment, this finding cannot be explained by longer birth hospitalizations in this cohort. Parental behavior may have reduced RSV exposure at young chronologic age in this cohort. Among infants 32–34 wGA with 2012 AAP environmental risk factors, the comparable and high RSV hospitalization rate through <6 months raises the question of whether RSV prophylaxis should be provided to this population beyond 90 days of age. The elevated RSV hospitalization rates among infants 35 wGA with 2012 AAP environmental risk factors at <3 months and 3 to <6 months also warrant consideration.
Enrolled infants who, according to the AAP guidelines in effect during the study, were eligible but did not receive RSV prophylaxis were more likely to be African American or Hispanic/Latino and of higher birthweight. Increased efforts to ensure that eligible minority and higher birthweight infants have access to RSV prophylaxis should be a priority of practicing health care providers.
Study strengths include the large study population that was prospectively recruited from diverse sites throughout the United States and shown to be generally similar to the US birth cohort of infants 32–35 wGA. Inclusion of 2 RSV seasons also increases study generalizability. Active surveillance and outpatient testing increased the accuracy of RSV disease incidence rates, as approximately 50% of RSV ED visits and hospitalizations did not have an RSV test performed as part of routine care. The primary study limitation was the fact that only 32- to 35-wGA infants who did not receive RSV prophylaxis were enrolled. The risk for serious RSV illness is likely higher in infants identified for prophylaxis by their providers. Although the preterm infants enrolled appeared generally similar to the US birth cohort of infants 32–35 wGA, Hispanic infants and infants of less educated mothers were underrepresented, perhaps due to a lower willingness to participate in research. Although there was active testing of all outpatient MAARI episodes and use of RSV test results from ED visits and hospitalizations, 5% of all LRI hospitalizations, 21% of all LRI ED visits and 28% of outpatient MAARI visits did not have an RSV test performed; as a result, RSV outpatient and ED visit rates were likely underestimated. Despite these limitations, this large prospective study demonstrates a substantial risk of severe RSV in US preterm infants born at 32–35 weeks gestation and helps inform US RSV immunoprophylaxis policy.
ACKNOWLEDGMENTS
We would like to thank the subjects, parents, investigators and staff who were involved in the conduct of the REPORT study. Editorial assistance was provided by Complete Healthcare Communications, Inc. (Chadds Ford, PA) and was funded by MedImmune.
Footnotes
This study was sponsored by MedImmune. Editorial assistance (formatting the manuscript for submission) was provided by Complete Healthcare Communications, Inc., (Chadds Ford, PA) and funded by MedImmune. C.S.A. is an employee of MedImmune. E.J.A. has received research funding to his institution on his behalf from MedImmune. E.A.F.S. has received grant support to his institution from MedImmune and Abbvie Inc and has received payment from MedImmune for lectures including service on speaker bureaus. X.W., H.E., F.S., and J.R.G. were employees of MedImmune at the time of study conduct and analysis. C.L.P. has no funding or conflicts of interest to disclose.
ClinicalTrials.gov Identifier: NCT00983606.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Simoes EA. Respiratory syncytial virus infection. Lancet. 1999;354:847–852. doi: 10.1016/S0140-6736(99)80040-3. [DOI] [PubMed] [Google Scholar]
- 2.Mutuc JD, Langley GE. Respiratory syncytial virus--United States, July 2007-June 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1203–1206. [PubMed] [Google Scholar]
- 3.Zachariah P, Shah S, Gao D, et al. Predictors of the duration of the respiratory syncytial virus season. Pediatr Infect Dis J. 2009;28:772–776. doi: 10.1097/INF.0b013e3181a3e5b6. [DOI] [PubMed] [Google Scholar]
- 4.Panozzo CA, Fowlkes AL, Anderson LJ. Variation in timing of respiratory syncytial virus outbreaks: lessons from national surveillance. Pediatr Infect Dis J. 2007;26(11 suppl):S41–S45. doi: 10.1097/INF.0b013e318157da82. [DOI] [PubMed] [Google Scholar]
- 5.Boyce TG, Mellen BG, Mitchel EF, Jr, et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 6.Moler FW, Khan AS, Meliones JN, et al. Respiratory syncytial virus morbidity and mortality estimates in congenital heart disease patients: a recent experience. Crit Care Med. 1992;20:1406–1413. doi: 10.1097/00003246-199210000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Horn SD, Smout RJ. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J Pediatr. 2003;143(5 suppl):S133–S141. doi: 10.1067/s0022-3476(03)00509-2. [DOI] [PubMed] [Google Scholar]
- 8.Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011;30:510–517. doi: 10.1097/INF.0b013e3182184ae7. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. User guide to the 2010 Natality public use file. Available at: http://ftp.cdc.gov/pub/Health_statistics/NCHs/Dataset_Documentation/DVS/natality/UserGuide2010.pdf. Accessed January 21, 2014.
- 10.Qin C, Hsia J, Berg CJ. Variation between last-menstrual-period and clinical estimates of gestational age in vital records. Am J Epidemiol. 2008;167:646–652. doi: 10.1093/aje/kwm345. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. CDC WONDER On-line Database. Linked Birth/Infant Death Records, 2007–2008. Available at: http://wonder.cdc.gov/lbd-current.html. Accessed January 21, 2014.
- 12.MedImmune, LLC, Gaithersburg, MD: 2013. Synagis® (palivizumab).Full Prescribing Information. [Google Scholar]
- 13.American Academy of Pediatrics. Respiratory syncytial virus. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012. pp. 609–617. [Google Scholar]
- 14.Committee on Infectious Diseases. From the American Academy of Pediatrics: Policy statements--modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–1701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics. Respiratory syncytial virus. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009. pp. 560–569. [Google Scholar]
- 16.Blanken MO, Koffijberg H, Nibbelke EE, et al. Dutch RSV Neonatal Network. Prospective validation of a prognostic model for respiratory syncytial virus bronchiolitis in late preterm infants: a multicenter birth cohort study. PLoS One. 2013;8:e59161. doi: 10.1371/journal.pone.0059161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueras-Aloy J, Carbonell-Estrany X, Quero J IRIS Study Group. Case-control study of the risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born at a gestational age of 33-35 weeks in Spain. Pediatr Infect Dis J. 2004;23:815–820. doi: 10.1097/01.inf.0000136869.21397.6b. [DOI] [PubMed] [Google Scholar]
- 18.Figueras-Aloy J, Carbonell-Estrany X, Quero-Jiménez J, et al. IRIS Study Group. FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J. 2008;27:788–793. doi: 10.1097/INF.0b013e3181710990. [DOI] [PubMed] [Google Scholar]
- 19.Law BJ, Langley JM, Allen U, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J. 2004;23:806–814. doi: 10.1097/01.inf.0000137568.71589.bd. [DOI] [PubMed] [Google Scholar]
- 20.Fryzek JP, Martone WJ, Groothuis JR. Trends in chronologic age and infant respiratory syncytial virus hospitalization: an 8-year cohort study. Adv Ther. 2011;28:195–201. doi: 10.1007/s12325-010-0106-6. [DOI] [PubMed] [Google Scholar]
- 21.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 22.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holman RC, Curns AT, Cheek JE, et al. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics. 2004;114:e437–e444. doi: 10.1542/peds.2004-0049. [DOI] [PubMed] [Google Scholar]
- 24.Paramore LC, Ciuryla V, Ciesla G, et al. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics. 2004;22:275–284. doi: 10.2165/00019053-200422050-00001. [DOI] [PubMed] [Google Scholar]
- 25.Stockman LJ, Curns AT, Anderson LJ, et al. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr Infect Dis J. 2012;31:5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 26.Legoff J, Kara R, Moulin F, et al. Evaluation of the one-step multiplex real-time reverse transcription-PCR ProFlu-1 assay for detection of influenza A and influenza B viruses and respiratory syncytial viruses in children. J Clin Microbiol. 2008;46:789–791. doi: 10.1128/JCM.00959-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 28.Heikkinen T, Valkonen H, Lehtonen L, et al. Hospital admission of high risk infants for respiratory syncytial virus infection: implications for palivizumab prophylaxis. Arch Dis Child Fetal Neonatal Ed. 2005;90:F64–F68. doi: 10.1136/adc.2003.029710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forbes ML, Hall CB, Jackson A, et al. Comparative costs of hospitalisation among infants at high risk for respiratory syncytial virus lower respiratory tract infection during the first year of life. J Med Econ. 2010;13:136–141. doi: 10.3111/13696990903583404. [DOI] [PubMed] [Google Scholar]
- 30.Carbonell-Estrany X, Quero J IRIS Study Group. Hospitalization rates for respiratory syncytial virus infection in premature infants born during two consecutive seasons. Pediatr Infect Dis J. 2001;20:874–879. doi: 10.1097/00006454-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 31.McEvoy C, Venigalla S, Schilling D, et al. Respiratory function in healthy late preterm infants delivered at 33-36 weeks of gestation. J Pediatr. 2013;162:464–469. doi: 10.1016/j.jpeds.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoo AF, Dezateux C, Henschen M, et al. Development of airway function in infancy after preterm delivery. J Pediatr. 2002;141:652–658. doi: 10.1067/mpd.2002.128114. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich L, Pitrez PM, Stein RT, et al. Growth rate of lung function in healthy preterm infants. Am J Respir Crit Care Med. 2007;176:1269–1273. doi: 10.1164/rccm.200703-476OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics. 2010;126:115–128. doi: 10.1542/peds.2009-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeung CY, Hobbs JR. Serum-gamma-G-globulin levels in normal premature, post-mature, and “small-for-dates” newborn babies. Lancet. 1968;1:1167–1170. doi: 10.1016/s0140-6736(68)91865-5. [DOI] [PubMed] [Google Scholar]
- 36.Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143(5 suppl):S118–S126. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 37.Lacaze-Masmonteil T, Truffert P, Pinquier D, et al. Lower respiratory tract illness and RSV prophylaxis in very premature infants. Arch Dis Child. 2004;89:562–567. doi: 10.1136/adc.2003.028282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simoes EA, King SJ, Lehr MV, et al. Preterm twins and triplets. A high-risk group for severe respiratory syncytial virus infection. Am J Dis Child. 1993;147:303–306. doi: 10.1001/archpedi.1993.02160270065020. [DOI] [PubMed] [Google Scholar]
- 39.Resch B, Pasnocht A, Gusenleitner W, et al. Rehospitalisations for respiratory disease and respiratory syncytial virus infection in preterm infants of 29-36 weeks gestational age. J Infect. 2005;50:397–403. doi: 10.1016/j.jinf.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Park HW, Lee BS, Kim AR, et al. Epidemiology of respiratory syncytial virus infection in infants born at less than thirty-five weeks of gestational age. Pediatr Infect Dis J. 2012;31:e99–e104. doi: 10.1097/INF.0b013e318257f619. [DOI] [PubMed] [Google Scholar]
- 41.Liese JG, Grill E, Fischer B, et al. Munich RSV Study Group. Incidence and risk factors of respiratory syncytial virus-related hospitalizations in premature infants in Germany. Eur J Pediatr. 2003;162:230–236. doi: 10.1007/s00431-002-1105-7. [DOI] [PubMed] [Google Scholar]
- 42.Law B, Macdonald N, Langley J, et al. Severe respiratory syncytial virus infection among otherwise healthy prematurely born infants: What are we trying to prevent? Paediatr Child Health. 1998;3:402–404. doi: 10.1093/pch/3.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winterstein AG, Knox CA, Kubilis P, et al. Appropriateness of age thresholds for respiratory syncytial virus immunoprophylaxis in moderate-preterm infants: a cohort study. JAMA Pediatr. 2013;167:1118-1124 doi: 10.1001/jamapediatrics.2013.2636. [DOI] [PubMed] [Google Scholar]
- 44.Carbonell X, Fullarton JR, Gooch KL, et al. The evolution of risk factors for respiratory syncytial virus-related hospitalisation in infants born at 32–35 weeks’ gestational age: time-based analysis using data from the FLIP-2 study. J Perinat Med. 2012;40:685–691. doi: 10.1515/jpm-2011-0248. [DOI] [PubMed] [Google Scholar]
- 45.Olsen IE, Lawson ML, Meinzen-Derr J, et al. Use of a body proportionality index for growth assessment of preterm infants. J Pediatr. 2009;154:486–491. doi: 10.1016/j.jpeds.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


