Abstract
In this work, we evaluated the ability of 3D co-cultures with mesenchymal stem cells (MSCs) to redifferentiate monolayer expanded articular chondrocytes (ACs) and produce cartilaginous extracellular matrix at varying stages of the dedifferentiation process and further examined the dependency of this effect on the culture medium composition. Primary bovine ACs were expanded in monolayers for up to nine population doublings to obtain seven cell stocks with gradually increasing levels of dedifferentiation. Culture expanded ACs were then seeded as monocultures and co-cultures with rabbit bone marrow-derived MSCs (30:70 ratio of ACs-to-MSCs) on porous scaffolds. Parallel cultures were established for each cell population in serum-containing growth medium and serum-free induction medium supplemented with dexamethasone and TGF-β3. After 3 weeks, all groups were analyzed for DNA content, glycosaminoglycan (GAG) and hydroxyproline (HYP) production, and chondrogenic gene expression. Significant enhancements in cellularity, GAG content and GAG/HYP ratio, and chondrogenic phenotype were observed in the induction medium compared to growth medium at all levels of AC expansion. Furthermore, primary co-cultures showed similarly enhanced chondrogenesis compared to monocultures in both culture media, whereas passaged ACs benefitted from co-culturing only in the induction medium. We conclude that co-cultures of ACs and MSCs can produce superior in vitro engineered cartilage in comparison to pure AC cultures, due to both heterotypic cellular interactions and decreased need for monolayer expansion of biopsied chondrocytes. While the initial level of AC dedifferentiation affected the quality of the engineered constructs, co-culture benefits were realized at all stages of AC expansion when suitable chondroinductive culture medium was used.
Introduction
Articular cartilage is responsible for the low friction and even distribution of loads in all synovial joints, making the normal pain-free movement possible.1 Mature cartilage is avascular and has a low cell density and metabolic activity. Therefore, damage to this tissue is unable to elicit an adequate healing response to produce functional repair of even small defects, and surgical interventions are often required. To improve articular cartilage repair, chondrogenic cell populations are traditionally brought to the defect site in the form of marrow stimulation, auto- and allograft implantation, and ex vivo expanded autologous chondrocyte transplantation.2 However, these strategies tend to result in the formation of mechanically and functionally inferior repair tissue, resulting in further complications in the long term.3 Therefore, improved tissue engineering strategies are needed to reach the full potential of cell-based therapies.4
Articular chondrocytes (ACs) and mesenchymal stem cells (MSCs) are the most common cell sources in cartilage engineering.5 Although easily harvested and expanded, multipotent MSCs require extensive in vitro manipulation to achieve stable chondrogenic differentiation,6 and the end results often bear signs of fibrocartilage and hypertrophy rather than mature articular cartilage.7,8 On the other hand, risk of donor site morbidity limits the number of harvested ACs,9 and unfortunately, these cells dedifferentiate rapidly during monolayer culture, acquiring a fibroblast-like phenotype and producing inferior cartilaginous tissue in comparison to primary cells.10,11 Minimally expanded human ACs seem to be more potent cartilage producers than MSCs both in vitro and in vivo,12,13 but it is not clear if this holds true for more dedifferentiated ACs.
Typical cartilage engineering schemes consist of two steps: chondrogenic cells are first expanded in serum-containing growth medium and cartilaginous tissue formation is subsequently achieved in serum-free induction medium commonly supplemented with known chondrogenic factors, such as dexamethasone and TGF-β.14 Prior research has shown that while AC dedifferentiation in monolayer cultures is a gradual process,15 already four population doublings can eradicate the intrinsic chondrogenicity of the resulting cell population.16 However, highly expanded ACs can regain their chondrogenicity in the induction medium, and this redifferentiation process can be further enhanced by other suitable external stimuli like hypoxia17 and mechanical loading.18 Recently, trophic factors produced by co-cultured MSCs have been implicated as a novel promising chondroinductive signal to enhance AC proliferation, phenotype, and extracellular matrix (ECM) production in 3D cultures.19,20 The benefits of such co-cultures have been demonstrated using chondrocytes and MSCs from various sources,21,22 but to the best of our knowledge, there are no detailed reports on the effectiveness of this approach at varying stages of AC dedifferentiation. In the current study, we investigated the relationship of chondrocyte redifferentiation capacity in AC-MSC co-cultures with the level of AC expansion and further examined the dependency of this trophic effect on the culture medium composition.
Materials and Methods
Experimental design
Primary bovine ACs were expanded in monolayers for up to nine population doublings to obtain seven cell stocks with gradually increasing levels of dedifferentiation. Culture expanded ACs were then seeded as monocultures and co-cultures with rabbit bone marrow-derived MSCs (30:70 ratio of ACs-to-MSCs) on electrospun poly(ɛ-caprolactone) (PCL) scaffolds. Parallel cultures were established for each cell population in serum-containing growth medium, and serum-free induction medium supplemented with dexamethasone and TGF-β3. After 3 weeks, all groups were analyzed for DNA content, cartilaginous ECM production, and chondrogenic gene expression.
Scaffold preparation
PCL fibers with ∼10 μm diameter were electrospun into a millimeter thick nonwoven mat as previously described.23 Discoid scaffolds with 8 mm diameter were then cut using a dermal biopsy punch. The scaffolds were sterilized by exposure to ethylene oxide (Andersen Sterilizers) for 14 h and aerated overnight to remove residual fumes. To remove air bubbles and improve cell adhesion, sterilized scaffolds were prewetted in a graded ethanol series, washed with phosphate-buffered saline (PBS), and incubated in general culture medium (high-glucose DMEM, 10% fetal bovine serum [BenchMark FBS; Gemini Bio-Products], penicillin/streptomycin/fungizone [PSF]) for 4 days before use.
Cell harvest and expansion
Bovine ACs and rabbit MSCs were harvested as previously described.19 ACs were obtained from 7- to 10-day-old calves (Research 87), less than 24 h after slaughter. Briefly, articular cartilage from femoral condyles of three donor animals was collected, minced to ∼1×1×1 mm, washed with PBS, and digested in the chondrocyte growth medium (DMEM, 10% FBS, 1% nonessential amino acids, 50 μg/mL ascorbic acid, 46 μg/mL L-proline, 20 mM HEPES, PSF) containing 2 mg/mL collagenase type II (Worthington Biochemical Corporation). Digestions were incubated on a shaker table at 37°C for 16 h and passed through cell strainers. Harvested primary ACs were then aliquoted and cryopreserved. Every 2 days, two to four frozen aliquots were plated at a density of 6600 cells/cm2 in the growth medium. Medium was changed once at 4 days, and semiconfluent cultures were passaged the first time at 6 days. Subsequent passaging occurred every 2 days with a constant plating density. Fourteen days after the first plating, all cultures were terminated simultaneously to form seven AC stocks with gradually increasing expansion levels to be used for scaffold seeding.
Bone marrow-derived MSCs were obtained from 5-week-old New Zealand White rabbits (Charles River Laboratories). Under general anesthesia, bone marrow from the tibia was aspirated into 10-mL syringes containing 1000 U heparin to prevent coagulation. The marrow was suspended in general medium and plated in tissue culture flasks. Nonadherent cells were washed away after 72 h, and cultures were maintained until confluent. Cells from six donor animals were pooled together, aliquoted, and cryopreserved. Frozen MSCs were plated at a density of 3300 cells/cm2 in general medium and passaged once at day 5. After 5 more days of culture, an MSC stock (passage 3) was formed for scaffold seeding.
Cell seeding and 3D culture
Seeding suspensions of ∼1×106 cells/mL were established for the MSCs, each of the AC passages, and corresponding co-cultures with 30:70 AC-to-MSC cell ratio. The cell ratio was chosen based on our previous studies, and the xenogeneic culture model was used to facilitate AC gene expression analysis without interference from co-cultured MSCs.19 Prewetted scaffolds were press-fitted into custom-made cylindrical polycarbonate cassettes designed to confine the seeding suspension, and cells in the chondrocyte growth medium were pipetted onto each scaffold to achieve a final seeding density of 4.5×106 cells/mL scaffold volume. Cells were allowed to adhere for 4 h before more medium was gently added to completely cover the cassettes. After 24 h, scaffolds were removed from the cassettes and divided into the growth medium and induction medium (high-glucose DMEM, 1% ITS+ premix [BD Biosciences], 50 μg/mL ascorbic acid, 40 μg/mL L-proline, 100 nM dexamethasone, 10 ng/mL TGF-β3 [PeproTech], PSF) in 12-well culture plates. Cultures were continued for 3 weeks, with half of the medium replenished two to three times a week. MSC controls were kept only in the induction medium.
Biochemical assays
Four replicate scaffolds from each culture group were harvested and washed in PBS. A 3 mm biopsy punch was used to obtain individual samples from randomized scaffold locations for biochemical and histological analysis, and the rest of the scaffold was used for RNA isolation.
Two biopsy samples were pooled together from each scaffold and stored at −20°C until further processing for DNA, glycosaminoglycan (GAG), and hydroxyproline (HYP) assays. Thawed samples were digested in proteinase K solution (1 mg/mL proteinase K, 0.01 mg/mL pepstatin A, and 0.185 mg/mL iodoacetamide in a 50 mM tris(hydroxymethyl aminomethane)–1 mM ethylenediaminetetraacetic acid buffer, pH 7.6) in a 56°C water bath for 16 h. Cell and ECM components were extracted via two additional freeze-thaw cycles followed by 10 min sonication in a water bath.
DNA content of the scaffolds was determined using Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen). Briefly, cell lysate, assay buffer, and dye solution were combined in duplicates and allowed to incubate for 10 min at room temperature. Fluorescence was measured using excitation and emission wavelengths of 485 and 528 nm (FL×800 Fluorescence Microplate Reader; BioTek Instruments), respectively, and DNA concentrations were determined relative to a lambda DNA standard curve.
GAG content was determined using the colorimetric dimethylmethylene blue assay.24 Briefly, cell lysate and color reagent were combined in duplicates and allowed to incubate for 7 min at room temperature. Absorbance at 520 nm was measured (PowerWave×340 Microplate Reader; BioTek Instruments), and GAG concentrations were determined relative to a chondroitin sulfate standard curve.
HYP content, an indicator for total collagen, was determined in a colorimetric assay.25 Briefly, an aliquot of cell lysate was combined with an equal volume of 4 N NaOH and hydrolyzed by autoclaving for 20 min, 121°C (∼55 min total processing time). The solution was neutralized with HCl and acetic acid to pH 6 and divided into duplicate reactions. Chloramine-T and p-dimethylaminobenzaldehyde solutions were added sequentially, the absorbance at 570 nm was measured using a plate reader and HYP concentrations were determined relative to a trans-4-hydroxy-L-proline standard curve.
Histology
One biopsy sample from each scaffold was fixed for histology in 10% neutral buffered formalin (Fisher Scientific), then immersed in 70% ethanol before embedding in the HistoPrep freezing medium (Fisher Scientific). Frozen sections of 5 μm thick were cut using a cryostat (Leica CM 1850 UV; Leica Biosystems Nussloch GmbH), mounted onto glass slides, and placed on a 42°C slide warmer to facilitate adhesion. Sections were stained with Alcian Blue, Picrosirius Red, and Fast Green to visualize the distribution of GAG, collagen, and cells, respectively, in the 3D constructs. Images were obtained using a light microscope with a digital camera attachment (Axio Imager.Z2 equipped with AxioCam MRc5; Carl Zeiss MicroImaging GmbH).
Real-time reverse transcription–polymerase chain reaction
Total RNA was isolated from pelleted cell seeding stocks and minced (∼1×1×1 mm pieces) 3D constructs, using RNeasy mini kit (Qiagen). Briefly, samples were immersed in lysis buffer and incubated at room temperature for 30 min with periodic vortexing. Cell lysate was then passed through a QIAshredder homogenization column and stored at −80°C until further processing. An equal volume of 70% ethanol was added to thawed lysates and RNA isolation was continued following the animal cell protocol provided by the manufacturer. Reverse transcription was then carried out to synthesize cDNA from purified RNA samples using Oligo(dT) primers (Promega) and SuperScript III reverse transcriptase (Invitrogen). Finally, cDNA was subjected to real-time PCR (Applied Biosystems 7300 Real-Time PCR System) using SYBR Green detection (PerfeCTa SYBR Green FastMix, ROX; Quanta Biosciences) with custom-designed primers (Integrated DNA Technologies).
Primer sequences are given in Table 1. Target gene expression was first normalized to the expression of the housekeeping gene GAPDH in the same sample (ΔCt), then to the average expression of that target gene measured in the p0 1 day AC seeding stock (ΔΔCt). Finally, the 2−ΔΔCt method was used to convert normalized gene expression levels to fold differences, and statistics were calculated on these values.26 Similarly, 2−ΔCt was used to calculate the ratios of collagen II/collagen I expression and bovine-specific/cross-species GAPDH signal within individual samples.
Table 1.
Forward and Reverse Primers Used for Quantitative RT-PCR
| Gene | Primer sequence | Product length | GenBank No. |
|---|---|---|---|
| Bovine-specific | |||
| Col1a2 | F: 5′-CGGGTCTTGCTGGTCATCAT-3′ | 125 | NM_174520.2 |
| R: 5′-TGCACCAGGCTGTCCAATG-3′ | |||
| Col2a1 | F: 5′-AGTGGAAGAGCGGAGACTACTG-3′ | 233 | NM_001001135.2 |
| R: 5′-GTTGGGAGCCAGGTTGTCAT-3′ | |||
| Col3a1 | F: 5′-CCTTGAAGCTGATGGGGTCA-3′ | 105 | NM_001076831.1 |
| R: 5′-ATTCCCCAGTGTGTTTTGTGC-3′ | |||
| Acan | F: 5′-AGAGAGCCAAACAGCCGACA-3′ | 270 | NM_173981.2 |
| R: 5′-TAGTCCTGGGCATTGTTGTTGA-3′ | |||
| COMP | F: 5′-TCGCGTTCGCTGCATCAATA-3′ | 201 | NM_001166517.1 |
| R: 5′-TGGAAGGAGCCCACGGTAT-3′ | |||
| GAPDH | F: 5′-GAGTCCACTGGGGTCTTCACT-3′ | 251 | NM_001034034.2 |
| R: 5′-GCGTGGACAGTGGTCATAAGTC-3′ | |||
| Cross-species | |||
| GAPDH | F: 5′-CCATCTTCCAGGAGCGAGAT-3′ | 186 | NM_001034034.2 |
| R: 5′-GGTTCACGCCCATCACAAAC-3′ | NM_001082253.1 | ||
F, forward; R, reverse; Col1a2, collagen type I; Col2a1, collagen type II; Col3a1, collagen type III; Acan, aggrecan; COMP, cartilage oligomeric matrix protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistics
Results are presented as means±standard deviations. Statistical analysis was performed with an IBM SPSS 20.0.0 software package. Biochemical assay data were analyzed using one-way ANOVA followed by Tukey's post-hoc test, whereas RT-PCR data were analyzed using the Kruskal–Wallis test with stepwise stepdown multiple comparisons. Differences were considered significant at 95% confidence level. For the sake of presentation clarity, results for 3D cultures are shown with only four levels of AC passaging. However, statistical analysis and data interpretation are based on all seven expansion levels, and quantitative measurements for all experimental groups are included in supplementary data (Supplementary Tables S1–S10; Supplementary Data are available online at www.liebertpub.com/tec).
Results
Monolayer expansion of ACs
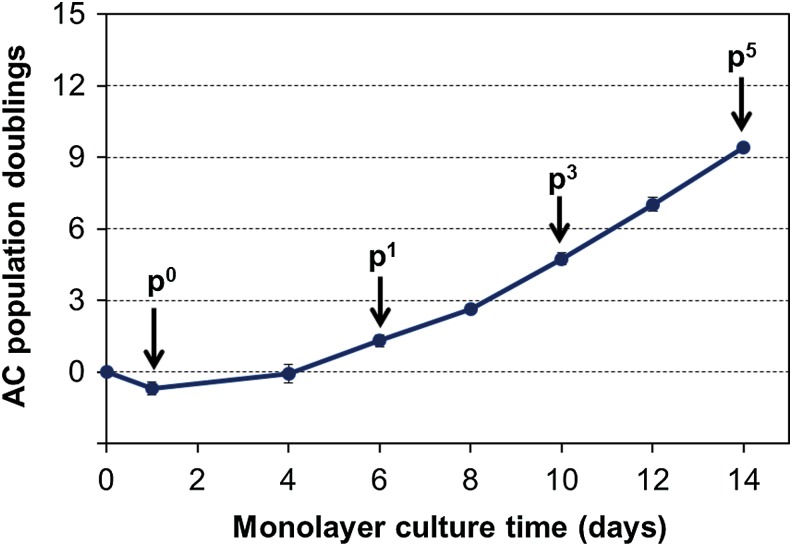
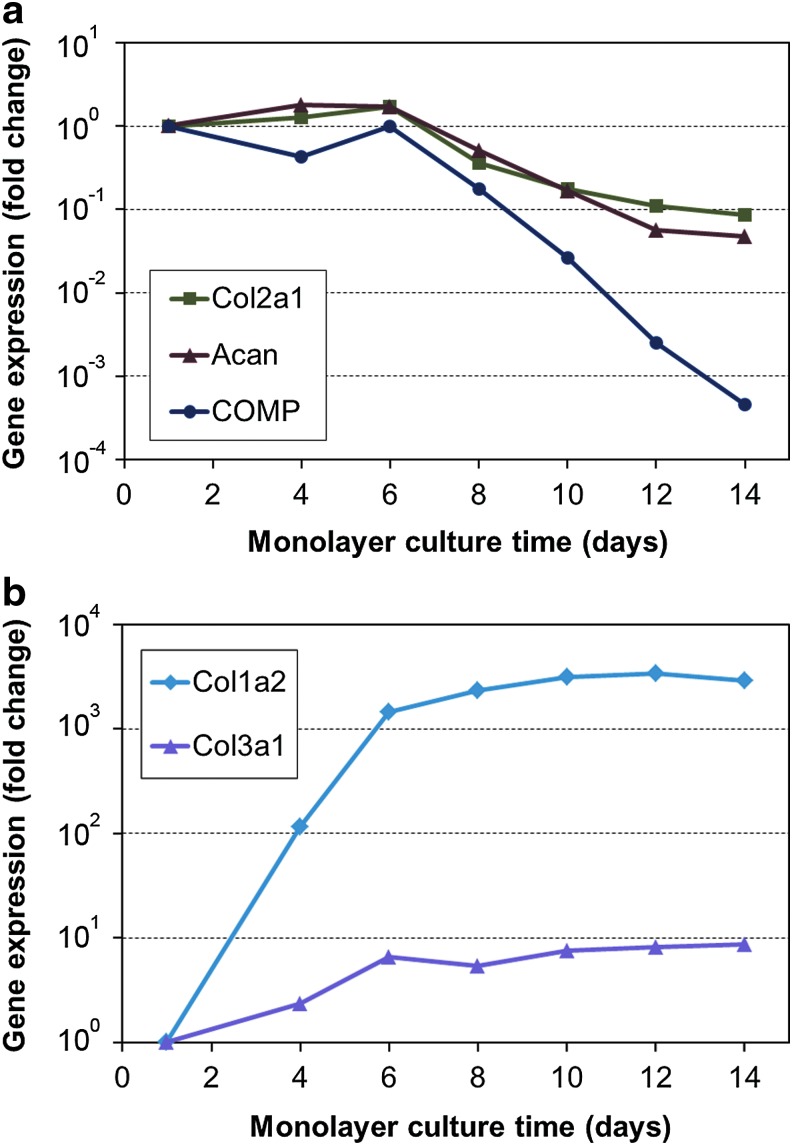
Proliferation of ACs during monolayer expansion is depicted in Figure 1. Some nonviable and poorly adherent cells were eliminated from the frozen cell stocks upon initial plating, resulting in ∼40% cell loss in the p0 seeding stock. For all other groups, the first medium change was performed after 4 days, and at that time, the cells had obtained a more flattened morphology and started to proliferate. Furthermore, a gradual increase in the proliferation rate was observed at each passage. Gene expression analysis (Fig. 2) showed immediate increase in dedifferentiation markers collagen type I and type III upon plating, whereas chondrogenic markers collagen type II, aggrecan, and COMP started to decline only after the first passage at day 6.
FIG. 1.
Articular chondrocyte (AC) proliferation in monolayer cultures. Each marker represents cumulative population doublings (mean±standard deviation [SD]) measured from 2 to 10 independent platings. Arrows indicate passage numbers for subsequent three dimensional (3D) cell seeding stocks discussed in the main article (data related to the unlabeled seeding stocks are included in the supplementary files). Color images available online at www.liebertpub.com/tec
FIG. 2.
Progression of AC dedifferentiation in monolayer cultures. Fold change in gene expression of selected (a) chondrogenic and (b) dedifferentiation markers relative to the p0 seeding stock. Each marker represents an individual seeding stock used in the subsequent 3D cultures. The analyzed genes were collagen type I (Col1a2), type II (Col2a1) and type III (Col3a1), aggrecan (Acan), and cartilage oligomeric matrix protein (COMP). Color images available online at www.liebertpub.com/tec
Scaffold cellularity
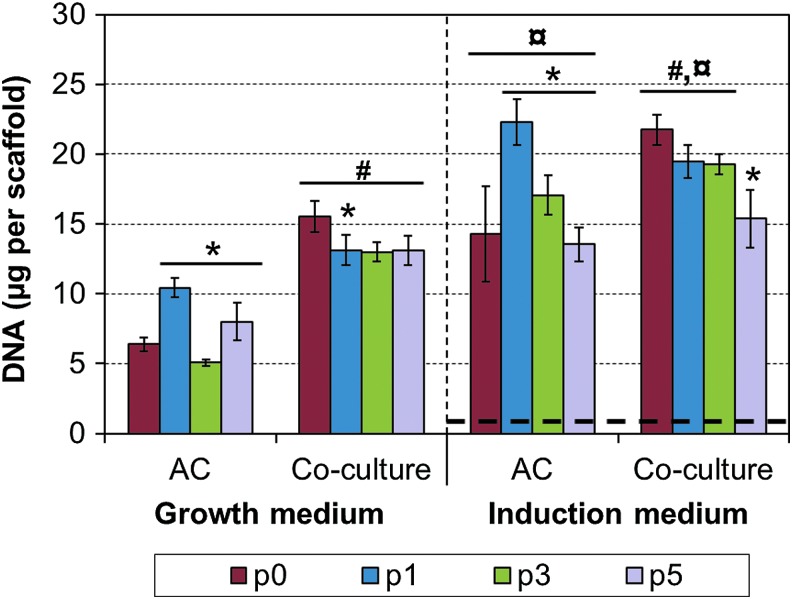
DNA contents in the 3D scaffolds were measured at the end of the 21-day culture period. Clear increase in cellularity was observed for all experimental groups, except the pure MSC control, which had very low DNA content (Fig. 3). Co-culturing and induction medium resulted in significant increases in cellularity in comparison to pure AC cultures and growth medium, respectively. In addition, highest cellularities were observed with p1 ACs and primary co-cultures, and cellularity tended to decrease with higher passages.
FIG. 3.
DNA contents of the engineered cartilage constructs. Results are presented as mean±SD with n=4. The bolded dashed line represents the mesenchymal stem cells (MSC) control group, which was cultured in the induction medium only. The used seeding protocol results in ∼3 μg of DNA per scaffold at the beginning of the cultures.19 #, ¤, and * denote statistically significant difference to the corresponding monoculture and growth medium (same passage) and to the lower AC expansion level (same cell population and culture medium), respectively (p<0.05). Color images available online at www.liebertpub.com/tec
ECM production
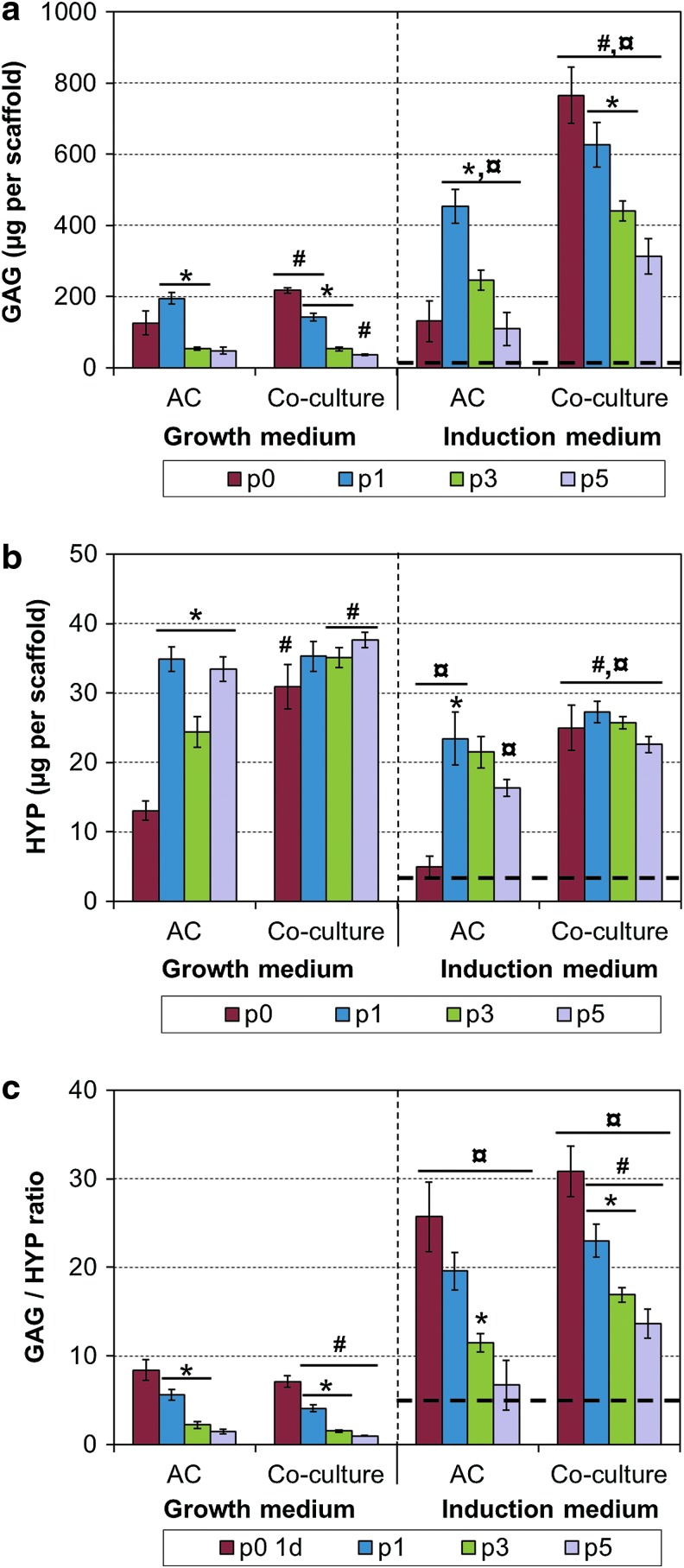
Cultured constructs were analyzed for their contents of major cartilaginous ECM components, that is, sulfated GAGs and total collagen (HYP). Induction medium increased GAG and decreased collagen production in comparison to the growth medium (Fig. 4). Co-culturing typically increased both GAG and HYP contents, especially with primary cultures. Highly passaged cells showed strong decline in GAG contents, whereas HYP contents remained relatively stable from p1 to p5. MSC controls produced only small amounts of ECM. The GAG/HYP ratio was calculated for each construct as an indicator for hyaline or fibrocartilage nature of the produced matrix. A steady decline was observed upon increasing levels of AC passaging, whereas the use of the induction medium highly increased the ratio (Fig. 4c). The effect of co-culturing was strongest with highly passaged cells, increasing the ratio in the induction medium but decreasing the ratio in the growth medium. MSC controls had low GAG/HYP ratio, similar to p5 chondrocytes.
FIG. 4.
Extracellular matrix production in 3D cultures. (a) GAG content, (b) HYP content, and (c) GAG/HYP ratio. Results are presented as mean±SD with n=4. The bolded dashed line represents the MSC control group, which was cultured in the induction medium only. #, ¤, and * denote statistically significant difference to the corresponding monoculture and growth medium (same passage) and to the lower AC expansion level (same cell population and culture medium), respectively (p<0.05). GAG, glycosaminoglycan; HYP, hydroxyproline. Color images available online at www.liebertpub.com/tec
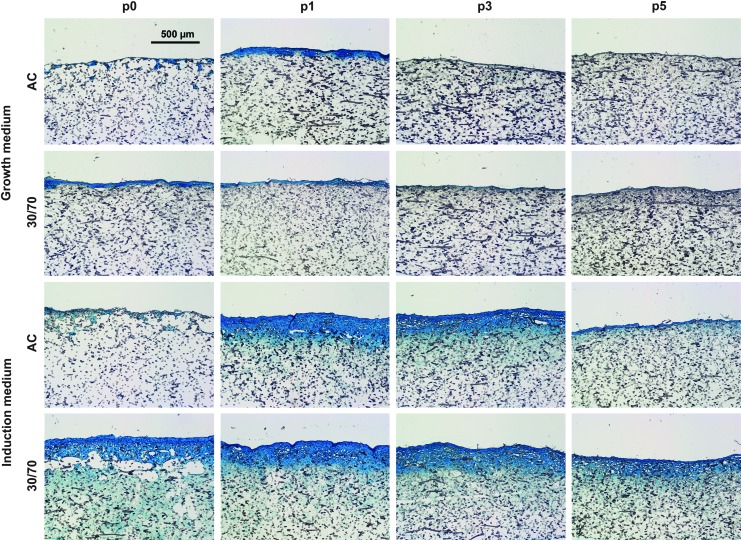
Histology
Histological evaluation of the 3D constructs corroborated with the quantitative ECM analysis. GAG production was highly increased in induction medium, and the overall amount of cartilaginous tissue was highest using p1 ACs (Fig. 5). Dense GAG-rich matrix with embedded cells was largely confined to the seeding surface of the scaffolds, whereas acellular collagenous matrix was more evenly distributed throughout the porous structure (data not shown).
FIG. 5.
Histological evaluation of GAG production and tissue distribution in 3D cultures. Scale bar represents 500 μm. Alcian Blue staining.
Gene expression in 3D scaffolds
The cellular proportions and chondrogenic phenotype of the cultured constructs were analyzed using real-time RT-PCR. The ratios of bovine-specific to cross-species PCR signal for a housekeeping gene GAPDH were equal with bovine AC monocultures and xenogeneic co-cultures (1.26±0.08 and 1.25±0.09, respectively) across all expansion levels and medium compositions, indicating negligible proportion of rabbit MSCs present in the co-cultures at the end of a 21-day culture period.
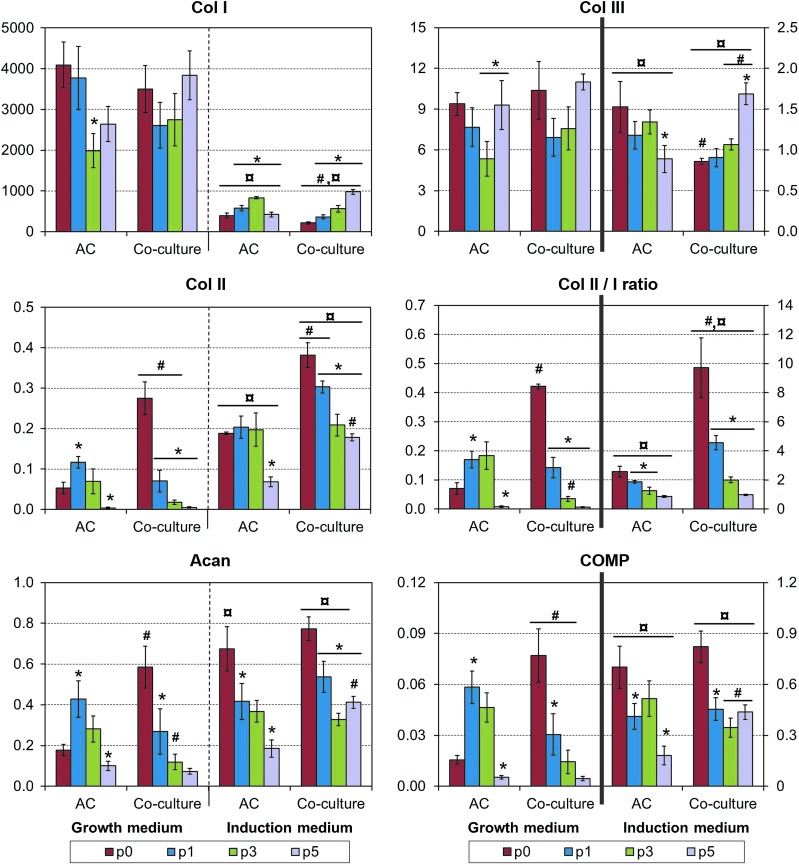
Induction medium decreased collagen type I and increased collagen type II expression, resulting in highly increased collagen II/I expression ratio (Fig. 6). The highest ratios were observed with primary co-cultures, with a gradual decrease upon increasing levels of AC passaging. Aggrecan and COMP expression acted similarly to collagen II. Again, induction medium and primary co-cultures increased these chondrogenic markers, with a gradual decrease on increased AC passaging. Collagen type III expression was analyzed as a dedifferentiation marker, and it was clearly decreased in the induction medium. Co-culturing or the level of AC passaging did not have a consistent effect on this marker. In addition, certain degree of redifferentiation (relative to the corresponding seeding stocks) was observed in chondroinductive cultures using p3–p5 ACs, but none of the 3D constructs were able to regain the initial chondrogenic phenotype of primary cells.
FIG. 6.
Fold change in gene expression of selected chondrogenic markers in 3D cultures. Results are normalized (except for Col II/I ratio) in relation to the p0 seeding stock and presented as mean±SD with n=4. #, ¤, and * denote statistically significant difference to the corresponding monoculture and growth medium (same passage) and to the previous AC expansion level (same cell population and culture medium), respectively (p<0.05). Note the different scales on primary and secondary Y-axes. Color images available online at www.liebertpub.com/tec
Discussion
Despite over 20 years of tissue engineering efforts, stable regeneration of articular cartilage defects still remains a challenge.27 One of the major difficulties encountered is the frequent formation of fibrous repair tissue instead of proper hyaline cartilage, indicating the need for optimized cell sources and in vitro culture conditions. Co-cultures of ACs and MSCs have recently shown promise for cartilage engineering, avoiding some of the common problems seen in the corresponding monocultures.5 The current study evaluated the ability of such 3D co-cultures to redifferentiate ACs and produce cartilaginous ECM at varying stages of culture expansion and further examined the dependency of this trophic effect on the culture medium composition.
Tissue engineering approaches using autologous chondrocytes to repair clinical cartilage defects typically require a minimum of 20-fold expansion of harvested cell numbers in monolayers before seeding into 3D scaffolds and eventual transplantation back to the patient.28,29 That would correspond to more than four population doublings, and approximately passage 3 in the current experimental setup. Extended cell cultures typically result in a significant loss of the chondrogenic phenotype in vitro and the ability to form hyaline cartilage in vivo.10,30 The problem of dedifferentiating chondrocytes is further reflected in the fact that although clinical products for autologous chondrocyte transplantation are designed with an upper limit to the level of monolayer expansion (the exact process parameters are not publicly available), the resulting constructs still exhibit signs of dedifferentiation.31
As current gene expression analysis indicated, robust redifferentiation of culture expanded ACs remains an elusive goal, and it should therefore be beneficial to limit the degree of dedifferentiation in the first place. Recent proposals to achieve this include the use of physiological oxygen tension,32 chondrogenic growth factors,33 ECM-coated culture substrates,34 and constant maintenance of high cell densities35 during monolayer culture. Surprisingly, however, some culture schemes have shown enhanced 3D chondrogenesis following treatments that increased proliferation rates but also favored dedifferentiation in monolayers.36,37
In the current study, rapidly increasing collagen type I and type III expression levels indicated that primary ACs started to dedifferentiate immediately on monolayer plating, although typical chondrogenic markers remained high until the first passaging at 6 days of culture. Whereas diffusional nutrient and oxygen limitations38,39 commonly confine 3D cultured ACs to the periphery of porous scaffolds, the seeded cells adhere, continue to proliferate, and slowly invade the available macropores. The observed increase in cellularity within our 3D constructs is in contrast to relatively flat or even decreasing cell numbers typical to hydrogel and pellet cultures.40,41 A possible down-side to this proliferation is the progression of the dedifferentiation process even in 3D. Indeed, signs of chondrogenic redifferentiation were seen only with our most expanded AC seeding populations and only in induction medium.
Surprisingly, 3D monocultures in the growth medium using the p1 ACs resulted in more matrix production and exhibited more chondrogenic phenotype in comparison to constructs derived from primary ACs. Possible reasons for this might include lower seeding efficiency and an initial adaptation period to the in vitro culture conditions with the primary cells, corresponding to the observed decline in cell numbers in the beginning of monolayer cultures. In line with our findings, Ng et al.42 recently showed enhanced mechanical and biochemical cartilage properties using p1 rather than primary chondrocytes in hydrogel cultures. Most importantly, however, primary ACs were highly responsive to the trophic effects of MSCs in our co-culture models, yielding engineered constructs with significantly higher GAG contents as well as GAG/HYP and collagen type II/I ratios in comparison to culture expanded cells. Previous xenogeneic studies have shown that the proportion of ACs dramatically increases during 3D culture due to high proliferation rate and MSC apoptosis, and the final constructs therefore originate mainly from the ACs.19–22 Furthermore, this phenomenon has been demonstrated using cells from multiple species and tissue sources and was again confirmed in the current study. Thus, it is interesting that passaged ACs benefitted from the co-culturing (in terms of ECM quality and cell phenotype) only in the induction medium, and our ongoing studies indicate that co-cultures are also more responsive to transient low-dose exposure to TGF-β3 in comparison to AC monocultures.43 Consequently, the trophic effect seems to be at least partially dependent on the differentiation status of the MSCs. On the other hand, the use of the induction medium increased the quality of our 3D constructs similarly at all levels of AC expansion in both mono- and co-cultures.
Assuming a fixed number of harvested chondrocytes and final seeding density, replacing 70% of ACs with MSCs in the current co-cultures would correspond to roughly one less passage in monolayer. Furthermore, a recent study by Sabatino et al.44 demonstrated enhanced in vivo cartilage formation in co-culture constructs with as little as 5% (primary) AC fraction. Co-culturing can therefore be an effective way to limit the degree of AC expansion and concomitant dedifferentiation in cartilage engineering. Taking into account previous reports on the robust chondrogenic potential of co-cultures in hypoxic45 and inflammatory conditions46 typical to injured cartilage, the use of co-cultures shows great promise for clinical applications.
Conclusions
Major enhancements in cellularity, ECM quantity and quality, and chondrogenic gene expression of AC-MSC co-cultures were observed in the induction medium compared to the growth medium at all levels of AC expansion. Primary co-cultures showed increased chondrogenesis compared to AC monocultures in both culture media, whereas passaged ACs benefitted from co-culturing with MSCs only in the induction medium. Co-cultures decrease the need for AC expansion, thus enabling the use of less dedifferentiated seeding stocks, resulting in improved quality of cartilage-engineered constructs.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health grant R01 AR057083.
Disclosure Statement
No competing financial interests exist.
References
- 1.Buckwalter J.A., Mankin H.J., and Grodzinsky A.J.Articular cartilage and osteoarthritis. Instr Course Lect 54,465, 2005 [PubMed] [Google Scholar]
- 2.Farr J., Cole B., Dhawan A., Kercher J., and Sherman S.Clinical cartilage restoration evolution and overview. Clin Orthop 469,2696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chahal J., Thiel G.V., Hussey K., and Cole B.J.Managing the patient with failed cartilage restoration. Sports Med Arthrosc 21,62, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Kock L., van Donkelaar C.C., and Ito K.Tissue engineering of functional articular cartilage: the current status. Cell Tissue Res 347,613, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leijten J.C.H., Georgi N., Wu L., van Blitterswijk C.A., and Karperien M.Cell sources for articular cartilage repair strategies: shifting from monocultures to cocultures. Tissue Eng Part B 19,31, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Freyria A.M., and Mallein-Gerin F.Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury 43,259, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Dickhut A., Pelttari K., Janicki P., Wagner W., Eckstein V., Egermann M., and Richter W.Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol 219,219, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Hellingman C.A., Koevoet W., and van Osch G.Can one generate stable hyaline cartilage from adult mesenchymal stem cells? A developmental approach. J Tissue Eng Regen Med 6,e1, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Matricali G.A., Dereymaeker G.P.E., and Luyten F.P.Donor site morbidity after articular cartilage repair procedures: a review. Acta Orthop Belg 76,669, 2010 [PubMed] [Google Scholar]
- 10.Dell'Accio F., De Bari C., and Luyten F.P.Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum 44,1608, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Pelttari K., Lorenz H., Boeuf S., Templin M.F., Bischel O., Goetzke K., Hsu H.Y., Steck E., and Richter W.Secretion of matrix metalloproteinase 3 by expanded articular chondrocytes as a predictor of ectopic cartilage formation capacity in vivo. Arthritis Rheum 58,467, 2008 [DOI] [PubMed] [Google Scholar]
- 12.De Bari C., Dell'Accio F., and Luyten F.P.Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum 50,142, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Giovannini S., Diaz-Romero J., Aigner T., Heini P., Mainil-Varlet P., and Nesic D.Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater 20,245, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Mauck R.L., Yuan X., and Tuan R.S.Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14,179, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Romero J., Nesic D., Grogan S.P., Hein P., and Mainil-Varlet P.Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells. J Cell Physiol 214,75, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Giovannini S., Diaz-Romero J., Aigner T., Mainil-Varlet P., and Nesic D.Population doublings and percentage of s100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. J Cell Physiol 222,411, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Schrobback K., Klein T.J., Crawford R., Upton Z., Malda J., and Leavesley D.I.Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res 347,649, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury T.T., Salter D.M., Bader D.L., and Lee D.A.Integrin-mediated mechanotransduction processes in tgf beta-stimulated monolayer-expanded chondrocytes. Biochem Biophys Res Commun 318,873, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Meretoja V.V., Dahlin R.L., Kasper F.K., and Mikos A.G.Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials 33,6362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu L., Leijten J.C.H., Georgi N., Post J.N., van Blitterswijk C.A., and Karperien M.Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A 17,1425, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Acharya C., Adesida A., Zajac P., Mumme M., Riesle J., Martin I., and Barbero A.Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol 227,88, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Wu L., Prins H.J., Helder M.N., van Blitterswijk C.A., and Karperien M.Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A 18,1542, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Dahlin R.L., Meretoja V.V., Ni M., Kasper F.K., and Mikos A.G.Hypoxia and flow perfusion modulate proliferation and gene expression of articular chondrocytes on porous scaffolds. AIChE J 59,3158, 2013 [Google Scholar]
- 24.Farndale R.W., Buttle D.J., and Barrett A.J.Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883,173, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Stegeman H., and Stalder K.Determination of hydroxyproline. Clin Chim Acta 18,267, 1967 [DOI] [PubMed] [Google Scholar]
- 26.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative pcr and the 2^(-delta delta ct) method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Huey D.J., Hu J.C., and Athanasiou K.A.Unlike bone, cartilage regeneration remains elusive. Science 338,917, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brittberg M., Peterson L., Sjogren-Jansson E., Tallheden T., and Lindahl A.Articular cartilage engineering with autologous chondrocyte transplantation—a review of recent developments. J Bone Joint Surg Am 85A,109, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Mandl E.W., Van der Veen S.W., Verhaar J.A.N., and Van Osch G.Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity. Tissue Eng 10,109, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Salzmann G.M., Sauerschnig M., Berninger M.T., Kaltenhauser T., Schonfelder M., Vogt S., Wexel G., Tischer T., Sudkamp N., Niemeyer P., Imhoff A.B., and Schottle P.B.The dependence of autologous chondrocyte transplantation on varying cellular passage, yield and culture duration. Biomaterials 32,5810, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Albrecht C., Tichy B., Nurnberger S., Hosiner S., Zak L., Aldrian S., and Marlovits S.Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: a comparative study. Osteoarthritis Cartilage 19,1219, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Henderson J.H., Ginley N.M., Caplan A.I., Niyibizi C., and Dennis J.E.Low oxygen tension during incubation periods of chondrocyte expansion is sufficient to enhance postexpansion chondrogenesis. Tissue Eng Part A 16,1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claus S., Aubert-Foucher E., Demoor M., Camuzeaux B., Paumier A., Piperno M., Damour O., Duterque-Coquillaud M., Galera P., and Mallein-Gerie F.Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer cultures. J Cell Biochem 111,1642, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Pei M., and He F.Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol 227,2163, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenzweig D.H., Matmati M., Khayat G., Chaudhry S., Hinz B., and Quinn T.M.Culture of primary bovine chondrocytes on a continuously expanding surface inhibits dedifferentiation. Tissue Eng Part A 18,2466, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claus S., Mayer N., Aubert-Foucher E., Chajra H., Perrier-Groult E., Lafont J., Piperno M., Damour O., and Mallein-Gerin F.Cartilage-characteristic matrix reconstruction by sequential addition of soluble factors during expansion of human articular chondrocytes and their cultivation in collagen sponges. Tissue Eng Part C 18,104, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Jakob M., Demarteau O., Schafer D., Hintermann B., Dick W., Heberer M., and Martin I.Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem 81,368, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Lewis M.C., MacArthur B.D., Malda J., Pettet G., and Please C.P.Heterogeneous proliferation within engineered cartilaginous tissue: the role of oxygen tension. Biotechnol Bioeng 91,607, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Zhou S., Cui Z., and Urban J.P.G.Nutrient gradients in engineered cartilage: Metabolic kinetics measurement and mass transfer modeling. Biotechnol Bioeng 101,408, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Babur B.K., Ghanavi P., Levett P., Lott W.B., Klein T., Cooper-White J.J., Crawford R., and Doran M.R.The interplay between chondrocyte redifferentiation pellet size and oxygen concentration. PLoS One 8,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckley C.T., Vinardell T., and Kelly D.J.Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived mscs and articular chondrocytes. Osteoarthritis Cartilage 18,1345, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Ng K.W., Lima E.G., Bian L.M., O'Conor C.J., Jayabalan P.S., Stoker A.M., Kuroki K., Cook C.R., Ateshian G.A., Cook J.L., and Hung C.T.Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A 16,1041, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahlin R.L., Ni M., Meretoja V.V., Kasper F.K., and Mikos A.G.Tgf-β3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials 35,123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabatino M.A., Santoro R., Gueven S., Jaquiery C., Wendt D.J., Martin I., Moretti M., and Barbero A.Cartilage graft engineering by co-culturing primary human articular chondrocytes with human bone marrow stromal cells. J Tissue Eng Regen Med 2012[Epub ahead of print]; DOI: 10.1002/term.1661 [DOI] [PubMed] [Google Scholar]
- 45.Meretoja V.V., Dahlin R.L., Wright S., Kasper F.K., and Mikos A.G.The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 34,4266, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooke M.E., Allon A.A., Cheng T., Kuo A.C., Kim H.T., Vail T.P., Marcucio R.S., Schneider R.A., Lotz J.C., and Alliston T.Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthritis Cartilage 19,1210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.