Abstract
Despite the widespread use of replication-incompetent recombinant adenovirus (Ad) vectors as candidate vaccine platforms, the mechanism by which these vectors elicit CD8+ T cell responses remains poorly understood. Our data demonstrate that induction and maintenance of CD8+ T cell responses by Ad vector immunization is longitudinally dependent on CD4+ T cell help for a prolonged period. Depletion of CD4+ T cells in wild type mice within the first 8 d following Ad immunization resulted in dramatically reduced induction of Ag-specific CD8+ T cells, decreased T-bet and eomesodermin expression, impaired KLRG1+ effector differentiation, and atypical expression of the memory markers CD127, CD27, and CD62L. Moreover, these CD8+ T cells failed to protect against a lethal recombinant Listeria monocytogenes challenge. Depletion of CD4+ T cells between weeks 1 and 4 following immunization resulted in increased contraction of memory CD8+ T cells. These data demonstrate a prolonged temporal requirement for CD4+ T cell help for vaccine-elicited CD8+ T cell responses in mice. These findings have important implications in the design of vaccines aimed at eliciting CD8+ T cell responses and may provide insight into the impaired immunogenicity of vaccines in the context of AIDS and other CD4+ T cell immune deficiencies.
Introduction
Adenovirus (Ad) vectors have garnered significant attention as candidate vaccine platforms because of their large transgene coding capacity and potent immunogenicity. Ad vector–based vaccines are being pursued for a number of viral infections, including Ebola (1), influenza (2), hepatitis C (3, 4), rabies (5), and HIV-1 (6). We recently evaluated an adenovirus serotype 26 (Ad26) vector–based vaccine for HIV-1 in clinical trials (7, 8), and preclinical studies with Ad26-based vaccine regimens in nonhuman primates resulted in partial protection against acquisition of infection as well as virologic control following SIVmac251 challenges (9–11). Virologic control correlated with vaccine-elicited SIV-specific CD8+ T cell responses (9–12). However, relatively little is known about the CD4+ T cell requirement to generate CD8+ T cell memory responses after vaccination.
Prior reports have evaluated Ad vectors as candidate vaccine and gene therapy platforms and have identified a role for CD8+ T cells in the clearance of transduced cells (13). Several follow-up studies have demonstrated prolonged transgene expression in the absence of CD4+ T cells at the time of vector administration, thus providing evidence that CD4+ T cells play an important role in priming the CD8+ T cell response following Ad vector administration (13–16). More recent studies have demonstrated that the frequency of Ag-specific CD8+ T cells was impaired in the absence of CD4+ T cells at the time of vector administration (17, 18). Lack of CD4+ T cells also resulted in primary CD8+ T cell responses of low magnitude and function in several disease and vaccination models (19–22). In contrast, in certain viral and bacterial infections, CD8+ T cell responses were induced in the absence of CD4+ T cells, although the long-term functional potential and maintenance were still impaired (23–30). CD4+ T cell help has also been reported to be required at the time of priming to elicit CD8+ T cell responses with normal recall potential upon secondary Ag exposure (31–33). These studies show the requirement of CD4+ T cell help at the time of CD8+ T cell priming, but the temporal requirements of CD4+ T cell help for the generation of CD8+ T cell responses have not previously been determined.
In the current study, we sought to determine the temporal requirements of CD4+ T cell help for the development, maintenance, and functionality of memory CD8+ T cells induced by Ad26 (34) and chimeric Ad5 with hypervariable regions 1-7 of Ad48 (Ad5HVR48) (35) vectors expressing SIV Gag, SIV Env, and lymphocytic choriomeningitis virus (LCMV) GP Ags. We selected Ad26 and Ad5HVR48 vectors for detailed study because they are both currently being evaluated in phase I clinical trials as vaccine candidates. We found that CD4+ T cell help was required not only at the time of priming, but also for 8 d after immunization to drive the induction and optimal effector differentiation of the primary CD8+ T cell response. Moreover, CD4+ T cell help was required for 4 wk after immunization for controlling the contraction of memory CD8+ T cells.
Materials and Methods
Mice, immunizations, and challenge
Six- to ten-week-old C57BL/6, B6.SJL-ptprca (CD45.1+), B6.129S2-Cd4tm1Mak/J (CD4 knockout [KO]), B6.129S2-H2dlAb1-Ea/J (MHC II KO), B6.129S2-Cd40lgtm1Imx/J (CD40L KO), and B6.129P2-Cd40tm1Kik/J (CD40 KO) animals were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were immunized with the previously described E1/E3 deleted Ad26, or Ad5HVR48(1-7) vectors expressing SIV Gag or SIV Env from the strain SIVmac239 or LCMV GP (11, 34–36). Mice were immunized i.m. in the quadriceps with 109 viral particles of each vector in a volume of 100 μl divided equally between the two legs. For coadministration of SIV Gag and SIV Env–expressing vectors, the final injection volume of 100 μl was held constant. Mice were challenged with 1.75 × 105 to 2.5 × 105 CFU of recombinant Listeria monocytogenes expressing the LCMV epitope GP33-41 (Lm-GP33) by i.v. injection (a gift from Dr. Hao Shen) (37). Precise dose was back calculated following each experiment by plating on BHI-Agar plates, as described previously (38). All animal experiments were performed in accordance with Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee guidelines.
mAb administration
The monoclonal anti-CD4 Ab GK1.5 (BioXcell) was administered with two i.p. injections of 500 μg in a 500-μl volume on sequential days. One day after the second injection, blood was collected and CD4+ T cell depletion was confirmed (data not shown) by staining with the noncompeting anti-CD4 Ab clone RM4-4 (BD Biosciences).
Tissue processing and flow cytometry
Single-cell suspensions of tissues were generated as previously described, with slight modification (39). Liver tissue was not treated with EDTA or collagenase. PBMCs were isolated from whole blood using Ficoll-Hypaque density centrifugation at 1900 rpm for 20 min. MHC class I tetramer staining was performed using an H-2Db tetramer loaded with the immunodominant AL11 peptide (AAVKNWMTQTL) or GP33-41 peptide (KAVYNFATM) as described previously (40). Biotinylated class I monomers were provided by the National Institutes of Health Tetramer Core Facility (Emory University, GA). Background staining of cells from naive animals was ≤0.1%. Surface staining was performed with anti-CD8α (53-6.7), -CD4 (RM4-5), -CD44 (IM7), -CD127 (A7R34), -CD62L (MEL-14), -KLRG1 (2F1), -CD122 (TM-β1), and -CD27 (LG.3A10). Transcription factor staining was performed by first permeabilizing the cells with the Foxp3 Fixation/Permeabilization Kit (eBioscience) and subsequently staining with anti-T-bet (4B10). Annexin V staining was performed using an Annexin V staining kit (BioLegend). All Abs were purchased from BD Biosciences, eBioscience, or BioLegend. Vital exclusion dye was purchased from Invitrogen. After fixation, samples were acquired on an LSR II flow cytometer (BD Biosciences), and data were analyzed using FlowJo version 9.3.3 (Tree Star).
Intracellular cytokine staining
Intracellular cytokine staining was performed as previously described with slight modification (41). Briefly, 2 × 106 splenocytes were restimulated for 1.5 h at 37°C with 2 μg/ml AL11 peptide or 1 μg/ml of an overlapping SIVmac239 Gag or SIVmac239 Env peptide pool. At this time, anti-CD28 (37.51) and -CD49d (R1-2) Abs were added. After this incubation, Brefeldin A (BD Biosciences) was added and samples incubated for an additional 4.5 h at 37°C. Cells were subsequently washed, stained with surface Abs, permeabilized with Cytofix/Cytoperm (BD Biosciences), and stained with an anti–IFN-γ (XMG1.2) Ab.
CFSE labeling and adoptive cell transfer
Splenocytes were processed as described above. CD8+ T cells were enriched by negative selection using the CD8a+ Enrichment Kit II following the manufacturer’s instructions (>90% purity; Miltenyi Biotec). CFSE labeling was performed as described previously (42). After labeling, 1.5 × 104 Db/AL11+ CD8+ T cells were transferred with i.v. injection into congenic marker differentiated naive animals. For Fig. 8, CFSE labeling was not performed, and 5 × 104 Db/AL11+ CD8+ T cells were transferred; all other preparation steps were identical.
FIGURE 8.
CD40 signaling is required for programming CD8+ T cells with anamnestic potential. (A–C) C57BL/6, CD40L KO, and CD40 KO animals were immunized i.m. with 109 vp of Ad26-Gag (n = 8 per group pooled from two independent experiments). (A) The number of Db/AL11+ CD8+ T cells in the spleen of immunized animals on day 28 after immunization. (B) Splenocytes were harvested on day 28 after immunization and the number of IFN-γ+ CD8+ T cells was quantified following stimulation with an overlapping SIV Gag peptide pool. (C) KLRG1 and CD127 expression were assessed on splenic Db/AL11+ CD8+ T cells on day 28 after immunization. (D and E) C57BL/6, CD40L KO, and CD40 KO animals were immunized i.m. with 109 vp of Ad26-Gag and on day 56 after priming were boosted with 109 vp of Ad5HVR48-Gag (n = 4 per group from one experiment). (D) The frequency of Db/AL11+ CD8+ T cells per 106 PBMCs after the boost was assessed. (E) The fold-change in frequency of Db/AL11+ CD8+ T cells per 106 PBMCs from 1 d before boost to day 7 after boost was assessed. (F) C57BL/6 or CD40L KO animals were immunized i.m. with 109 vp of Ad26-Gag. On day 50 after immunization, 5 × 104 Db/AL11+ CD8+ T cells were enriched by negative selection and transferred i.v. to congenically marked (CD45.1+) naive recipients (n = 8–9 per group from two independent experiments). One day after transfer, recipient mice were immunized i.m. with 109 vp of Ad5HVR48-Gag. On day 12 after secondary immunization, the number of donor Db/AL11+ CD45.2+ CD8+ T cells in PBMCs, spleen, and liver was assessed. Mean ± SEM are shown. #p = 0.06, *p < 0.05.
RNA extraction, cDNA synthesis, and quantitative PCR
Splenocytes were processed as described above. CD8+ T cells were enriched by negative selection using the CD8a+ enrichment kit II following the manufacturer’s instructions (>90% purity; Miltenyi Biotec). Db/AL11+ CD8+ T cells were subsequently sorted to >95% purity on a FACSAria (BD Biosciences). Cells were centrifuged at 10,000 rpm for 5 min, and pellets were resuspended in 1 ml TRIzol (Invitrogen) and stored at −80°C for further processing. RNA extraction and cDNA synthesis were performed as described previously (43, 44). RNA extraction was performed using the RNAdvance Tissue Isolation kit (Agencourt). cDNA synthesis was performed using the Ovation Pico WTA v2 kit (NuGEN) per the manufacturer’s instructions. Quantitative PCR was performed using the SYBR Green quantification system (Qiagen). The following primers were used: Gapdh forward 5′-AGGTCGGTGTGAACGGATTTG -3′ and reverse 5′-TGTAGACCATGTAGTTGAGGTCA -3′ (45); Prdm1 forward 5′-CACACAGGAGAGAAGCCACA-3′ and reverse 5′-TTGATTCGGGTCAGATCCTC-3′ (46); Bcl6 forward 5′-CGCAACTCTGAAGAGCCACCTGCG-3′ and reverse 5′-TTTGTGACGGAAATGCAGGTTA-3′ (47); Tbx21 forward 5′-AGCAAGGACGGCGAATGTT-3′ and reverse 5′-GGGTGGACATATAAGCGGTTC-3′ (48); Eomes forward 5′-TGAATGAACCTTCCAAGACTCAGA-3′ and reverse 5′-GGCTTGAGGCAAAGTGTTGACA-3′ (49); Id2 forward 5′-ACCAGAGACCTGGACAGAAC-3′ and reverse 5′-AAGCTCAGAAGGGAATTCAG-3′ and Id3 forward 5′-GACTCTGGGACCCTCTCTC-3′ and reverse 5′-ACCCAAGTTCAGTCCTTCTC-3′ (50); and Tcf7 forward 5′-AGCTTTCTCCACTCTACGAACA-3′ and reverse 5′-AATCCAGAGAGATCGGGGGTC-3′ (51). Data were acquired on a StepOnePlus Real-Time PCR System (Applied Biosystems). Cycle conditions were: 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Gene expression was normalized across samples by Gapdh expression levels.
Statistical analysis
Statistical analysis was performed using a two-tailed nonparametric Mann–Whitney U test or Mantel–Cox test using Prism version 6.0c (GraphPad Software). In all cases, the CD4+ T cell–deficient animals or CD40/L KO animals were compared with the wild type and untreated controls.
Results
CD4+ T cell help is required for the induction of CD8+ T cell responses following Ad vector immunization
We initially assessed the requirement for CD4+ T cell help for the induction of primary CD8+ T cell responses following Ad26 vector immunization (34). C57BL/6, CD4 KO, or MHC class II KO mice were immunized i.m. with 109 viral particles (vp) of Ad26 expressing SIV Gag (Ad26-Gag). After immunization, robust Gag-specific CD8+ T cell responses were detected in wild type animals by CD8+ T cells binding to the immunodominant Db-restricted AL11 peptide (Db/AL11) tetramer (40) (Fig. 1A, 1B). This response peaked at day 14 after immunization and subsequently contracted, consistent with prior observations (34, 40). In contrast, in CD4+ T cell–deficient animals (CD4 KO or MHC class II KO), the AL11 epitope-specific CD8+ T cell responses were largely abrogated (p < 0.01; Fig. 1A, 1B).
FIGURE 1.
CD4+ T cell help is required for the development of CD8+ T cell responses following i.m. Ad vaccination. (A and B) C57BL/6, CD4 KO, or MHC class II KO mice were immunized i.m. with 109 vp of Ad26-Gag or were unimmunized (naive C57BL/6). Animals were bled longitudinally, and SIV Gag-specific CD8+ T cells were quantified by tetramer staining. Representative plots (A) and group averages (B) for Db/AL11+ cells as a percent of CD8+ T cells are shown. Data are from n = 8 per group (pooled from two independent experiments). (C and D) C57BL/6, CD4 KO, or MHC class II KO mice were coimmunized i.m. with 109 vp each of Ad26-Gag and Ad26-Env. Splenocytes were harvested on day 28 after immunization and stimulated with the indicated peptide pool. Representative plots of IFN-γ+ CD8+ T cells (C) and group averages (D) are shown. Data are from n = 8 per group (pooled from two independent experiments). (E) C57BL/6, CD4 KO, or MHC class II KO mice were coimmunized i.m. with 109 vp each of Ad5HVR48-Gag and Ad5HVR48-Env. Splenocytes were harvested on day 28 after immunization and stimulated with the indicated peptide pool. Percent of IFN-γ+ cells as a fraction of CD8+ T cells are shown. Data are from n = 8 per group (pooled from two independent experiments). Mean ± SEM are shown. *p < 0.01.
To confirm that these observations were generalizable to multiple epitopes, C57BL/6, CD4 KO, and MHC class II KO animals were immunized with 109 vp each of Ad26 vectors expressing SIV Gag and SIV Env. Twenty-eight days after immunization, splenocytes were harvested, and IFN-γ production by CD8+ T cells was assessed by ex vivo restimulation with overlapping SIV Gag and SIV Env peptide pools. Gag- and Env-specific responses were detected in wild type animals by the percentage of SIV-specific IFN-γ+ CD8+ T cells (Fig. 1C, 1D). The frequency of Gag- and Env-specific IFN-γ+ CD8+ T cells was significantly impaired in CD4 KO and MHC class II KO mice as compared with wild type animals (p < 0.01; Fig. 1C, 1D). These data confirm that the defect seen in CD8+ T cell responses in the absence of CD4+ T cells by Db/AL11 tetramer binding was generalizable to multiple Ags.
To confirm that our observations with Ad26-based vectors were generalizable to other Ad vector serotypes, C57BL/6, CD4 KO, and MHC class II KO animals were immunized with 109 vp each of Ad5HVR48-Gag and Ad5HVR48-Env (11, 35). Concordant with the data generated after Ad26 immunization, the Gag- and Env-specific CD8+ IFN-γ+ T cell responses following Ad5HVR48 immunization were significantly impaired on day 28 after immunization in CD4 KO and MHC II KO animals as compared with wild type animals (p < 0.01; Fig. 1E). Collectively, these data demonstrate that the induction of primary CD8+ T cell responses following Ad vector immunization critically requires CD4+ T cell help at the time of priming.
Longitudinal interruption of CD4+ T cell help impairs the expansion and increases contraction of CD8+ T cell responses
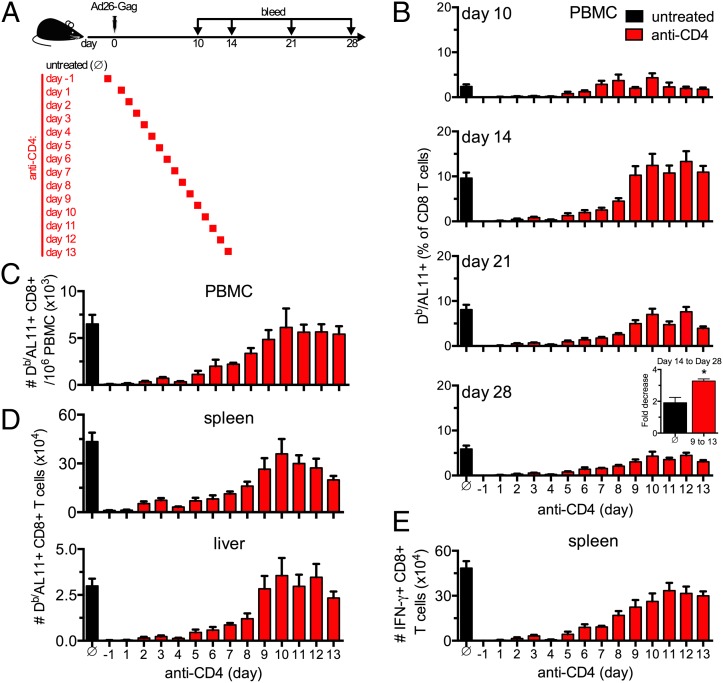
We next sought to determine for how long CD4+ T cell help was required after immunization for the induction of CD8+ T cell responses. To accomplish this, CD4+ T cells were depleted by administration of the monoclonal anti-CD4 Ab GK1.5 on two consecutive days starting at various time points following immunization (52). Depletion of CD4+ T cells was begun prior to immunization (day −1) or on day 1–13 after immunization (Fig. 2A). All mice were immunized i.m. with 109 vp of Ad26-Gag on day 0, and PBMCs were evaluated longitudinally to assess Db/AL11+ CD8+ T cell responses.
FIGURE 2.
Temporal requirement for CD4+ T cell help for the induction and expansion of CD8+ T cell responses following Ad vaccination. (A) C57BL/6 mice were depleted of CD4+ T cells by i.p. administration of mAb GK1.5 (anti-CD4) beginning on days −1, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, or 13 and immunized i.m. with 109 vp of Ad26-Gag on day 0. Untreated controls are designated by a circle with a diagonal line though it. (B) Mice from each treatment group were bled at the indicated time points, and AL11-specific CD8+ T cells were assessed by Db/AL11 staining. Inset, the fold-decrease in the number of AL11-specific CD8+ T cells in blood from day 14 to 28 after immunization is shown. Groups of mice administered with anti-CD4 beginning on day 9–13 were pooled for analysis. (C and D) On day 28 after immunization, the total number of AL11 peptide-specific CD8+ T cells per 106 PBMCs (C) or per tissue (D) were quantified by Db/AL11 staining. (E) On day 28 after immunization, the total number of AL11 peptide-specific CD8+ T cells was assessed by ex vivo restimulation with AL11 peptide followed by intracellular cytokine staining for IFN-γ. Data are from n = 7–22 per group (pooled from two to six independent experiments). Mean ± SEM are shown. *p < 0.001.
Db/AL11+ CD8+ T cell responses in the untreated group were detected on day 7 (data not shown), peaked on day 14, and contracted by day 28 (Fig. 2B, black bar). Depletion of CD4+ T cells prior to immunization completely ablated the Db/AL11+ CD8+ T cell response (Fig. 2B–D), consistent with the data from CD4 KO and MHC II KO animals in the previous experiment (Fig. 1A). Administration of an isotype control Ab had no effect on CD4+ T cell frequency or the Db/AL11-specific CD8+ T cell response (data not shown). Depletion of CD4+ T cells starting on day 1–8 after immunization resulted in dramatically lower Db/AL11+ CD8+ T cell responses from day 14 onward (p < 0.05; Fig. 2B, 2C). Earlier depletion of CD4+ T cells led to more pronounced impairment of Db/AL11+ CD8+ T cell responses. Mice that were depleted of CD4+ T cells on or after day 9 exhibited initial CD8+ T cell response magnitudes that were comparable to those for untreated animals (Fig. 2B, 2C). These data suggest a critical requirement for CD4+ T cell help for the induction of CD8+ T cell responses for 8 d following immunization.
We next examined Db/AL11+ CD8+ T cell responses in various tissues. Consistent with the data from peripheral blood, Db/AL11+ CD8+ T cell responses in both spleen and liver on day 28 were also reduced in animals depleted of CD4+ T cells with similar kinetics (Fig. 2D). Moreover, the absolute number of functional AL11-specific IFN-γ+ CD8+ splenocytes on day 28 was also reduced in animals depleted of CD4+ T cells as compared with untreated controls (Fig. 2E).
We next asked whether CD4+ T cells were also important during the contraction phase of the CD8+ T cell responses. Animals depleted of CD4+ T cells beginning on day 9–13 exhibited equivalent initial Db/AL11+ CD8+ T cell magnitudes as compared with untreated controls (day 14). However, we observed greater contraction of the Gag-specific CD8+ T cell responses (p < 0.001; Fig. 2B inset) in the mice depleted of CD4+ T cells on day 9–13 as compared with untreated controls, suggesting a requirement for longitudinal CD4+ T cell help for controlling the contraction of CD8+ T cell responses.
Longitudinal interruption of CD4+ T cell help alters effector differentiation and survival of CD8+ T cells
We next examined the phenotype of Db/AL11+ CD8+ T cells from mice that were depleted of CD4+ T cells on day 4–13 after immunization. Of note, depletion prior to day 4 resulted in insufficient Ag-specific CD8+ T cells for analysis. Terminal effector and memory precursor cells were differentiated by surface marker expression of KLRG1+ and CD127+, respectively, as reported previously (53–55). On day 28 after immunization, the frequency of Db/AL11+ CD8+ T cells that were KLRG1+CD127− terminal effector cells was reduced in mice depleted of CD4+ T cells, with a corresponding increase in the frequency that were KLRG1-CD127+ memory precursor cells (p < 0.05; Fig. 3A, 3B). The earlier CD4+ T cells were depleted, the lower the frequency of terminal effector cells and the greater the frequency of potential memory precursor cells. This trend was also observed in Db/AL11+ CD8+ T cells from the blood and liver (Fig. 3C, 3D). No differences in the proportion of Db/AL11+ CD8+ T cells that were KLRG1+CD127+ or KLRG1−CD127− was observed between the groups (Fig. 3A). Depletion of CD4+ T cells after day 8 did not appear to affect these phenotypic markers. Overall, there was a decrease in the absolute number of both the KLRG1+CD127− and KLRG1−CD127+ populations of Db/AL11+ CD8+ T cells in the spleens of mice depleted of CD4+ T cells prior to day 8 (Fig. 3E). Thus, the altered frequency of KLRG1+CD127− and KLRG1−CD127+ cells is not due to a specific survival defect of one subset, but instead may reflect altered differentiation of CD8+ T cells in mice depleted of CD4+ T cells postimmunization.
FIGURE 3.
Effector differentiation of CD8+ T cells is dependent on CD4+ T cell help following Ad vaccination. C57BL/6 mice were depleted of CD4+ T cells by i.p. administration of mAb GK1.5 (anti-CD4) beginning on days 4, 5, 6, 7, 8, 9, 10, 11, 12, or 13 or were left untreated, and were immunized i.m. with 109 vp of Ad26-Gag on day 0. On day 28 after immunization, animals were sacrificed and tissues were harvested. KLRG1 and CD127 expression on Db/AL11+ CD8+ T cells was assessed. (A and B) Representative flow plots (A) and group averages (B) of KLRG1 and CD127 expression on Db/AL11+ CD8+ T cells in the spleen. (C and D) Group summary of KLRG1 and CD127 expression of Db/AL11+ CD8+ T cells in blood (C) and liver (D). (E) Absolute number of KLRG1+CD127− or KLRG1−CD127+ Db/AL11+ CD8+ T cells in the spleen. Anti-CD4 treatment on days −1, 1, 2, and 3 after immunization had insufficient numbers of Db/AL11+ cells for analysis. Data are from n = 8–16 per group (pooled from two to four independent experiments), except for PBMC anti-CD4 day 9, 10, and 12 groups, which are n = 3–4 per group (one experiment). Mean ± SEM are shown. *p < 0.05.
Given the increase in the percentage of Db/AL11+ CD8+ T cells that were KLRG1−CD127+ following CD4+ T cell depletion from day 4–7 after immunization, we sought to determine whether CD4+ T cell depletion also altered the expression of other memory-associated surface markers. Expression of CD62L and CD27 has been correlated with a long-term memory phenotype (39, 56). We examined the expression of these markers on Db/AL11+ CD8+ T cells from the spleen at day 28 after immunization of untreated controls or animals that were depleted of CD4+ T cells on day 5 (reduced peak CD8+ T cell response and reduced terminal effector phenotype), day 8 (reduced peak CD8+ T cell response but normal terminal effector phenotype), or day 11 (normal peak CD8+ T cell response and normal terminal effector phenotype) after immunization. Depletion of CD4+ T cells on day 8 or 11 after immunization progressively led to a significant reduction in CD27 expression on Db/AL11-specific CD8+ T cells (p < 0.05; Fig. 4A). Conversely, depletion of CD4+ T cells resulted in an increase in the fraction of Db/AL11+ CD8+ T cells that were CD62L+ as compared with the untreated controls (p < 0.03; Fig. 4A), and earlier CD4+ T cell depletion resulted in higher CD62L expression. These data suggest that the absence of CD4+ T cell help led to dysfunctional expression of memory-associated markers and did not in fact accelerate conversion to memory precursor cells.
FIGURE 4.
CD8+ T cells in mice depleted of CD4+ T cells exhibit an abnormal phenotype after i.m. Ad vaccination. C57BL/6 mice were depleted of CD4+ T cells by i.p. administration of mAb GK1.5 (anti-CD4) on days 5, 8, or 11 or left untreated and immunized i.m. with 109 vp of Ad26-Gag. On day 28 after immunization, animals were sacrificed and splenocytes were isolated. (A and B) Expression level of the indicated protein as a percentage (A) or mean fluorescence intensity (B) on gated Db/AL11+ cells. Dashed lines indicate CD44lo CD8+ (naive) T cells, and solid-shaded histograms indicate Db/AL11+ CD8+ T cells. Data are from n = 8–16 per group (pooled from two to four independent experiments). (C) Gene expression of sorted Db/AL11+ CD8+ T cells from untreated animals or animals treated with GK1.5 (anti-CD4) on days 5 or 8 after immunization was determined by quantitative real-time PCR. Gene expression was normalized to Gapdh. Data are from n = 4–6 per group (pooled from two independent experiments). (D) Annexin V expression and uptake of vital dye by Db/AL11+ cells was assessed. Data are from n = 8 per group (pooled from two independent experiments). Mean ± SEM are shown. #p = 0.067, *p < 0.05.
To characterize further the effect of CD4+ T cell depletion on effector phenotypes, we investigated the expression of transcriptional regulators that drive CD8+ T cell effector differentiation. The transcription factors T-bet, Eomesodermin (Eomes), and T cell factor 1, as well as the transcriptional regulators Blimp-1, Bcl-6, Id2, and Id3 have reported roles in dictating effector versus memory differentiation of CD8+ T cells (49, 50, 57–63). On day 28 after immunization, we assessed the expression of these transcriptional regulators in sorted Db/AL11+ CD8+ T cells from the spleen of untreated controls, or in mice depleted of CD4+ T cells on days 5 or 8 after immunization. We observed by quantitative RT-PCR that Tbx21 (encodes T-bet) expression by Db/AL11+ CD8+ T cells from mice depleted of CD4+ T cells on day 5 was 4-fold downregulated relative to the untreated controls (p = 0.067; Fig. 4C). This group also exhibited a 10-fold downregulation of Eomes and Bcl6 as compared with untreated controls (p < 0.04; Fig. 4C). We observed only modest differences in the expression of Prdm1 (encodes Blimp-1), Id2, Id3, and Tcf7 (encodes T cell factor 1; Fig. 4C). No significant differences were observed in mice depleted of CD4+ T cells on day 8 after immunization. We confirmed the reduced expression of T-bet on Db/AL11+ CD8+ T cells by flow cytometry from animals depleted of CD4+ T cells as compared with untreated controls (p < 0.001; Fig. 4B). Eomes and T-bet have been demonstrated to act in concert to promote effector differentiation and expression of CD122 (49). Consistent with this, in animals depleted of CD4+ T cells on day 5 after immunization, there was decreased expression of CD122 as compared with untreated controls (p = 0.038; Fig. 4A). These data suggest that the defect in effector phenotype seen after depletion of CD4+ T cells is consistent with aberrant expression of transcriptional regulators involved in effector CD8+ T cell differentiation.
Our earlier findings identified that the contraction of Db/AL11+ CD8+ T cell responses were increased following CD4+ T cell depletion postimmunization (Fig. 2B). To investigate the mechanism of this increased contraction, we evaluated expression of the apoptotic marker Annexin V on CD8+ T cells on day 28 after immunization from mice depleted of CD4+ T cells on day 5, 8, or 11 postimmunization. We observed that the Ag-specific CD8+ T cells from these mice depleted of CD4+ T cells exhibited increased Annexin V expression (54%, 41%, and 21%, respectively), as compared with 15% of Db/AL11+ CD8+ T cells from the untreated control animals (p < 0.04; Fig. 4D). These data suggest that CD4+ T cells play a key role the induction and survival of Ag-specific CD8+ T cell responses (27).
CD8+ T cells primed with reduced CD4+ T cell help fail to protect against lethal recombinant L. monocytogenes challenge
We next sought to determine the effects of longitudinal depletion of CD4+ T cells on the ability of vaccine-elicited CD8+ T cells to protect against a lethal recombinant L. monocytogenes challenge. To accomplish this, we used Ad26-GP followed by challenge on day 30 following immunization with Lm-GP33 (Fig. 5A). Longitudinal depletion of CD4+ T cells following immunization with 109 vp of Ad26-GP resulted in a reduced frequency of Db/GP33+ CD8+ T cells (Fig. 5B), consistent with our previous findings (Fig. 2). No Db/GP33+ CD8+ T cells were detected when depletion of CD4+ T cells was performed on days −1 or 5, with progressively less impairment after depletion of CD4+ T cells on days 8 or 11. Depletion of CD4+ T cells on day 8 after immunization resulted in altered expression on KLRG1 and CD127 on Db/GP33+ CD8+ T cells, and to a lesser degree when depletion of CD4+ T cells was performed on day 11 (Fig. 5C).
FIGURE 5.
CD8+ T cells primed with reduced CD4+ T cell help fail to protect against lethal recombinant L. monocytogenes challenge. (A) C57BL/6 mice were depleted of CD4+ T cells by i.p. administration of mAb GK1.5 (anti-CD4) on day 8 or left untreated, and immunized with 109 vp of Ad26-GP. On day 30 after immunization, mice were challenged with 1.75 × 105 to 2.5 × 105 CFU Lm-GP33. (B) Frequency of Db/GP33+ CD8+ T cells in the blood on day 30 after immunization. (C) KLRG1 and CD127 expression on Db/GP33+ CD8+ T cells in the blood on day 30 after immunization. (D) Survival of mice after Lm-GP33 challenge. Data are from n = 16–32 per group (A–C) or n = 7–19 per group (D) pooled from two to four independent experiments. Mean ± SEM are shown. #p = 0.08, *p < 0.05.
Challenge of unvaccinated mice with Lm-GP33 resulted in 100% mortality by day 4 (Fig. 5D). In contrast, mice immunized 30 d prior with Ad26-GP exhibited a significant delay and reduction in mortality compared with unvaccinated controls (p < 0.0001; Fig. 5D). Mice immunized with Ad26-GP and depleted of CD4+ T cells on day 8 (impaired frequency and altered phenotype) exhibited mortality comparable to that in unvaccinated control animals (p = 0.13) and significantly impaired protective efficacy compared with vaccinated untreated mice (p = 0.02; Fig. 5D). These data demonstrate that depletion of CD4+ T cells 8 d after immunization resulted in CD8+ T cells that failed to protect against a lethal recombinant L. monocytogenes challenge.
Prolonged requirement for CD4+ T cell help for controlling the contraction of CD8+ T cell responses
Because depletion of CD4+ T cells on days 9–13 during the expansion phase of the immune response led to an increased contraction of Ag-specific CD8+ T cells (Fig. 2B) and increased apoptosis (Fig. 4D), we sought to determine how long CD4+ T cell help was required following immunization to regulate the contraction of Ag-specific CD8+ T cells. Mice were depleted of CD4+ T cells on day −1, 7, 14, 21, or 28 relative to immunization with 109 vp of Ad26-Gag on day 0 (Fig. 6A). Consistent with previous experiments, depletion of CD4+ T cells on day −1 resulted in complete abrogation of Db/AL11+ CD8+ T cell responses, and depletion on day 7 resulted in markedly lower Db/AL11+ CD8+ T cell responses relative to untreated controls (Fig. 6B). Depletion of CD4+ T cells on days 14, 21, or 28 resulted in more rapid decay of the Db/AL11+ CD8+ T cell response as compared with untreated mice as a function of the day of depletion (Fig. 6B). On day 90, the frequency of Db/AL11+ CD8+ T cells in untreated control animals was 3.74% of CD8+ T cells, whereas the frequency of Db/AL11+ CD8+ T cells was significantly lower in animals depleted of CD4+ T cells on day −1 (0.04%, below the limit of detection; p = 0.002), day 7 (1.97%; p = 0.005), day 14 (2.0%; p = 0.016), or day 21 (1.84%; p = 0.0007), and a trend was observed in animals depleted of CD4+ T cells on day 28 (2.79%; p = 0.11; Fig. 6B). When the magnitude of the response on day 90 was normalized to the peak of the response on day 14, the degree of contraction of the Db/AL11+ CD8+ T cell responses was significantly greater in mice depleted of CD4+ T cells on days 14 or 21 after immunization (p < 0.05), and a trend was observed in mice depleted of CD4+ T cells on day 28 after immunization (p = 0.08; data not shown). Thus, depletion of CD4+ T cells on day 7, 14, or 21 led to a significant reduction in the frequency of Db/AL11+ CD8+ T cells on day 90 as compared with untreated controls. However, the effect of this increased contraction of vaccine-elicited CD8+ T cells on protective efficacy remains to be determined.
FIGURE 6.
Normal contraction of the CD8+ T cell response requires prolonged CD4+ T cell help. (A) C57BL/6 mice were depleted of CD4+ T cells by i.p. administration of mAb GK1.5 (anti-CD4) on days −1, 7, 14, 21, or 28 or left untreated, and immunized i.m. with 109 vp of Ad26-Gag. (B) Animals were bled longitudinally, and Gag-specific CD8+ T cells were quantified by Db/AL11 tetramer staining. (C) Frequency of KLRG1 and CD127 expression on Db/AL11+ CD8+ T cells in blood. Data are from n = 8–12 per group (pooled from two to three independent experiments). Mean ± SEM are shown. *p < 0.05.
Given our previous observations of abnormal phenotypes of Db/AL11+ CD8+ T cells following early interruption of CD4+ T cell help (Figs. 3, 4), we assessed the long-term expression of KLRG1 and CD127 in animals depleted of CD4+ T cells on days −1, 7, 14, 21, or 28. Depletion of CD4+ T cells on day 7 resulted in CD8+ T cells that did not undergo the normal phenotypic evolution observed in the untreated controls. The frequency of Db/AL11+ CD8+ T cells that expressed KLRG1 and CD127 failed to increase over time in the mice depleted of CD4+ T cells on day 7 and was significantly lower at days 50 and 90 after immunization as compared with the untreated controls (p < 0.05; Fig. 6C). Animals depleted of CD4+ T cells on days 14, 21, or 28 after immunization exhibited a less abnormal phenotypic evolution (Fig. 6C), consistent with the early need for CD4+ T cell help to program this process. These data demonstrate a prolonged role for CD4+ T cells in controlling the contraction of Ag-specific CD8+ T cell responses.
CD8+ T cells that receive at least 8 d of CD4+ T cell help exhibit normal anamnestic potential
Given that the provision of CD4+ T cell help for at least 8 d after immunization resulted in CD8+ T cells that exhibited a largely normal phenotype, we sought to determine whether these cells also had acquired the normal memory characteristic of anamnestic expansion upon Ag re-exposure. Therefore, we performed adoptive transfer experiments of AL11-specific CD8+ T cells from mice depleted of CD4+ T cells into naive animals followed by secondary Ag exposure. As outlined in Fig. 7A, CD45.1+ congenic mice immunized with 109 vp of Ad26-Gag were either untreated or depleted of CD4+ T cells on days 8, 11, or 14. On day 28 after immunization, splenic CD8+ T cells were purified and equal numbers of CFSE-labeled Db/AL11+ CD8+ T cells were transferred into CD45.2+ congenic mice. One day after cell transfer, recipient animals were immunized with 109 vp of Ad5HVR48-Gag, and the expansion of Db/AL11+ CD45.1+ CD8+ T cells was evaluated. Eight days after immunization, the Db/AL11+ CD8+ CD45.1+ donor T cells from untreated controls and the donor cells from mice depleted of CD4+ T cells expanded to nearly equivalent levels (Fig. 7B, 7D). All the Db/AL11+ CD8+ CD45.1+ donor T cells had also completely diluted CFSE by day 8 after boost (Fig. 7C), which is consistent with the robust expansion of the population. These data suggest two possible options: either CD4+ T cells are not required to program anamnestic responses in the Ad system (17) or this CD4+ T cell–mediated anamnestic programming occurs within 8 d of immunization (27, 64, 65).
FIGURE 7.
CD8+ T cells primed with at least 8 d of CD4+ T cell help proliferate upon secondary Ag exposure. (A) CD45.1+ mice were depleted of CD4+ T cells by i.p. administration of mAb GK1.5 (anti-CD4) on days 8, 11, or 14 or left untreated, and immunized i.m. with 109 vp of Ad26-Gag (day 0). On day 28 postimmunization, CD8+ T splenocytes were pooled, enriched by negative selection, and labeled with CFSE; 1.5 × 104 Db/AL11+ CD8+ T cells were transferred by tail vein injection into naive CD45.2+ recipients. One day after transfer, animals were immunized i.m. with 109 vp of Ad5HVR48-Gag. (B) Representative plots of Db/AL11+ CD45.1+ donor cells as a fraction of CD8+ T cells in the spleen on days 8 or 14. (C) Representative histograms of CFSE dilution in the donor Db/AL11+ CD45.1+ CD8+ T cell population on day 8 after boost (open histogram) or the donor CD45.1+ CD8+ T cell population with no boosting immunization (solid histogram). (D) Absolute number of Db/AL11+ CD45.1+ CD8+ T cells in the spleen on days 8 or 14 after immunization. Frequency of donor Db/AL11+ CD45.1+ CD8+ T cells at the time of immunization (day 0) is based on 10% engraftment. Data are from n = 6–13 recipient mice per group on day 8 (pooled from three independent experiments) and n = 5 recipient mice per group on day 14 (from one experiment). Mean ± SEM are shown.
CD40 signaling pathway is not critical for initial CD8+ T cell priming but is required for anamnestic CD8+ T cell expansion
Several reports have identified the CD40 signaling pathway as a major mechanism for the provision of CD4+ T cell help, both for the generation of primary CD8+ T cell responses and for the programming of anamnestic responses (21, 31–33, 66, 67). Therefore, we evaluated the importance of this signaling pathway for the generation of Ad vector–elicited CD8+ T cell responses. C57BL/6, CD40L KO, and CD40 KO mice were immunized i.m. with 109 vp of Ad26-Gag. On day 28 after immunization, no significant differences in the number of Db/AL11+ CD8+ T cells between wild type and CD40 pathway–deficient (CD40L KO or CD40 KO) animals were observed (Fig. 8A). Consistent with this, the absolute number of Gag-specific CD8+ T cells that secreted IFN-γ was comparable between the wild type and CD40L KO and CD40 KO animals (Fig. 8B). Moreover, splenic Db/AL11+ CD8+ T cells from wild type, CD40L KO, and CD40 KO animals expressed equivalent levels of KLRG1 and CD127 (Fig. 8C). Collectively, these data suggest that signaling via CD40 is not critical for the initial generation of Ad vector–elicited primary CD8+ T cell responses.
To determine the role of CD40 signaling in the generation of anamnestic CD8+ T cell responses, Ad26-Gag primed C57BL/6, CD40L KO, and CD40 KO animals were boosted with 109 vp of Ad5VHR48-Gag on day 56. The frequency of Db/AL11+ CD8+ T cells in the blood prior to priming was equivalent between groups (Fig. 8D). Upon boosting, the Db/AL11+ CD8+ T cell population in wild type animals expanded 15-fold by day 7 after boosting and had begun to contract by day 14 (Fig. 8D, 8E). However, in CD40L KO and CD40 KO animals, the Db/AL11+ CD8+ T cell population expanded significantly less by day 7 after boosting than in wild type animals (p < 0.03; Fig. 8D, 8E).
Finally, to determine whether signaling via CD40 was required at the time of priming or boosting, on day 50, equal numbers of Db/AL11+ CD8+ T cells from wild type or CD40L KO Ad26-Gag-immunized animals were transferred into congenic (CD45.1+) naive hosts (Fig. 8F). After an Ad5HVR48-Gag immunization, the recall potential of donor Db/AL11+ CD8+ T cells was assessed. The number of donor Db/AL11+ CD8+ T cells from CD40L KO animals was significantly reduced after Ad5HVR48-Gag immunization in peripheral blood, spleen, and liver compared with donor Db/AL11+ CD8+ T cells from wild type animals (p < 0.05; Fig. 8F). These data suggest that although CD40-derived signals are not required for the generation of a primary CD8+ T cell response, CD40 is critical for programming anamnestic potential upon secondary Ag challenge. Collectively these experiments demonstrate a prolonged and multifaceted role for CD4+ T cell help to generate optimal Ad vector–induced CD8+ T cell responses (Fig. 9).
FIGURE 9.
Schematic representation of longitudinal requirement for CD4+ T cell help. Summary of longitudinal requirement for CD4+ T cell help for the induction of CD8+ T cell responses, effector differentiation, and normal contraction following Ad vector vaccination. The requirement for CD4+ T cell help was prolonged but waned over time.
Discussion
In this study, we demonstrate that immunization with Ad vectors requires CD4+ T cell help for the induction and optimal sustainment of CD8+ T cell responses, not only at the time of priming but also for a prolonged period of time following immunization. We observed that CD4+ T cell help was required for 8 d after immunization for primary induction of CD8+ T cell responses and normal effector differentiation. Moreover, CD4+ T cells were critical for 4 wk after immunization for controlling the contraction of CD8+ T cell responses. CD40 signaling was required to program CD8+ T cells with anamnestic potential, but did not appear critical for initial CD8+ T cell priming. Taken together, these data demonstrate that the need for CD4+ T cell help to generate Ad vector–induced CD8+ T cell responses is multifaceted and prolonged over time (Fig. 9).
Our data suggest that Ad vector–based vaccines and potentially also other vaccines can exhibit reduced cellular immunogenicity in patients with reduced CD4+ T cell function, such as in patients with advanced AIDS or following hematopoietic stem cell transplantation as a result of insufficient CD4+ T cell help. Consistent with this hypothesis, patients with HIV-1 infection and patients undergoing hematopoietic stem cell transplantation have been reported to develop impaired Ab responses after vaccination against influenza virus, hepatitis B virus, Haemophilus influenza type B, tetanus-reduced diphtheria-reduced pertussis, and yellow fever virus (68–72). Most previous studies have focused on vaccine-elicited Ab responses, and the requirement for CD4+ T cell help to generate CD8+ T cell responses by vaccination has remained poorly understood. Our data suggest that prolonged CD4+ T cell help is required for the induction and sustainment of optimal vaccine-elicited cellular immune responses.
Prior studies have reported that CD4+ T cell help is required at the time of priming to generate robust Ad vector–elicited CD8+ T cell responses (17, 18). However, the timing of CD4+ T cell help for the development of primary CD8+ T cell responses has not been previously reported to the best of our knowledge. Our data confirm and extend the observation of a requirement for CD4+ T cell help at the time of vaccine priming with multiple serotypes of Ad vectors. Longitudinal depletion of CD4+ T cells identified a prolonged role for CD4+ T cell help in the expansion and contraction of CD8+ T cell responses. In contrast, Yang et al. (17) did not detect any differences in CD127, CD62L, and CD122 between Ad5-elicited Ag-specific CD8+ T cells that did or did not receive CD4+ T cell help. This difference might reflect the inherent difference between the ability of Ad5- and Ad26-induced CD8+ T cells to express memory markers, as has been reported recently (36, 73). Furthermore, the largest differences in phenotype observed in the current study related to KLRG1 expression, which was not assessed in this prior study (17).
A number of reports have identified that pathogens and vaccines can be broadly divided into two categories based on whether CD4+ T cell help is required for induction of primary CD8+ T cell responses (reviewed in Ref. 30). Most candidate vaccine platforms (including Ad vectors) fall into the category of requiring CD4+ T cell help for the induction of primary CD8+ T cell responses. In contrast, the widely used viral infection model LCMV generates a primary CD8+ T cell response in the absence of CD4+ T cell help (23). After LCMV infection of CD4 KO animals, the CD8+ T cell response is initially of normal magnitude, but has impaired memory potential characterized by T-bet–mediated suppression of CD127, CD27, and CD62L expression (74). In contrast, we identified that after Ad vector immunization, the absence of CD4+ T cells resulted in impaired effector differentiation (KLRG1 and CD122) and atypical memory marker expression (CD127, CD27, and CD62L), and this corresponded to a decrease in T-bet and Eomes expression. These data suggest that in the absence of CD4+ T cell help Ad vector–induced CD8+ T cell responses fail to receive the necessary signals to upregulate T-bet and Eomes, and hence do not properly initiate the program of effector differentiation. Thus, it appears that in addition to an ability to drive CD4+ T cell–independent expansion, a fundamental difference between these two classes of antigenic stimuli is their intrinsic ability to induce effector T cell differentiation.
Impaired maintenance of the CD8+ T cell population in the absence of CD4+ T cells has been reported in other systems (27, 29, 65). In these prior studies, CD4+ T cells were removed at only a single time postinfection; therefore, the temporal requirements for CD4+ T cell help could not be assessed. Consistent with these reports, we observed accelerated contraction of CD8+ T cells following longitudinal depletion of CD4+ T cells. We extended these observations by demonstrating a prolonged requirement for CD4+ T cell help that lasted at least 2 wk and declined over time. Future studies will be required to determine how this accelerated contraction alters protective efficacy of these vaccine elicited CD8+ T cells.
CD4+ T cells have a well-established role in programming memory CD8+ T cells to be capable of expansion upon secondary Ag exposure, and CD40-derived signals are a major mechanism of this process (20, 27, 31–33, 64). However, a previous report has demonstrated in the Ad system that CD8+ T cells have unimpaired anamnestic potential when primed in the absence of CD4+ T cells (17). Given no detectable AL11-specific CD8+ T cell responses in our system when CD4+ T cells were depleted before priming, it was not possible to investigate directly a role for CD4+ T cell programming of anamnestic potential at priming; however, we observed no defect in anamnestic expansion when CD4+ T cells were depleted after priming. However, we observed impaired recall responses in CD8+ T cells from mice that lacked the CD40 signaling pathway. There are two possible conclusions from these data. First, anamnestic programming occurs rapidly following priming (27, 64, 65). Alternatively, anamnestic programming in the Ad system occurs independent of CD4+ T cell help (17), but still requires CD4 T cell–independent CD40 signaling, which has been reported following influenza infection (75). Future experiments will be required to elucidate the relationships among CD4+ T cell help, CD40 signaling, and the programming of anamnestic potential following Ad vector immunization.
This study illustrates the multifaceted role of CD4+ T cells in the development and maintenance of Ad vector–induced CD8+ T cell responses. CD4+ T cell help at different points in time was required for CD8+ T cell priming, effector differentiation, and regulating contraction (Fig. 9). The relative importance of CD4+ T cell help gradually waned over time following immunization, but was required for 8 d for CD8+ T cell priming and 28 d for regulating contraction. Thus, it appears that CD4+ T cells do not simply program functional CD8+ T cells through a single or transient signaling event, but instead modulate CD8+ T cell frequency and functionality via an active process over a prolonged period of time. Future efforts to optimize CD4+ T cell responses, especially in situations of impaired CD4+ T cell frequency such as HIV infection, could lead to increased immunogenicity and durability of CD8+ T cell responses elicited by Ad vector–based vaccines and possibly other vaccine platforms as well.
Acknowledgments
We thank Kevin Carlson and Leila Eslamizar for assistance in cell sorting, W. Nicholas Haining and Kathleen Yates for assistance in RNA extraction and cDNA synthesis, Nathanial L Simmons for technical assistance, and the National Institutes of Halth Tetramer Core Facility (Emory University, GA) for providing the Db/AL11 and Db/GP33 monomers.
This work was supported by National Institutes of Health Grants AI078526 and AI096040 (to D.H.B.) and the Bill and Melinda Gates Foundation (OPP1033091). N.M.P. is the recipient of a Herchel Smith Graduate Fellowship from Harvard University.
- Ad
- adenovirus
- Ad26
- adenovirus serotype 26
- Ad5HVR48
- chimeric Ad5 with hypervariable regions 1-7 of Ad48
- Gag
- SIVmac239 Gag
- Env
- SIVmac239 Env
- LCMV
- lymphocytic choriomeningitis virus
- Lm-GP33
- recombinant Listeria monocytogenes expressing GP33-41 of LCMV GP
- KO
- knockout
- vp
- viral particles.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sullivan N. J., Hensley L., Asiedu C., Geisbert T. W., Stanley D., Johnson J., Honko A., Olinger G., Bailey M., Geisbert J. B., et al. 2011. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat. Med. 17: 1128–1131 [DOI] [PubMed] [Google Scholar]
- 2.Alexander J., Ward S., Mendy J., Manayani D. J., Farness P., Avanzini J. B., Guenther B., Garduno F., Jow L., Snarsky V., et al. 2012. Pre-clinical evaluation of a replication-competent recombinant adenovirus serotype 4 vaccine expressing influenza H5 hemagglutinin. PLoS ONE 7: e31177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes E., Folgori A., Capone S., Swadling L., Aston S., Kurioka A., Meyer J., Huddart R., Smith K., Townsend R., et al. 2012. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 4: 115ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colloca S., Barnes E., Folgori A., Ammendola V., Capone S., Cirillo A., Siani L., Naddeo M., Grazioli F., Esposito M. L., et al. 2012. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci. Transl. Med. 4: 115ra112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang Z. Q., Yang Y., Wilson J. M., Ertl H. C. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219: 220–227 [DOI] [PubMed] [Google Scholar]
- 6.Shiver J. W., Fu T. M., Chen L., Casimiro D. R., Davies M. E., Evans R. K., Zhang Z. Q., Simon A. J., Trigona W. L., Dubey S. A., et al. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415: 331–335 [DOI] [PubMed] [Google Scholar]
- 7.Baden L. R., Walsh S. R., Seaman M. S., Tucker R. P., Krause K. H., Patel A., Johnson J. A., Kleinjan J., Yanosick K. E., Perry J., et al. 2013. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J. Infect. Dis. 207: 240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch D. H., Liu J., Peter L., Abbink P., Iampietro M. J., Cheung A., Alter G., Chung A., Dugast A.-S., Frahm N., et al. 2013. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001). J. Infect. Dis. 207: 248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barouch D. H., Liu J., Li H., Maxfield L. F., Abbink P., Lynch D. M., Iampietro M. J., SanMiguel A., Seaman M. S., Ferrari G., et al. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J., O’Brien K. L., Lynch D. M., Simmons N. L., La Porte A., Riggs A. M., Abbink P., Coffey R. T., Grandpre L. E., Seaman M. S., et al. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457: 87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch D. H., Liu J., Lynch D. M., O’Brien K. L., La Porte A., Simmons N. L., Riggs A. M., Clark S., Abbink P., Montefiori D. C., et al. 2009. Protective efficacy of a single immunization of a chimeric adenovirus vector-based vaccine against simian immunodeficiency virus challenge in rhesus monkeys. J. Virol. 83: 9584–9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephenson K. E., Li H., Walker B. D., Michael N. L., Barouch D. H. 2012. Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following simian immunodeficiency virus challenges of vaccinated rhesus monkeys. J. Virol. 86: 9583–9589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y., Ertl H. C., Wilson J. M. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1: 433–442 [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Haecker S. E., Su Q., Wilson J. M. 1996. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum. Mol. Genet. 5: 1703–1712 [DOI] [PubMed] [Google Scholar]
- 15.Yang Y., Xiang Z., Ertl H. C., Wilson J. M. 1995. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 92: 7257–7261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X., Robinson M. B., Pabin C., Batshaw M. L., Wilson J. M. 2000. Transient depletion of CD4 lymphocyte improves efficacy of repeated administration of recombinant adenovirus in the ornithine transcarbamylase deficient sparse fur mouse. Gene Ther. 7: 1761–1767 [DOI] [PubMed] [Google Scholar]
- 17.Yang T. C., Millar J., Groves T., Zhou W., Grinshtein N., Parsons R., Evelegh C., Xing Z., Wan Y., Bramson J. 2007. On the role of CD4+ T cells in the CD8+ T-cell response elicited by recombinant adenovirus vaccines. Mol. Ther. 15: 997–1006 [DOI] [PubMed] [Google Scholar]
- 18.Holst P. J., Bartholdy C., Stryhn A., Thomsen A. R., Christensen J. P. 2007. Rapid and sustained CD4(+) T-cell-independent immunity from adenovirus-encoded vaccine antigens. J. Gen. Virol. 88: 1708–1716 [DOI] [PubMed] [Google Scholar]
- 19.von Herrath M. G., Yokoyama M., Dockter J., Oldstone M. B., Whitton J. L. 1996. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 70: 1072–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett S. R., Carbone F. R., Karamalis F., Miller J. F., Heath W. R. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186: 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiesel M., Joller N., Ehlert A.-K., Crouse J., Spörri R., Bachmann M. F., Oxenius A. 2010. Th cells act via two synergistic pathways to promote antiviral CD8+ T cell responses. J. Immunol. 185: 5188–5197 [DOI] [PubMed] [Google Scholar]
- 22.Chan K., Lee D. J., Schubert A., Tang C. M., Crain B., Schoenberger S. P., Corr M. 2001. The roles of MHC class II, CD40, and B7 costimulation in CTL induction by plasmid DNA. J. Immunol. 166: 3061–3066 [DOI] [PubMed] [Google Scholar]
- 23.Ahmed R., Butler L. D., Bhatti L. 1988. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J. Virol. 62: 2102–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanolkar A., Fuller M. J., Zajac A. J. 2004. CD4 T cell-dependent CD8 T cell maturation. J. Immunol. 172: 2834–2844 [DOI] [PubMed] [Google Scholar]
- 25.Williams M. A., Tyznik A. J., Bevan M. J. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441: 890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buller R. M., Holmes K. L., Hügin A., Frederickson T. N., Morse H. C., III 1987. Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature 328: 77–79 [DOI] [PubMed] [Google Scholar]
- 27.Sun J. C., Bevan M. J. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300: 339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matloubian M., Concepcion R. J., Ahmed R. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68: 8056–8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J. C., Williams M. A., Bevan M. J. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5: 927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiesel M., Oxenius A. 2012. From crucial to negligible: functional CD8⁺ T-cell responses and their dependence on CD4⁺ T-cell help. Eur. J. Immunol. 42: 1080–1088 [DOI] [PubMed] [Google Scholar]
- 31.Bennett S. R., Carbone F. R., Karamalis F., Flavell R. A., Miller J. F., Heath W. R. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393: 478–480 [DOI] [PubMed] [Google Scholar]
- 32.Schoenberger S. P., Toes R. E., van der Voort E. I., Offringa R., Melief C. J. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393: 480–483 [DOI] [PubMed] [Google Scholar]
- 33.Ridge J. P., Di Rosa F., Matzinger P. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393: 474–478 [DOI] [PubMed] [Google Scholar]
- 34.Abbink P., Lemckert A. A. C., Ewald B. A., Lynch D. M., Denholtz M., Smits S., Holterman L., Damen I., Vogels R., Thorner A. R., et al. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81: 4654–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts D. M., Nanda A., Havenga M. J. E., Abbink P., Lynch D. M., Ewald B. A., Liu J., Thorner A. R., Swanson P. E., Gorgone D. A., et al. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441: 239–243 [DOI] [PubMed] [Google Scholar]
- 36.Penaloza-MacMaster P., Provine N. M., Ra J., Borducchi E. N., McNally A., Simmons N. L., Iampietro M. J., Barouch D. H. 2013. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J. Virol. 87: 1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foulds K. E., Zenewicz L. A., Shedlock D. J., Jiang J., Troy A. E., Shen H. 2002. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168: 1528–1532 [DOI] [PubMed] [Google Scholar]
- 38.Shen H., Slifka M. K., Matloubian M., Jensen E. R., Ahmed R., Miller J. F. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc. Natl. Acad. Sci. USA 92: 3987–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wherry E. J., Teichgräber V., Becker T. C., Masopust D., Kaech S. M., Antia R., von Andrian U. H., Ahmed R. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4: 225–234 [DOI] [PubMed] [Google Scholar]
- 40.Barouch D. H., Pau M. G., Custers J. H. H. V., Koudstaal W., Kostense S., Havenga M. J. E., Truitt D. M., Sumida S. M., Kishko M. G., Arthur J. C., et al. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172: 6290–6297 [DOI] [PubMed] [Google Scholar]
- 41.Liu J., Ewald B. A., Lynch D. M., Nanda A., Sumida S. M., Barouch D. H. 2006. Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies. J. Virol. 80: 11991–11997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parish C. R., Glidden M. H., Quah B. J. C., Warren H. S. 2009. Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. Curr. Protoc. Immunol. Chapter 4: Unit 4.9. [DOI] [PubMed] [Google Scholar]
- 43.Quigley M., Pereyra F., Nilsson B., Porichis F., Fonseca C., Eichbaum Q., Julg B., Jesneck J. L., Brosnahan K., Imam S., et al. 2010. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 16: 1147–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnitz R. A., Imam S., Yates K., Haining W. N. 2013. Isolation of RNA and the synthesis and amplification of cDNA from antigen-specific T cells for genome-wide expression analysis. Methods Mol. Biol. 979: 161–173 [DOI] [PubMed] [Google Scholar]
- 45.Nelson E. D., Kavalali E. T., Monteggia L. M. 2008. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J. Neurosci. 28: 395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinay D. S., Kim C. H., Chang K. H., Kwon B. S. 2010. PDCA expression by B lymphocytes reveals important functional attributes. J. Immunol. 184: 807–815 [DOI] [PubMed] [Google Scholar]
- 47.Saito M., Novak U., Piovan E., Basso K., Sumazin P., Schneider C., Crespo M., Shen Q., Bhagat G., Califano A., et al. 2009. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. USA 106: 11294–11299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He S., Wang J., Kato K., Xie F., Varambally S., Mineishi S., Kuick R., Mochizuki K., Liu Y., Nieves E., et al. 2012. Inhibition of histone methylation arrests ongoing graft-versus-host disease in mice by selectively inducing apoptosis of alloreactive effector T cells. Blood 119: 1274–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Intlekofer A. M., Takemoto N., Wherry E. J., Longworth S. A., Northrup J. T., Palanivel V. R., Mullen A. C., Gasink C. R., Kaech S. M., Miller J. D., et al. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6: 1236–1244 [DOI] [PubMed] [Google Scholar]
- 50.Yang C. Y., Best J. A., Knell J., Yang E., Sheridan A. D., Jesionek A. K., Li H. S., Rivera R. R., Lind K. C., D’Cruz L. M., et al. 2011. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat. Immunol. 12: 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cretney E., Xin A., Shi W., Minnich M., Masson F., Miasari M., Belz G. T., Smyth G. K., Busslinger M., Nutt S. L., Kallies A. 2011. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12: 304–311 [DOI] [PubMed] [Google Scholar]
- 52.Goronzy J., Weyand C. M., Fathman C. G. 1986. Long-term humoral unresponsiveness in vivo, induced by treatment with monoclonal antibody against L3T4. J. Exp. Med. 164: 911–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaech S. M., Tan J. T., Wherry E. J., Konieczny B. T., Surh C. D., Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4: 1191–1198 [DOI] [PubMed] [Google Scholar]
- 54.Joshi N. S., Cui W., Chandele A., Lee H. K., Urso D. R., Hagman J., Gapin L., Kaech S. M. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27: 281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaech S. M., Cui W. 2012. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendriks J., Gravestein L. A., Tesselaar K., van Lier R. A., Schumacher T. N., Borst J. 2000. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 1: 433–440 [DOI] [PubMed] [Google Scholar]
- 57.Cannarile M. A., Lind N. A., Rivera R., Sheridan A. D., Camfield K. A., Wu B. B., Cheung K. P., Ding Z., Goldrath A. W. 2006. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat. Immunol. 7: 1317–1325 [DOI] [PubMed] [Google Scholar]
- 58.Zhou X., Yu S., Zhao D.-M., Harty J. T., Badovinac V. P., Xue H.-H. 2010. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity 33: 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ichii H., Sakamoto A., Hatano M., Okada S., Toyama H., Taki S., Arima M., Kuroda Y., Tokuhisa T. 2002. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat. Immunol. 3: 558–563 [DOI] [PubMed] [Google Scholar]
- 60.Shin H., Blackburn S. D., Intlekofer A. M., Kao C., Angelosanto J. M., Reiner S. L., Wherry E. J. 2009. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity 31: 309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kallies A., Xin A., Belz G. T., Nutt S. L. 2009. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity 31: 283–295 [DOI] [PubMed] [Google Scholar]
- 62.Szabo S. J., Kim S. T., Costa G. L., Zhang X., Fathman C. G., Glimcher L. H. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669 [DOI] [PubMed] [Google Scholar]
- 63.Pearce E. L., Mullen A. C., Martins G. A., Krawczyk C. M., Hutchins A. S., Zediak V. P., Banica M., DiCioccio C. B., Gross D. A., Mao C.-A., et al. 2003. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302: 1041–1043 [DOI] [PubMed] [Google Scholar]
- 64.Janssen E. M., Lemmens E. E., Wolfe T., Christen U., von Herrath M. G., Schoenberger S. P. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421: 852–856 [DOI] [PubMed] [Google Scholar]
- 65.Choo D. K., Murali-Krishna K., Anita R., Ahmed R. 2010. Homeostatic turnover of virus-specific memory CD8 T cells occurs stochastically and is independent of CD4 T cell help. J. Immunol. 185: 3436–3444 [DOI] [PubMed] [Google Scholar]
- 66.Wilson E. B., Livingstone A. M. 2008. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J. Immunol. 181: 7445–7448 [DOI] [PubMed] [Google Scholar]
- 67.Yang Y., Wilson J. M. 1996. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science 273: 1862–1864 [DOI] [PubMed] [Google Scholar]
- 68.Peters V. B., Sood S. K. 1994. Immunity to Haemophilus influenzae type b polysaccharide capsule in children with human immunodeficiency virus infection immunized with a single dose of Haemophilus vaccine. J. Pediatr. 125: 74–77 [DOI] [PubMed] [Google Scholar]
- 69.Small T. N., Zelenetz A. D., Noy A., Rice R. D., Trippett T. M., Abrey L., Portlock C. S., McCullagh E. J., Vanak J. M., Mulligan A. M., Moskowitz C. H. 2009. Pertussis immunity and response to tetanus-reduced diphtheria-reduced pertussis vaccine (Tdap) after autologous peripheral blood stem cell transplantation. Biol. Blood Marrow Transplant. 15: 1538–1542 [DOI] [PubMed] [Google Scholar]
- 70.Veit O., Niedrig M., Taillard C. C., Cavassini M., Mossdorf E., Schmid P., Bae H. G., Litzba N., Staub T., Hatz C., Furrer H. 2009. Immunogenicity and safety of yellow fever vaccination for 102 HIV-infected patients. Clin. Infect. Dis. 48: 659–666 [DOI] [PubMed] [Google Scholar]
- 71.Landrum M. L., Hullsiek K. H. K., Ganesan A., Weintrob A. C., Crum-Cianflone N. F., Barthel R. V., O’Connell R. J., Fieberg A., Chun H. M., Marconi V. C., et al. Infectious Disease Clinical Research Program HIV Working Group 2010. Hepatitis B vaccination and risk of hepatitis B infection in HIV-infected individuals. AIDS 24: 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beck C. R., McKenzie B. C., Hashim A. B., Harris R. C., Nguyen-Van-Tam J. S., University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group 2012. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J. Infect. Dis. 206: 1250–1259 [DOI] [PubMed] [Google Scholar]
- 73.Tan W. G., Jin H.-T., West E. E., Penaloza-Macmaster P., Wieland A., Zilliox M. J., McElrath M. J., Barouch D. H., Ahmed R. 2013. Comparative analysis of SIV Gag specific effector and memory CD8 T cells induced by different adenovirus vectors. J. Virol. 87: 1359–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Intlekofer A. M., Takemoto N., Kao C., Banerjee A., Schambach F., Northrop J. K., Shen H., Wherry E. J., Reiner S. L. 2007. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 204: 2015–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson S., Zhan Y., Sutherland R. M., Mount A. M., Bedoui S., Brady J. L., Carrington E. M., Brown L. E., Belz G. T., Heath W. R., Lew A. M. 2009. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity 30: 218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]