Abstract
Immunosuppressive status against infections in monocytes from neonates and elderly subjects has been reported. The interaction between dengue virus and monocytes/macrophages plays an important role during dengue disease. The aim of this study was to determine the cytokine response of monocytes from individuals with different ages after infection with dengue virus. Monocyte/macrophage cultures from neonatal, adult, and elderly subjects (n=10 each group) were incubated with all four dengue virus types (DENV-1 to -4). After 1 and 3 days of culture, cytokine concentrations (TNF-α, IL-6, and IL-1β) were determined in culture supernatants by enzyme-linked immunosorbant assay. Increased production of all studied cytokines was induced by the different viral types in monocyte/macrophage cultures regardless of their source. However, lower cytokine concentrations were found in neonatal and elderly monocytes. The relative monocyte/macrophage immunosuppressive status observed in neonates and the elderly could be relevant during dengue infection in those age groups and important in innate and adaptive immunity responses against this virus.
Introduction
Monocytes/macrophages (Mo/MΦ) are one of the major target cells of dengue virus (DENV; genus Flavivirus, family Flaviviridae). These cells are responsible for the dissemination of DENV after its initial entry via the mosquito vector (15). It has been shown that soluble mediators released from DENV-infected Mo/MΦ exert prominent effects on the biologic properties of endothelial cells and the hematopoietic cell population (1,5,31), suggesting that the interaction of DENV with Mo/MΦ could play a role in the pathogenesis of DENV. Previous clinical reports have shown that dengue shock syndrome is rare in neonates (6) and that elderly people are at lower risk of presenting with hemorrhagic manifestations (12) during DENV infection—features that are related to the level of circulating proinflammatory cytokines. In this regard, tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) represent important Mo/MΦ soluble mediators and could play a role in the development of the disease (11,22,33,35). Since it has been shown that monocytes from neonates and the elderly have immunosuppressive status against infections (9,13,21,23,28,30,34,36), the cytokine (TNF-α, IL-6, and IL-1β) response of monocytes from neonates, young adults, and elderly subjects were analyzed during experimental DENV infection with each of the four DENV types (DENV-1 to -4).
Materials and Methods
Preparation of virus stock and virus titration
Each of the DENV laboratory prototype strains—DENV-1 (Hawaii), DENV-2 (New Guinea C), DENV-3 (H-87), and DENV-4 (H-241)—were propagated in C6/36HT mosquito cells that were cultured in Eagle's minimal essential medium (EMEM) containing 10% fetal bovine serum (FBS) prior to viral infection. The cell culture supernatant was harvested after 5 days of incubation, and cell debris were removed by centrifugation. The clarified virus stocks were aliquoted and stored at −70°C until used. Viruses were titrated by plaque formation assays on Vero cells (3). Briefly, cells were planted at a density of 1×106 cells/well in 24-well plates, and subsequently, serial dilutions of virus were added, and the mixtures were incubated at 37°C for 7 days. Afterwards, the plaques were visualized by staining with a 1% crystal violet dye solution. Virus concentrations are given as plaque-forming units (PFU)/mL. Virus stocks were free of endotoxin as determined by the limulus amebocyte lysate assay (Charles River Laboratories, Wilmington, MA).
Monocyte/macrophage cultures
Monocytes were isolated from heparinized peripheral blood obtained from human healthy male neonates (umbilical cord blood), young adults (35–45 years old), and elderly subjects (65–70 years old; n=10 per group). Individuals or parents were informed about the study procedures, and their consents were obtained before enrollment in the investigation following the ethical committee guidelines of the bioethical committee of the Medical School (Universidad del Zulia, Maracaibo, Venezuela). All individuals were tested for circulating DENV NS1 protein and anti-dengue IgM and IgG antibodies (Dengue NS1 Ag+Ab combo; SD Bioline, Seoul, South Korea). Positive subjects to any of these parameters were excluded from this study. Mononuclear cells were isolated by density centrifugation over 1.077 Histopaque (Sigma Chemical Co, St. Louis, MO). Total mononuclear leukocyte recovery from the interface were washed and suspended in RPMI 1640 medium containing 10% FBS and penicillin/streptomycin. Afterwards, 300 μL/well of a cellular suspension (4×106 cells/mL) were layered on 24-well plastic tissue culture plates (Nunc, Roskilde, Denmark) and incubated for 3 h at 37°C and 5% CO2. Nonadherent cells were washed out with warm medium, and adhered cells (approx 3×105 cells) were used for experiments. To assess the purity of the cultures, cells were reacted with an FITC-conjugated anti- human CD14 monoclonal antibody (Sigma Chemical Co.), and the percentage of CD14+cells was determined by microscopy with an epifluorescence system (Zeiss, Oberkochen, Germany).
Infection of Mo/MΦ cultures
Monocytes from each subject were infected with each DENV type at a multiplicity of infection of 1, and incubated for 1 and 3 days at 37°C, 5% CO2. Controls represent monocytes cultured with supplemented medium without virus. In addition, monocyte cultures were incubated with LPS (50 ng/mL; Sigma-Aldrich Company, St. Louis, MO) for the same period of time. All cultures were performed in triplicate. Intracellular viral antigens were detected by an indirect immunofluorescence assay using a DENV-specific polyclonal antibody (Chemicon/Millipore, Billerica, MA). Uninfected cultures from each group were used as negative controls.
Cytokine determination
Cytokine concentrations (TNF-α, IL-6, and IL-1β) were determined in supernatant cultures by enzyme-linked immunosorbant assay (ELISA) following the manufacturer's indications (R & D Systems, Minneapolis, MN). Cell monolayers were sonicated to determine total cellular protein content per well (Bio-Rad Laboratories, Inc., Hercules, CA) in order to normalize supernatant cytokine concentration (pg/mg of cellular protein). Indexes of neonatal, adult, and elderly cytokine productions were also determined by neonatal cytokine values/neonatal cytokine values, adult cytokine values/neonatal cytokine values, or elderly cytokine values/neonatal cytokine values. These indexes represent the cytokine values obtained by all DENV infection types or by LPS.
Statistical analysis
Data were expressed as mean±standard deviation. Differences between groups were determined by analysis of variance following by Bonferroni's post-hoc test. Statistical significance was considered when p<0.05.
Results
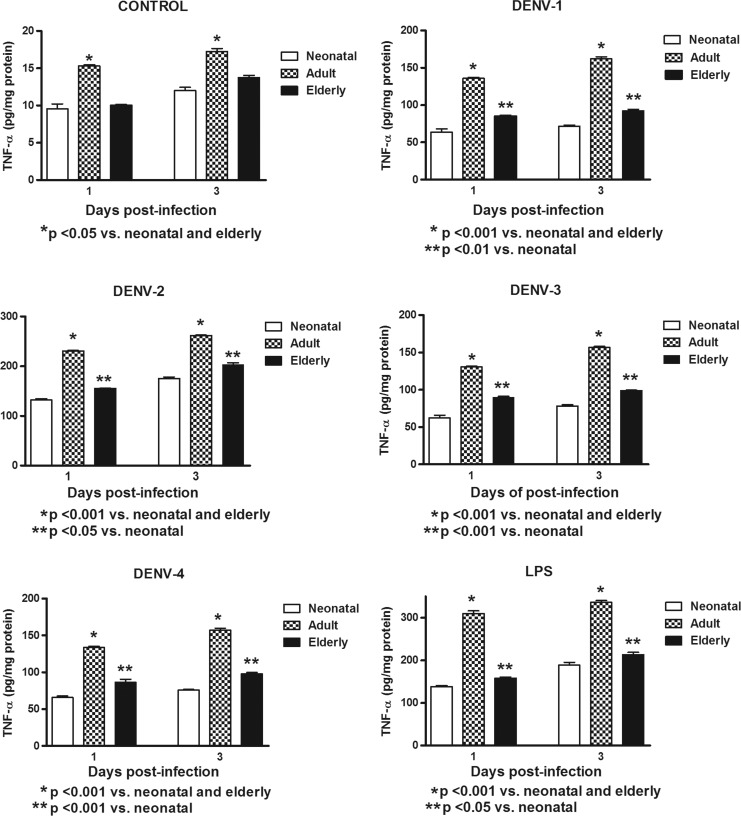
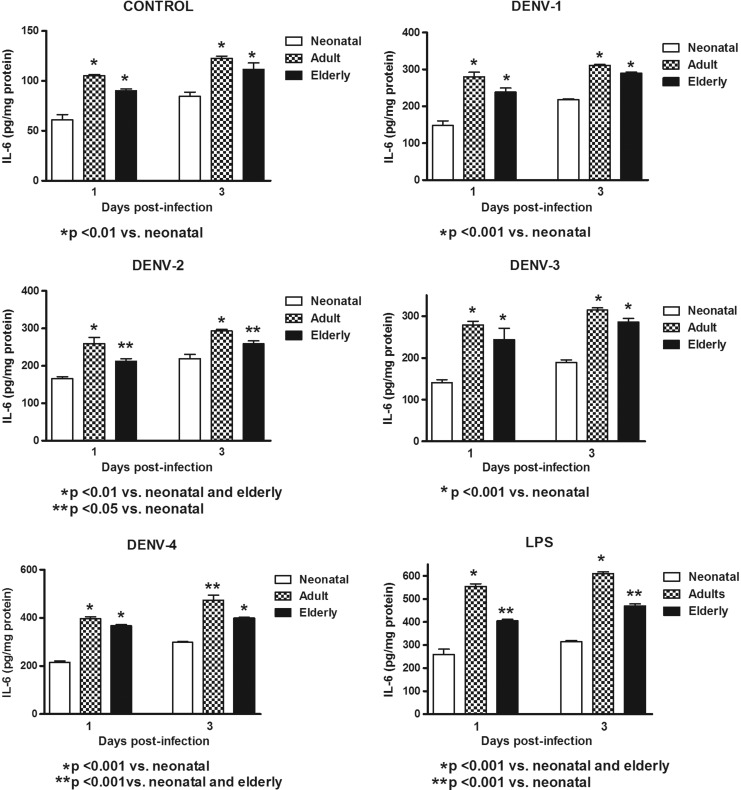
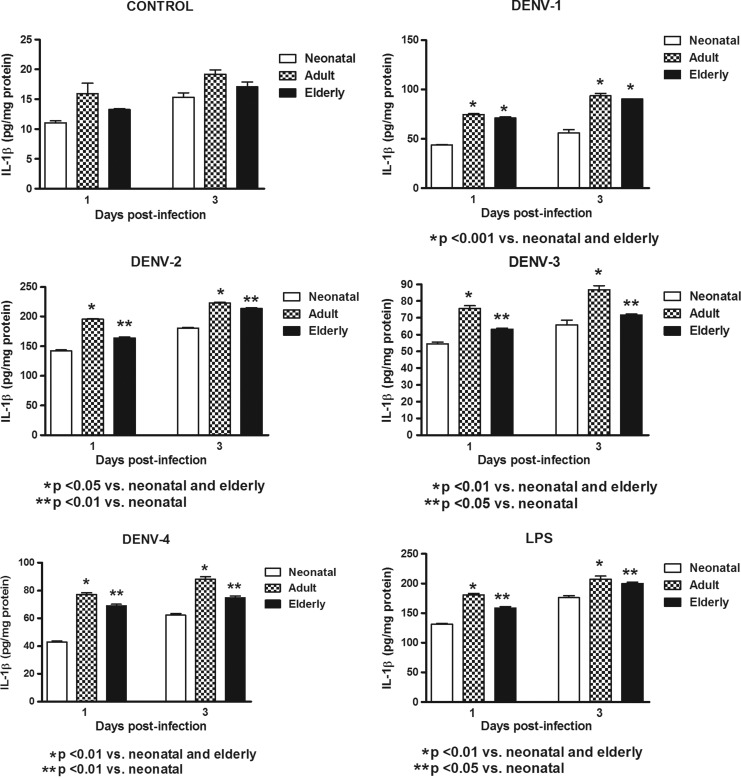
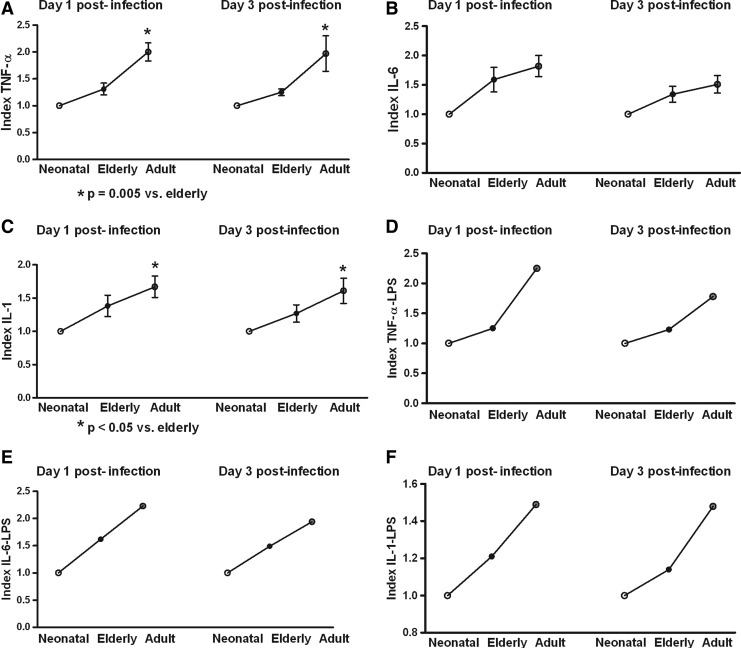
In this study, Mo/MΦ (purity>95%) were co-cultured with the laboratory strains of DENV-1 to -4 or with LPS. In order to determine the response capacity of Mo/MΦ according to the age of the donor, monocytes from neonates, young adults, and elderly individuals were tested for the cytokine response during infection by DENV. Increased production of TNF-α, IL-6, and IL-1β were induced by the different viral types in cultures from neonatal, adult, and elderly individuals. DENV-2 induced the highest production of TNF-α and IL-1β, and DENV-4 of IL-6. The grade of cytokine responses was influenced by monocyte source (Table 1). In general, lower values in TNF-α, IL-6, and IL-1β production (Figs. 1–3) were found in neonatal Mo/MΦ, followed by elderly Mo/MΦ, while the highest values were observed in adult Mo/MΦ. These findings were observed at days 1 and 3 postinfection, and the cytokine pattern of Mo/MΦ response induced by DENV was similar to that observed in LPS-treated cultures, as indicated by the index of elderly or adult cytokine values/neonatal cytokine values (Fig. 4).
Table 1.
Cytokine Production According to Viral Type at Days 1 and 3
| Cytokine/Day | DENV-1 | DENV-2 | DENV-3 | DENV-4 | Control |
|---|---|---|---|---|---|
| Neonatal | |||||
| TNF-α/1 | 63.76±4.25** | 132.1±2.40* | 62.33±3.54** | 65.7±1.95** | 9.57±0.66 |
| TNF-α/3 | 71.34±1.94** | 185.3±3.11* | 78.27±1.62** | 75.8±0.54** | 12.0±0.43 |
| IL-6/1 | 147.9±12.5** | 165.7±5.77** | 140.4±7.51** | 215.2±5.38* | 60.89±5.3 |
| IL-6/3 | 217±2.30** | 219±11.6** | 189±6.32** | 298.3±3.5* | 84.38±4.27 |
| IL-1β/1 | 43.84±0.36** | 145.2±1.64* | 54.59±0.93** | 43.06±0.6** | 11.04±0.33 |
| IL-1β/3 | 56.03±3.24** | 180.6±1.08* | 65.83±2.85** | 62.3±1.19** | 15.35±0.71 |
| Adult | |||||
| TNF-α/1 | 135.9±1.04** | 230.6±1.2* | 130.7±0.86** | 133.5±1.6** | 15.29±0.16 |
| TNF-α/3 | 162.1±2.65** | 261.7±1.03* | 156.6±1.46** | 157±2.77** | 17.26±0.41 |
| IL-6/1 | 279.5±12.7** | 259.4±16.5** | 279±8.59** | 396.7±7.49* | 105.2±1.21 |
| IL-6/3 | 310.8±2.60** | 293.3±4.69** | 314.7±5.23** | 473±21.63* | 122.7±2.29 |
| IL-1β/1 | 74.73±0.7** | 195.5±0.72* | 75.55±1.65** | 77.31±1.2** | 15.95±1.75 |
| IL-1β/3 | 93.59±2.32** | 223.1±1.13* | 86.72±2.41** | 88.17±1.9** | 19.2±0.72 |
| Elderly | |||||
| TNF-α/1 | 85.09±1.38** | 155.2±0.58* | 90.06±1.04** | 86.27±4.3** | 10.04±0.07 |
| TNF-α/3 | 92.29±2.03** | 202.8±4.41* | 98.78±0.81** | 97.8±1.91** | 13.76±0.28 |
| IL-6/1 | 238.3±10.7** | 212.4±6.65** | 243.7±27.3** | 367.1±5.14* | 90.03±2.08 |
| IL-6/3 | 289.4±3.43** | 259.3±7.57** | 286±8.60** | 397.7±4.80* | 111.6±6.43 |
| IL-1β/1 | 71.21±0.94** | 164±0.36* | 63.15±0.69** | 68.96±1.2** | 13.26±0.16 |
| IL-1β/3 | 83.17±0.21** | 213.7±1.14* | 71.63±0.67** | 74.9±1.23** | 17.1±0.81 |
Boldface numbers represent the cytokine high values observed during a particular dengue type infection.
p<0.01 versus all other groups; **p<0.001 versus control (uninfected Mo/MΦ cultures).
FIG. 1.
Induction of tumor necrosis factor alpha (TNF-α) by different dengue viral (DENV) types and LPS according to monocyte source. Decreased cytokine values in neonatal and elderly monocyte cultures infected with all viral types compared to adult monocyte cultures were observed at days 1 and 3. These responses were similar to those observed with LPS stimulation.
FIG. 2.
Induction of interleukin-6 (IL-6) by different DENV types and LPS according to monocyte source. Decreased cytokine values in neonatal and elderly monocyte cultures infected with all viral types compared to adult monocyte cultures were observed at days 1 and 3. These responses were similar to those observed with LPS stimulation.
FIG. 3.
Induction of interleukin-1 beta (IL-1β) by different DENV types and LPS according to monocyte source. Decreased cytokine values in neonatal and elderly monocyte cultures infected with all viral types compared to adult monocyte cultures were observed at days 1 and 3. These responses were similar to those observed with LPS stimulation.
FIG. 4.
Index elderly or adult cytokine values/neonatal cytokine values. In general, cytokine values from neonatal cultures were lower than those observed in elderly and adult monocyte/macrophage cultures in DENV infections (A), (B), and (C), with the highest values in adult cultures. Similar cytokine responses were observed in LPS-treated cultures (D), (E), and (F). These indexes represent the cytokine values obtained from all viral infection types or from LPS.
Discussion
During inflammatory reactions, increased production of TNF-α, IL-6, and IL-1β can be produced in inflamed tissues by Mo/MΦ. The sustained inflammation after bacterial infection in neonates, resulting in inflammatory sequelae, is well known (13), suggesting an altered monocyte function. This study assessed the potential contribution of Mo/MΦ from neonates, young adults, and elderly subjects in the production of cytokines after infection with DENV. Although in general all tested cytokines were increased after DENV infection compared to control cells (uninfected Mo/MΦ) in all age groups, the viral–neonatal Mo/MΦ interaction showed decreased production of cytokines when compared to other Mo/MΦ groups (i.e., elderly and adult monocytes), suggesting a diminished response to DENV by neonate Mo/MΦ. It is well known that neonates are born with quantitative and qualitative defects in both adaptive and innate immune responses (23,36). Thus, the neonatal Mo/MΦ response observed in this study may be related to intrinsic functional deficiency of neonatal status. In this regard, lymphocyte subset percentages in cord blood from neonates and neonatal cytokine responses to bacterial antigens were observed to be diminished when compared to those observed in adults (25). Plasma cytokine concentrations (IL-1 α, IL-1β, and IL-6) and cytokine production by neonatal monocytes after in vitro lipopolysaccharide stimulation have been found to be decreased compared to plasma and monocytes from adults (28). In addition, blocking cytokine condition has been reported in neonates. Plasma levels of interleukin-1 receptor antagonist (IL-1ra) and IL-1ra produced in vitro by neonatal peripheral blood monocytes stimulated with LPS were significantly higher than plasma and monocytes from adults (27). The reduced response of neonatal Mo/MΦ to DENV could also be related to immunosuppression activities from growth factors. It has been reported that Insulin-like growth factor-I may be a key regulator of neonatal immune responses in maturation processes and inflammation by suppressing proinflammatory Th1 responses (29). It has also been demonstrated that an increment of distinct inhibitory receptors on neonatal peripheral blood immune cells could play a role in the regulation of the neonatal immune system (36). Decreased production of TNF-α, IL-6, and IL-1β by neonatal Mo/MΦ after interaction with DENV could have an effect in the pathogenesis of DENV in this age group, since these cytokines play important roles in the development of the disease (11,22,33,35). However, besides the immunosuppressant status observed in neonatal Mo/MΦ, human cord blood mononuclear cells are capable of increasing expression/secretion of high mobility group box 1 protein (HMGB1) triggered by diverse stimuli. HMGB1 mediates the response to infection, injury, and inflammation, inducing dendritic cells maturation and Th-1 responses (7). This could be important in the neonatal response of Mo/MΦ to DENV, since this virus has been shown to induce the translocation of HMGB1 from the nucleus to the cytoplasm in human monocytes, which is followed by further proinflammatory events (24). In addition, monocytes and T-lymphocytes from neonates are capable, like those from adults, of recognizing the presence of pathogens through Toll-like receptors (TLRs) (8), and these receptors play a role in the susceptibility and severity of complicated DENV infection (10). The clinical relevance of the observed decreased IL-1β, IL-6, and TNF-α production in neonatal Mo/MΦ remains to be clarified, since several studies have shown that dengue shock syndrome is rare in neonates (6), and this condition has been related to increased circulation of proinflammatory cytokines.
On the other hand, ageing may contribute to the immune dysregulation that affects the elderly (32). It has been demonstrated that the number of myeloid dendritic cells progressively declines with age, accompanied by a decrease of CD34+precursors and impaired ability of dendritic cells to produce IL-12 upon stimulation (9). In this study, Mo/MΦ from elderly subjects had decreased production of cytokines after DENV infection compared to young adult leukocytes, suggesting impairment in the production of cytokines in older individuals. Monocyte alterations in elderly subjects have been previously described. Monocytes from elderly individuals had decreased accessory function for PHA-stimulated T-cells from young individuals (30). Lower cytokine production and low regulation of co-stimulatory proteins such as CD80 (essential for optimal activation of T-cells) on monocytes from older adults were observed for all TLR ligands tested when compared to cells from young individuals (34). Therefore, the impaired response to DENV by elderly monocytes found in this study could be involved in the course of DENV in elder individuals (21). In this regard, it has been reported that DENV infection in the elderly is related to a higher likelihood of being hospitalized, and those individuals are at higher risk for death in comparison to infants, youth, and young adults, besides being at lower risk of presenting with hemorrhagic manifestations (12). However, elder individuals with impaired production of Mo/Mφ-produced IL-1β, IL-6, and TNF-α, as suggested by our results, could have high concentrations of these cytokines, since other cells such as T-lymphocytes and NK cells are capable of producing these cytokines during DENV infection (15,33).
The proinflammatory cytokines are a key factor in the pathogenesis of dengue. TNF-α, IL-6, and IL-1β play important roles during DENV infection, and the low response for those cytokines by DENV-infected monocytes from neonates and elderly people could be important in the development of the disease. TNF-α, IL-6, and IL-1β have been involved in DENV hemorrhagic manifestations. In this regard, a rapid increase in the levels of cytokines, especially TNF-α, plays a key role in inducing unique clinical manifestations of dengue hemorrhagic fever such as plasma leakage, shock, and hemorrhagic manifestations (20). DENV infection leads to increased platelet-derived IL-1β that contributes to increased vascular permeability (17). IL-6 has been associated with disease severity, especially in dengue hemorrhagic fever/dengue shock syndrome patients (4). Therefore, decreased levels of those cytokines (compared to adult response) could be involved in decreased hemorrhagic manifestations. In this regard, clinical reports have shown that dengue shock syndrome is rare in neonates (6) and that elderly people are at lower risk of presenting with hemorrhagic manifestations (12) during dengue infection, findings related to the level of circulating proinflammatory cytokines.
Interestingly, DENV-2 appears to have a higher stimulatory effect on TNF-α and IL-1β production by Mo/Mφ than the rest of the other DENV types. We do not have a clear explanation for this phenomenon, but the higher production of cytokines induced by DENV-2 (TNF-α, IL-1β) and by DENV-4 (IL-6) could involve these viral types in severe forms of DENV (2,16,19,33).
The interaction of viruses with proteins expressed on cell membranes could be an important factor to define cytokine cell response. DENV entry in target cells occurs via clatrhin-mediated endocytosis and the process is initiated after the binding of DENV virions to putative receptors that include DC-SIGN, the mannose receptor (MR), and glycosaminoglycans (GAG) such as heparan sulfate. To date, no DENV- or Flavivirus-specific receptors have been identified, and the binding step between DENV and DC-SIGN, MR, GAG, and other unidentified receptor(s) is not well understood. Other receptors such as pattern recognition receptors (PRR) including TLR3, TLR7, RIG-I, and MDA5 are induced during DENV infection and are involved in type I IFN induction after the infection. It has also been reported that CLEC5A is critical for DENV-induced lethal disease in animal models (14,26). The interaction of DENV with any of those proteins could stimulate the release of proinflammatory cytokines. However, to our knowledge, there are no reports on the differential expression of putative DENV receptors in cells from individuals in different stages of life (i.e., children, adults, elderly) that could be involved in the differential cytokine response observed in this study. In this study, Dengue intracellular viral antigens were detected in cells by a nonspecific dengue type antibody. Many positive cells were found, suggesting that viral entry occurred (data not shown). The presence of intracellular viral antigen could be involved in cytokine production. However, the interaction of DENV with CLEC5A does not result in viral entry, but stimulates the release of proinflammatory cytokines (14,26). Therefore, the viral stimulatory cytokine production observed in this study could be related to intra- and/or extracellular stimulation.
During this study, both neonatal and elderly Mo/MΦ had a lower cytokine response to DENV infection than young adult Mo/MΦ, suggesting that an immunosuppressive condition exists at both ends of life, which is probably due to different physiopathological mechanisms. The balance between proinflammatory/anti-inflammatory cytokine effects and other mechanisms of inflammation could mediate the final response to DENV and the course of the disease. In addition, the innate immune system initiates and supports adaptive immunity (18), suggesting that the initial response of neonatal and elderly Mo/MΦ could support different adaptive immunity responses than that observed in young adults. Further investigation is required to determine the cytokine profile response and the cellular expression of relevant proteins involved in cytokine production in DENV-infected patients according to the age of donors.
Acknowledgments
This work was supported by grants from Fondo de Investigación de la Seguridad Social (Spain), Consejería de Educación, Comunidad de Madrid, MITIC-CM (S-2010/BMD-2502), Instituto de Salud Carlos III, MEC (PIO51871, CIBERehd), and CONDES CC-0393-12 (Maracaibo, Venezuela).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Anderson R, Wang S, Osiowy C, and Issekutz AC. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J Virol 1997;71:4226–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atrasheuskaya A, Petzelbauer P, Fredeking TM, and Ignatyev G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol Med Microbiol 2003;35:33–42 [DOI] [PubMed] [Google Scholar]
- 3.Bergold GH, and Mazzali R: Plaque formation by arbovirus. J Gen Virol 1968;2:273–284 [DOI] [PubMed] [Google Scholar]
- 4.Butthep P, Chunhakan S, Yoksan S, Tangnararatchakit K, and Chuansumrit A. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic fever. Pediatr Infect Dis J 2012;31:e232–238 [DOI] [PubMed] [Google Scholar]
- 5.Chang DM, and Shaio MF. Production of interleukin-1 (IL-1) and IL-1 inhibitor by human monocyte exposed to dengue virus. J Infect Dis 1994;170:811–817 [DOI] [PubMed] [Google Scholar]
- 6.Choudhry SP, Gupta RK, and Kishan J. Dengue shock syndrome in new born—a case series. Indian Pediatr 2004;41:397–399 [PubMed] [Google Scholar]
- 7.Ciucci A, Gabriele I, Percario ZA, Affabris E, Colizzi V, and Mancino G. HMGB1 and cord blood: its role as immuno-adjuvant factor in innate immunity. PLoS One 2011;6:e23766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasari P, Zola H, and Nicholson IC. Expression of Toll-like receptors by neonatal leukocytes. Pediatr Allergy Immunol 2011;22:221–228 [DOI] [PubMed] [Google Scholar]
- 9.Della Bella S, Bierti L, Presicce P, et al. . Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol 2007;122:220–228 [DOI] [PubMed] [Google Scholar]
- 10.Djamiatun K, Ferwerda B, Netea MG, van der Ven AJ, Dolmans M, and Faradz SM. Toll-like receptor 4 polymorphisms in dengue virus-infected children. Am J Trop Med Hyg 2011;85:352–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espina LM, Valero NJ, Hernández JM, and Mosquera JA. Increased apoptosis and expression of tumor necrosis factor-alpha caused by infection of cultured human monocytes with dengue virus. Am J Trop Med Hyg 2003;68:48–53 [PubMed] [Google Scholar]
- 12.García-Rivera EJ, and Rigau-Pérez JG. Dengue severity in the elderly in Puerto Rico. Rev Panam Salud Publica 2003;13:362–368 [DOI] [PubMed] [Google Scholar]
- 13.Gille C, Dreschers S, Leiber A, et al. . The CD95/CD95L pathway is involved in phagocytosis-induced cell death of monocytes and may account for sustained inflammation in neonates. Pediatr Res 2013;73:402–408 [DOI] [PubMed] [Google Scholar]
- 14.Green A, Beatty PR, Hadjilaou A, and Harris E. Innate immunity to dengue virus infection and subversion of antiviral responses. J Mol Biol 2014;426:1148–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 1998;11:480–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang YH, Lei HY, Liu HS, et al. . Tissue plasminogen activator induced by dengue virus infection of human endothelial cells. J Med Virol 2003;70:610–616 [DOI] [PubMed] [Google Scholar]
- 17.Hottz ED, Lopes JF, Freitas C, et al. . Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 2013;122:3405–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kollmann TR, Crabtree J, Rein-Weston A, et al. . Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009;183:7150–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuno G, and Bailey RE. Cytokine responses to dengue infection among Puerto Rican patients. Mem Inst Oswaldo Cruz 1994;89:179–182 [DOI] [PubMed] [Google Scholar]
- 20.Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis 2007;30:329–340 [DOI] [PubMed] [Google Scholar]
- 21.Lee CC, Hsu HC, Chang CM, Hong MY, and Ko WC. Atypical presentations of dengue disease in the elderly visiting the ED. Am J Emerg Med 2013;31:783–787 [DOI] [PubMed] [Google Scholar]
- 22.Levy A, Valero N, Espina LM, Añez G, Arias J, and Mosquera J. Increment of interleukin 6, tumour necrosis factor alpha, nitric oxide, C-reactive protein and apoptosis in dengue. Trans R Soc Trop Med Hyg 2010;104:16–23 [DOI] [PubMed] [Google Scholar]
- 23.Martino D, Holt P, and Prescott S. A novel role for interleukin-1 receptor signaling in the developmental regulation of immune responses to endotoxin. Pediatr Allergy Immunol 2012;23:567–572 [DOI] [PubMed] [Google Scholar]
- 24.Ong SP, Lee LM, Leong YFI, Ng ML, and Chu JJH. Dengue virus infection mediates HMGB1 release from monocytes involving PCAF acetylase complex and induces vascular leakage in endothelial cells. PLoS One 2012;7:e41932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peoples JD, Cheung S, Nesin M, et al. . Neonatal cord blood subsets and cytokine response to bacterial antigens. Am J Perinatol 2009;26:647–657 [DOI] [PubMed] [Google Scholar]
- 26.Perera-Lecoin M, Meertens L, Carnec X, and Amara A. Flavivirus entry receptors: an update. Viruses 2014;6:69–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillay V, Savage N, and Laburn H. Interleukin-1 receptor antagonist in newborn babies and pregnant women. Pflugers Arch 1993;424:549–551 [DOI] [PubMed] [Google Scholar]
- 28.Pillay V, Savage N, and Laburn H. Circulating cytokine concentrations and cytokine production by monocytes from newborn babies and adults. Pflugers Arch 1994;428:197–201 [DOI] [PubMed] [Google Scholar]
- 29.Puzik A, Rupp J, Tröger B, Göpel W, Herting E, and Härtel C. Insulin-like growth factor-I regulates the neonatal immune response in infection and maturation by suppression of IFN-γ. Cytokine 2012;60:369–376 [DOI] [PubMed] [Google Scholar]
- 30.Rich EA, Mincek MA, Armitage KB, et al. . Accessory function and properties of monocytes from healthy elderly humans for T lymphocyte responses to mitogen and antigen. Gerontology 1993;39:93–108 [DOI] [PubMed] [Google Scholar]
- 31.Shaio MF, Cheng SN, Yuh YS, and Yang KD. Cytotoxic factor released by dengue virus-infected human monocytes. J Med Virol 1995;46:216–223 [DOI] [PubMed] [Google Scholar]
- 32.Shaw AC, Joshi S, Greenwood H, Panda A, and Lord JM. Aging of the innate immune system. Curr Opin Immunol 2010;22:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suharti C, van Gorp EC, Setiati TE, et al. . The role of cytokines in activation of coagulation and fibrinolysis in dengue shock syndrome. Thromb Headmost 2002;87:42–46 [PubMed] [Google Scholar]
- 34.van Duin D, and Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc 2007;55:1438–1444 [DOI] [PubMed] [Google Scholar]
- 35.Valero N, Espina LM, Añez G, Torres E, and Mosquera JA. Short report: increased level of serum nitric oxide in patients with dengue. Am J Trop Med Hyg 2002;66:762–764 [DOI] [PubMed] [Google Scholar]
- 36.Walk J, Westerlaken GH, van Uden NO, Belderbos ME, Meyaard L, and Bont LJ. Inhibitory receptor expression on neonatal immune cells. Clin Exp Immunol 2012;169:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]