Abstract
Objective: This study tested the hypothesis that feeding an exclusively human milk (EHM) diet to premature infants reduces the incidence of necrotizing enterocolitis (NEC) associated with enteral feeding.
Study Design: An observational study for infants born at less than 33 weeks of gestational age was performed in a single neonatal intensive care unit. An EHM diet prospectively eliminated bovine-based artificial milk, including bovine-based fortifier, through 33 weeks postmenstrual age (PMA). The clinical data from a 2.5-year interval of the EHM diet were compared with data from the previous 6.5 years for similar infants who received bovine-based milk products before 33 weeks PMA.
Results: In the EHM diet cohort, 148 of 162 infants (91%) received EHM through 33 weeks PMA. In order to achieve an EHM diet, 140 of 162 infants (86%) received their own mother's milk, and 98 of 162 infants (60%) received donor human milk. The EHM cohort was also fed a human milk-based fortifier to truly eliminate bovine products. The distribution of NEC onset in the EHM cohort was significantly different from that in the control cohort for the day of onset (p=0.042) and the PMA at onset (p=0.011). In the control cohort, NEC onset after Day 7 of life occurred in 15 of 443 infants (3.4%), significantly more than in the EHM cohort where NEC occurred in two of 199 infants (1%) (p=0.009).
Conclusions: Changing to an EHM milk diet through 33 weeks PMA reduced the incidence of NEC associated with enteral feeding.
Introduction
The American Academy of Pediatrics has recommended human milk ahead of artificial milk (formula) to feed infants born prematurely.1 The advantages of human milk cited by the American Academy of Pediatrics include reduction of occurrence of necrotizing enterocolitis (NEC), sepsis, and mortality, improved tolerance of feeding and earlier attainment of full enteral feeding, and improved long-term growth and neurodevelopment. The American Academy of Pediatrics has also recommended donor human milk (DHM) ahead of artificial milk, in part, due to a reduction in occurrence of NEC.1 The risk of developing NEC at any gestational age (GA) may be related to the choice of milk used as well as the quantity and timing of milk introduction. These three measures of milk feeding are collectively referred to as a “feeding practice.”2 The safety of human and artificial milk fed to premature infants and associated feeding practices have not been sufficiently evaluated.2 Consequently, the practice of introducing milk is variable from physician to physician and from neonatal intensive care unit (NICU) to NICU.3 Variability in practice and lack of attention to safe feeding practice persist despite the serious nature of NEC, the significant long-term morbidity for survivors of surgical NEC, high rates of mortality, and an incidence of NEC that has not declined.
A reduction in occurrence of NEC has been attributed to human milk,4–10 but a randomized controlled trial comparing bovine-based artificial milk to mother's own milk (MOM) has not been conducted.11 Only recently has complete elimination of bovine milk products from the premature infant's diet, including bovine-based milk fortifiers, been suggested as a possible explanation for the reduction of NEC.8 A dose-dependent reduction in NEC risk attributed to human milk has been observed in several retrospective studies.6,9,10,12 However, those studies were not designed to evaluate the baseline risk of developing NEC associated with an exclusively human milk (EHM) diet.
It is well known that the rate of NEC in the premature infant population increases with low birth weight and younger GA and declines after 32 weeks postmenstrual age (PMA).13,14 In the absence of empirical data establishing the safety of bovine products for premature human infants, we chose a threshold standard of 33 weeks GA and PMA, below which we recommended an EHM diet. In order to achieve an EHM diet we offered DHM in cases of maternal milk insufficiency, and a human milk-derived fortifier replaced a bovine-derived fortifier. Our hypothesis was that an EHM diet provided through 33 weeks PMA would reduce the incidence of NEC associated with enteral feeding.
Materials and Methods
Study site and data collection
The study site is a Level III NICU with inborn admissions located in the Midwestern region of the United States. Two cohorts of infants were included in this observational single-site study. The control cohort included all infants admitted to the NICU at less than 33 weeks GA from January 1, 2004 through June 30, 2010. The EHM cohort included all infants admitted to the NICU at less than 33 weeks GA from July 1, 2010 through December 31, 2012. Eligible infants were identified from the NICU electronic medical record database (NeoData©, Isoprime Corp., Lisle, IL). Data were collected retrospectively for both cohorts from the electronic medical record.
Enteral feeding
During the EHM study period neonatologists recommended an EHM diet for all infants born at less than 33 weeks GA. All NICU mothers were encouraged by the multidisciplinary NICU team to express their breastmilk using a hospital-grade electric breast pump. When MOM was insufficient to meet the feeding volumes required by the infant, pasteurized DHM obtained from a human milk bank was used to achieve and maintain EHM feedings. To ensure no exposure to bovine proteins prior to 33 weeks PMA, human milk was fortified with a commercial human milk-based product (Prolact+H2MF®; Prolacta Bioscience, City of Industry, CA). Fortification of human milk with bovine-based products was delayed until 33 weeks PMA or 1 week after establishment of full enteral feedings with EHM, whichever came later. The target enteral feeding volume was 150–160 mL/kg/day through 33 weeks PMA. After 33 weeks PMA or 1 week after establishment of full enteral feedings with EHM, an infant who had been receiving DHM was weaned to artificial milk, with or without MOM. After 33 weeks PMA and a minimum of 1 week of full enteral feedings, the parents of an infant who continued to receive MOM were offered a bovine-based fortifier. Artificial milk sources with intact bovine protein were provided after 33 weeks PMA when MOM was not available. The duration of EHM use was determined by PMA. Milk use was recorded daily by attending neonatologists in the nutrition section of the electronic medical record database. The study was not commercially supported, and the cost of nutritional products was borne by the treating hospital with no cost passed on to families.
Parenteral nutrition
Parenteral nutrition (PN) delivering 50–60 Kcal/kg/day with amino acids (2.5–3 g/kg/day) and lipids (2–3 g/kg/day) was provided promptly after delivery (age 1–2 hours). The PN macronutrients and fluid intake volumes increased daily after birth. PN was discontinued when enteral feeding achieved 140 mL/kg/day. Nutritional information and postnatal growth have previously been reported for the study-site infants.15,16 Umbilical catheters were frequently the initial form of vascular access for PN solutions. Enteral feedings were provided while umbilical catheters were in use. Peripherally inserted central catheters replaced umbilical catheters. The PN duration was determined for the control cohort from January 1, 2006 through June 30, 2010 and that for the EHM cohort from July 1, 2010 through December 31, 2012. The duration of PN was calculated for the EHM and control cohorts.
NEC
NEC was defined as stage 2, or more, using the classification of Bell et al.17 Potential cases of NEC were identified from the electronic medical record, and all potential episodes were extensively reviewed for accuracy of diagnosis, the day of onset, and the milk type that the infant received prior to developing NEC.
Blood use
Transfusions of packed red blood cells were provided to replace blood loss and for hemoglobin values less than 12 g/dL while infants required respiratory support. When no respiratory support was required, transfusions were provided for hemoglobin values less than 10 g/dL. Feedings continued without interruptions during the course of blood transfusions. Delayed umbilical cord clamping was not provided.
Statistical analysis
The probability statistic for frequency data was determined by χ2 test, Mann–Whitney U test, and analysis of variance methods using VassarStats programs.18
Human subjects research ethics
The United States Code of Federal Regulations, Title 45 (Public Welfare), Part 46 (Protection of Human Subjects), Section 46.101(b)(6) states that research is exempt from Institutional Review Board approval when it involves an evaluation of the quality of wholesome foods. Human milk is regarded as wholesome food for premature infants. The study and verification of exemption status were reviewed by the Research Institute of Deaconess Clinic and found to be compliant by the Deaconess Health Systems Research Oversight and Privacy Committee. Parents provided written consent for use of DHM and human milk-based fortification.
Results
EHM study cohort
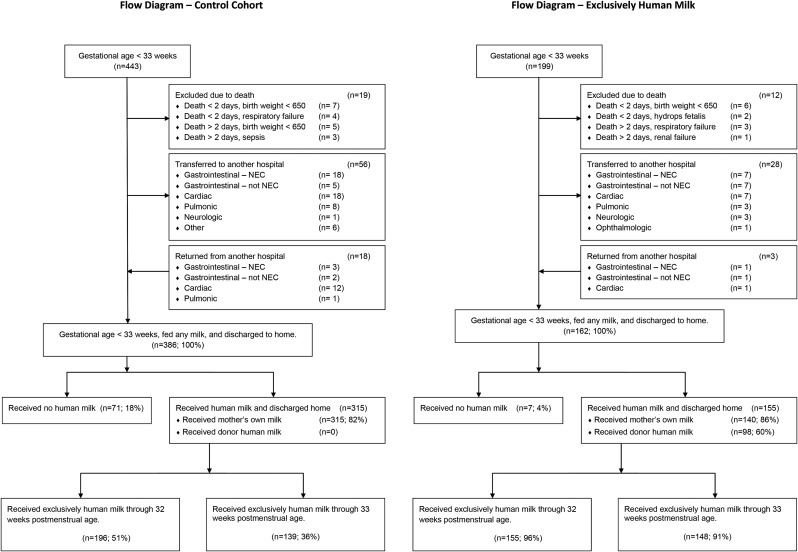
The EHM cohort included 162 infants who were discharged home; 148 (91%) received an EHM diet through 33 weeks PMA (Fig. 1).
FIG. 1.
Donor human milk was used in the exclusively human milk cohort, increasing the use of an exclusively human milk diet to over 90% of the cohort through 33 weeks postmenstrual age. NEC, necrotizing enterocolitis.
The EHM cohort included a greater proportion of infants with respiratory distress syndrome and GA of <26 weeks (Table 1). Infants in the EHM cohort <28 weeks GA were smaller, but the difference was not statistically different (Table 2).
Table 1.
The Cohort Fed Exclusively Human Milk Was Similar to the Control Cohort That Received Bovine Milk Products Prior to 33 Weeks Postmenstrual Age
| Characteristic | Control cohort | EHM cohort | p |
|---|---|---|---|
| Birth weight (kg) | 1.334 (0.436) | 1.361 (0.542) | 0.503 |
| Gestational age (weeks) | 29.7 (2.5) | 29.6 (3.0) | 0.920 |
| Multiples | 172 (39%) | 64 (32%) | 0.106 |
| Small for gestational age | 46 (10%) | 27 (14%) | 0.282 |
| Male | 225 (51%) | 110 (55%) | 0.306 |
| Ethnicity | |||
| White | 363 (82%) | 167 (84%) | 0.576 |
| Black | 45 (10%) | 16 (8%) | 0.388 |
| Other | 35 (8%) | 16 (8%) | 1 |
| Respiratory distress syndrome | 335 (76%) | 167 (84%) | 0.023 |
| Surfactant administration | 300 (68%) | 151 (76%) | 0.040 |
| Ventilation | 317 (72%) | 129 (65%) | 0.096 |
| High-frequency oscillatory | 57 (13%) | 38 (19%) | 0.042 |
| Jet | 11 (2%) | 10 (5%) | 0.147 |
| Postnatal steroid | 66 (15%) | 33 (17%) | 0.815 |
| UAC | 351 (79%) | 158 (79%) | 1 |
| UVC | 296 (67%) | 122 (61%) | 0.180 |
| PDA | 91 (21%) | 46 (23%) | 0.467 |
| PDA treated with indomethacin | 75 (17%) | 29 (15%) | 0.489 |
| Sepsis | |||
| Clinical, culture negative | 93 (21%) | 51 (26%) | 0.220 |
| Bacterial, culture positive | 40 (9%) | 5 (3%) | 0.004 |
| Fungal (Candida) | 6 (1%) | 0 (0%) | 0.185 |
| Discharged home | 387 (87%) | 162 (81%) | 0.053 |
| Acute transfer | 37 (8%) | 25 (13%) | 0.112 |
| Died before transfer | 19 (4%) | 12 (6%) | 0.426 |
| Total | 443 (100%) | 199 (100%) | |
Data are mean (SD) or number (%) values as indicated.
EHM, exclusively human milk; PDA, patent ductus arteriosus; UAC, umbilical arterial catheter; UVC, umbilical venous catheter.
Table 2.
Gestational Age and Birth Weight Distributions for the Control and the Exclusive Human Milk Cohorts
| Control cohort | Exclusively human milk cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gestational age | n | % | Birth weight | SD | n | % | Birth weight | SD | p |
| <26 | 41 | 9.3% | 0.650 | 0.128 | 29 | 14.6% | 0.590 | 0.132 | 0.189 |
| 26–27 | 59 | 13.3% | 0.916 | 0.203 | 20 | 10.1% | 0.883 | 0.194 | 0.626 |
| 28–29 | 102 | 23.0% | 1.169 | 0.233 | 38 | 19.1% | 1.177 | 0.245 | 0.050 |
| 30–32 | 241 | 54.4% | 1.623 | 0.310 | 112 | 56.3% | 1.709 | 0.402 | 0.023 |
| All | 443 | 100.0% | 1.334 | 0.436 | 199 | 100.0% | 1.361 | 0.542 | 0.920 |
Feeding
The age of reaching full enteral feeding was similar for the control and EHM cohorts: 16.7 (SD 8.3) and 17.4 (SD 10.2) days, respectively, which was not clinically or statistically significantly different (p=0.424). The youngest GA infants reached full feeding at older chronologic ages: 37 (SD 6.1) versus 45 (SD 11.8) days at 23–25 weeks GA (p=0.189); 25.3 (SD 9.4) versus 27.6 (SD 7.9) days at 26–27 weeks GA (p=0.459); 18.8 (SD 7.5) versus 21.2 (SD 6.7) at 28–29 weeks GA (p=0.118); and 13.3 (SD 5.2) versus 13.1 (SD 6.2) days at 30–32 weeks GA (p=0.792) for the control versus EHM cohorts, respectively.
NEC
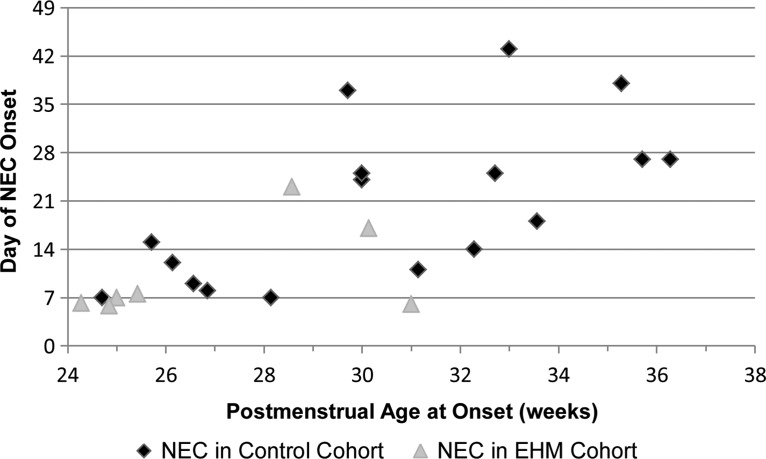
NEC occurred in 17 of 443 infants (3.8%) in the control cohort and in seven of 199 (3.5%) in the EHM cohort. Expressed by the PMA and chronologic age at the day of onset, there was a significant reduction of NEC in the EHM cohort for the day of onset (p=0.042) and the PMA at onset (p=0.011) (Fig. 2).
FIG. 2.
The incidence of late necrotizing enterocolitis (NEC) was reduced for infants receiving an exclusively human milk (EHM) diet through 33 weeks postmenstrual age.
The distribution of the age of onset of NEC was significantly different for the two cohorts (p=0.009). In the control cohort, 15 of 443 infants (3.4%) developed NEC after Day 7. In the EHM cohort, two of 199 infants (1.0%) developed NEC after Day 7. The occurrence of NEC prior to Day 8 of life was not different for the two cohorts (p=0.092). In the EHM cohort NEC was observed in five infants and occurred on Day 6 or Day 7. Their GAs were 23, 24, 24, 24, and 30 weeks, and they had received 18, 0, 0, 0, and 282 mL/kg of MOM in the previous 7 days, respectively. In the EHM cohort NEC was observed in two infants after Day 7. NEC appeared on Day 17 in an infant with trisomy 21 receiving 120 mL/kg/day of MOM (665 mL/kg in the previous 7 days), and NEC appeared on Day 23 in an infant with severe respiratory distress who had received therapeutic indomethacin beginning on Day 21. For this second infant, feeding stopped on Day 21 for indomethacin therapy, and prior to Day 21 the infant was receiving <30 mL/kg/day (94 mL/kg in the previous 7 days) of MOM.
Discussion
The risk of developing NEC from one NICU to another is quite variable, leading to speculation that the feeding practice of a NICU may contribute to the incidence of NEC. In addition to feeding practices, NEC has variously been attributed to intolerance of bovine milk protein, chorioamnionitis, intrauterine asphyxia, hypovolemia, congenital heart disease, abnormal intestinal development, medications, infections, blood transfusions, and other etiologies.13,19–21 The relative contribution of each remains unclear. The primary goal of the study was to determine the rate of NEC associated with an EHM diet. Past observational studies had suggested that NEC was reduced in proportion to the amount of human milk a premature infant received.6,9,10,12 The EHM feeding practice in this study eliminated all bovine-based artificial milk products and targeted those infants who were considered to be at greatest risk for developing NEC. The current study demonstrates that the EHM diet reduces occurrence of NEC during the phase of enteral nutrition. The single important practice change during the study period was the elimination of bovine milk products prior to 33 weeks PMA. The rate of enteral feeding advancement was unchanged in the EHM cohort as evidenced by the duration of PN: both groups received 17 days. However, a large randomized controlled trial of the EHM diet would provide stronger evidence that delaying introduction of bovine products reduces the incidence of NEC.
The EHM diet has been reported to significantly reduce the occurrence of NEC; however, in these previous studies the comparison cohorts had high baseline NEC rates (15.9% and 21%).8,22 In the present study, the baseline rate of NEC in the control cohort was low (3.8%). The distribution of NEC onset in the control cohort was scattered and resembled the chronologic age and PMA distribution of previously reported research.13 In contrast, the majority of the NEC in the EHM cohort occurred on Days 6 and 7, before much enteral milk was provided. The onset of NEC after Day 7, during the enteral nutrition phase, was rare for the EHM cohort (1%).
NEC in the premature infant is not a single disease entity derived from a single cause or condition. A bimodal age distribution of NEC was previously reported, with the early peak at 8 days and the later peak at 19 days of age in infants born <33 weeks PMA.23 A bimodal distribution suggests two major etiologies for NEC. In the present study, five of the seven infants in the EHM cohort developed NEC on Day 6 or Day 7 of life, during the early modal distribution of NEC. Three of the five infants had never been fed milk, one had received <5 mL of MOM, and one infant had received daily 15 mL/kg/day increases of MOM. Thus, four of the five infants who developed NEC before Day 8 in the EHM cohort had received very little milk. After Day 7, two infants received EHM and subsequently developed NEC. The first infant had trisomy 21 and consequently was at fourfold greater risk for developing NEC.24 The second infant had severe respiratory distress syndrome and received indomethacin for a significant patent ductus arteriosus. But, therapeutic indomethacin has been reported not to increase the risk of NEC.25 Factors independent of the enteral feeding strategy—genetic predisposition and compromised physiology—increased the risks for NEC for these two infants.
Predisposing factors should be considered when evaluating the potential association of an enteral feeding practice and the risk of NEC. The lack of NEC reduction during the earlier first mode is consistent with a trivial volume of enteral feeding in the first days of life for both cohorts, and the reduced NEC during the second mode was associated with elimination of bovine milk in the EHM cohort. Introduction of enteral feeding in the first days of life has been suggested to reduce NEC, and insufficient early enteral nutrition combined with PN has been linked to NEC in experimental piglet models of NEC, but early enteral feeding has not been demonstrated to reduce occurrence of NEC in premature infants.26–32 Therefore, factors other than the choice of milk require further evaluation for NEC that occurs in the first week of life.
Our findings agree with a prospective randomized multicenter trial of NEC and an EHM diet that compared bovine milk-based fortifier (BOV) and human milk-based fortifier added to MOM and DHM.8 This trial gathered 138 infants who had received an EHM diet in 12 NICUs. Eight of the 138 EHM infants (5.8%) in this trial developed NEC. Three of these eight, however, had inadvertently received BOV prior to the onset of NEC. If the three infants who had inadvertently received BOV are excluded, then only five of 135 infants (3.7%) developed NEC associated with an EHM diet. After this adjustment, the multicenter study and the current study observe a similar low rate of NEC associated with an EHM diet. Differences in enteral feeding strategies may contribute to the rate of NEC. The multicenter trial reported a comparison cohort that received BOV; 11 of 69 infants (15.9%) in the BOV cohort developed NEC.8 The BOV infants received bovine fortification at a lower threshold enteral feeding volume (100 mL/kg/day) compared with fortification in the control cohort of the present study (150 mL/kg/day). This observation also suggests that bovine-based fortification should be delayed in order to reduce the risk of NEC.
A recent single-site study33 provided detailed nutritional information for 102 Belgian infants who received early PN and an enteral diet of MOM and DHM. The goal of the Belgian study33 was to improve postnatal growth of premature infants by applying expert nutritional recommendations34 that included fortification of human milk. Similar to the previously described multicenter trial,8 infants in this study received a bovine-based fortifier when their enteral intake reached 100 mL/kg/day. Nine of 102 infants (9%) developed NEC. The greater rate of NEC in the Belgian single-site study33 compared with the present single-site study is suggestive that earlier bovine-based fortification of human milk increases the risk of NEC.
Inherent in all retrospective observational studies is the potential for other clinical practice changes occurring over time to influence the measured outcome. A relatively uniform practice pattern was maintained by the neonatologists through both cohorts, with a significant exception that infants in the EHM cohort received more fluid and nutrition in the first 2 days of life.16 This practice may have influenced the rate of NEC that occurred unrelated to enteral feeding at the end of the first week of life, but the sample size of the study was not sufficient to evaluate that effect. Although increasing early fluid administration may have increased NEC, the available evidence supports the observation that increasing early vascular volume reduces NEC.21 Additionally, over the duration of the study period there were incremental changes in the NICU's management of central vascular catheters in an effort to reduce bloodstream infections. Consequently, we have not concluded that the EHM diet caused the significant reduction in bacterial sepsis demonstrated in Table 1.
NEC persists in the very premature infant population, inversely proportional to GA and weight. A bimodal distribution of the age of onset suggests separate etiologies for early and late NEC. In the current study, the late-phase NEC was significantly reduced in a single NICU with homogeneous clinical care practices by delaying introduction of bovine milk products. The early-phase NEC persisted, especially at the youngest GAs, with no significant incidence change. The conclusion that the EHM diet reduces NEC is limited by the cohort study design and the persistence of early NEC. This early-phase NEC occurred prior to providing any significant volume of milk; therefore, the choice of milk as an etiology for early NEC lacks a plausible physiologic foundation. The EHM diet effectively reduced the occurrence of late NEC, which potentially exposes early NEC to investigation in future studies. Other early clinical care practices deserve evaluation for early NEC, for example, the timing and volume of early milk feeding or delayed cord clamping to reduce hypovolemic bowel ischemia.21 Future studies should attend to early and late NEC etiologies.
Acknowledgments
K.C. received research funding from the Australian Research Council (grant DP11110103125) and an Endeavour Fellowship.
Disclosure Statement
No competing financial interests exist.
References
- 1.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–e841 [DOI] [PubMed] [Google Scholar]
- 2.Grave GD, Nelson SA, Walker W, et al. . New therapies and preventive approaches for necrotizing enterocolitis: Report of a research planning workshop. Pediatr Res 2007;62:510–514 [DOI] [PubMed] [Google Scholar]
- 3.Henderson G, Craig S, Brocklehurst P, et al. . Enteral feeding regimens and necrotising enterocolitis in preterm infants: A multicentre case-control study. Arch Dis Child Fetal Neonatal Ed 2009;94:F120–F123 [DOI] [PubMed] [Google Scholar]
- 4.Lucas A, Cole T. Breast milk and neonatal necrotising enterocolitis. Lancet 1990;336:1519–1523 [DOI] [PubMed] [Google Scholar]
- 5.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: Beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999;103:1150–1157 [DOI] [PubMed] [Google Scholar]
- 6.Schanler RJ, Lau C, Hurst NM, et al. . Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. Pediatrics 2005;116:400–406 [DOI] [PubMed] [Google Scholar]
- 7.Boyd C, Quigley M, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: Systematic review and meta-analysis. Arch Dis Chld Fetal Neonatal Ed 2006;92:F169–F175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan S, Schanler R, Kim J, et al. . Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. J Pediatr 2010;156:562–567 [DOI] [PubMed] [Google Scholar]
- 9.Meinzen-Derr J, Poindexter B, Wrage L, et al. . Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol 2009;29:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corpeleijn WE, Kouwenhoven SM, Paap MC, et al. . Intake of own mother's milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life. Neonatology 2012;102:276–281 [DOI] [PubMed] [Google Scholar]
- 11.Denning PW, Maheshwari A. Necrotizing enterocolitis: Hope on the horizon. Clin Perinatol 2013;40:xvii–xix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sisk PM, Lovelady CA, Dillard RG, et al. . Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol 2007;27:428–433 [DOI] [PubMed] [Google Scholar]
- 13.Sharma R, Hudak ML, Tepas JJ 3rd, et al. . Impact of gestational age on the clinical presentation and surgical outcome of necrotizing enterocolitis. J Perinatol 2006;26:342–347 [DOI] [PubMed] [Google Scholar]
- 14.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: Past, present, and future. Clin Perinatol 2013;40:21–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann KR, Herrmann KR. Early parenteral nutrition and successful postnatal growth of premature infants. Nutr Clin Pract 2010;25:69–75 [DOI] [PubMed] [Google Scholar]
- 16.Herrmann K, Woolen S. Early parenteral nutrition and subsequent growth of premature infants. In: Watson R, Grimble G, Preedy V, et al. (eds.). Nutrition in Infancy, Volume 1. New York: Springer, 2013:185–206 [Google Scholar]
- 17.Bell MJ, Ternberg JL, Feigin RD, et al. . Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VassarStats: Website for statistical computation. www.vassarstats.net/ (accessed March13, 2013)
- 19.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon P, Christensen R, Weitkamp J, et al. . Mapping the new world of necrotizing enterocolitis (NEC): Review and opinion. EJ Neonatol Res 2012;2:146–172 [PMC free article] [PubMed] [Google Scholar]
- 21.Rabe H, Diaz-Rossello JL, Duley L, et al. . Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 2012;8:CD003248. [DOI] [PubMed] [Google Scholar]
- 22.Cristofalo EA, Schanler RJ, Blanco CL, et al. . Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr 2013;163:1592–1595.e1 [DOI] [PubMed] [Google Scholar]
- 23.Yee WH, Soraisham AS, Shah VS, et al. . Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129:e298–e304 [DOI] [PubMed] [Google Scholar]
- 24.Boghossian NS, Hansen NI, Bell EF, et al. . Survival and morbidity outcomes for very low birth weight infants with Down syndrome. Pediatrics 2010;126:1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones LJ, Craven PD, Attia J, et al. . Network meta-analysis of indomethacin versus ibuprofen versus placebo for PDA in preterm infants. Arch Dis Child Fetal Neonatal Ed 2011;96:F45–F52 [DOI] [PubMed] [Google Scholar]
- 26.Goldstein RM, Hebiguchi T, Luk GD, Taqi F, Guilarte TR, et al. . The effects of total parenteral nutrition on gastrointestinal growth and development. J Pediatr Surg 1985;20:785–791 [DOI] [PubMed] [Google Scholar]
- 27.Morgan W, 3rd, Yardley J, Luk G, et al. . Total parenteral nutrition and intestinal development: a neonatal model. J Pediatr Surg 1987;22:541–545 [DOI] [PubMed] [Google Scholar]
- 28.Dudley MA, Wykes LJ, Dudley AW Jr, et al. . Parenteral nutrition selectively decreases protein synthesis in the small intestine. Am J Physiol 1998;274:G131–G137 [DOI] [PubMed] [Google Scholar]
- 29.Niinikoski H, Stoll B, Guan X, et al. . Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr 2004;134:1467–1474 [DOI] [PubMed] [Google Scholar]
- 30.Bjornvad CR, Schmidt M, Petersen YM, et al. . Preterm birth makes the immature intestine sensitive to feeding-induced intestinal atrophy. Am J Physiol Regul Integr Comp Physiol 2005;289:R1212–R1222 [DOI] [PubMed] [Google Scholar]
- 31.Siggers J, Sangild PT, Jensen TK, et al. . Transition from parenteral to enteral nutrition induces immediate diet-dependent gut histological and immunological responses in preterm neonates. Am J Physiol Gastrointest Liver Physiol 2011;301:G435–G445 [DOI] [PubMed] [Google Scholar]
- 32.Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database Syst Rev 2013;3:CD000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senterre T, Rigo J. Optimizing early nutritional support based on recent recommendations in VLBW infants allows abolishing postnatal growth restriction. J Pediatr Gastroenterol Nutr 2011;53:536–542 [DOI] [PubMed] [Google Scholar]
- 34.Agostoni C, Buonocore G, Carnielli VP, et al. . Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 2010;50:85–91 [DOI] [PubMed] [Google Scholar]