Abstract
For influenza hemagglutination inhibition (HAI) assays, species selection of red blood cells (RBCs) is critical to determine antibody titers to influenza viruses reliably. We compared pandemic influenza virus A/H1N1 (pdH1N1) HAI titers using turkey or guinea pig RBCs. Turkey RBCs appear to be the more appropriate species choice for influenza A/pH1N1 HAI assays.
Introduction
Influenza viruses are a global threat to public health and, in the United States alone, millions of people are infected on average each year (25). However, inactivated and live attenuated influenza vaccines reduce the annual morbidity and mortality by inducing protective antibodies against circulating influenza viruses. The influenza virus hemagglutinin (HA) protein mediates receptor binding to sialic acids on the membranes of specific cell types, such as epithelial cells, in the respiratory tract (2,5,19,23), and also binds sialic acids on red blood cells (RBCs) resulting in hemagglutination. The ability of influenza-specific antibodies to bind to the HA protein to prevent hemagglutination of RBCs is the basis for the hemagglutination inhibition (HAI) assay, a quantitative and inexpensive approach to diagnose influenza infection serologically (11,24,26), and measure the humoral immune response following influenza vaccination. An HAI titer of 1:40 has been considered seroprotective (3), although this is somewhat arbitrary.
Selection of the appropriate species of RBCs for the HAI assay is important, since the affinity of the HA globular head for sialic acid varies among the different types and strains of influenza viruses (4,9,10,16,18). Sialic acid moieties are bound to galactose sugars through α(2,3)-linkages (SAα2,3Gal) and/or SAα2,6Gal, depending upon the host species. The proportion of these linkages differs across various species. For instance, horse RBCs predominately contain SAα2,3Gal, making it an ideal choice to determine HAI titers against A/H5N1 strains (4,8,10). In contrast, RBCs from turkeys and guinea pigs contain disproportionately more SAα2,6Gal than SAα2,3Gal (1,4,10,13,21). Both species' RBCs are commonly used to measure protection against A/H3N2 and A/pH1N1 viral strains, though assay sensitivity may differ between species (1,13,21). The composition of sialic acid receptors on RBCs can be enzymatically altered to influence detection of influenza hemagglutinin-specific antibody responses after influenza infection or vaccination (15,20). The goal of this study was to compare antibody titers of the influenza vaccine strain A/California/7/2009 (pdH1N1) in a cohort of older individuals from two different HAI data sets obtained with turkey or guinea pig RBCs.
Older subjects between 50 and 74 years old (n=106) received a single intramuscular (IM) dose of the 2010–2011 seasonal trivalent influenza vaccine (TIV; Fluarix). The Mayo Clinic Institutional Review Board granted approval for the study. Written, informed consent from subjects was obtained at the time of enrollment. Blood samples were collected prior to (day 0) and following vaccination (days 3, 28, and 75). Humoral immune responses were measured for each subject at all time points by measuring HAI titers against the influenza vaccine strain A/California/7/2009 (pdH1N1). The virus was propagated in the allantoic cavity of embryonated chicken eggs, and Vibrio cholerae filtrate (Sigma-Aldrich, St. Louis, MO) was used for receptor-destroying enzyme (RDE) treatment, as described elsewhere (22). Before the HAI assay was performed, subjects' sera were pretreated with receptor-destroying enzyme (1:4 dilution; Accurate Chemical and Scientific, Westbury, NY; Sigma-Aldrich) to inactivate nonspecific inhibitors of hemagglutination. Serial dilutions of treated serum samples were permitted to react with influenza virus at a fixed dose of 8 hemagglutinin units (HAU) per 50 μL, followed by the addition of either 0.5% turkey or 0.6% guinea pig RBCs (Lampire Biological Laboratories, Pipersville, PA). The virus was independently standardized against the respective RBCs, which may also influence the actual amount of virus included in each assay. All serum samples were tested in triplicate. HAI titers were read after a 45 min (turkey) or 1 h (guinea pig) incubation time. The HAI titer was reported as the reciprocal of the highest dilution of serum in which complete inhibition of hemagglutination occurred. Influenza A/H1N1 antiserum (Centers for Disease Control and Prevention, Atlanta, GA) was used as a positive reference antiserum for the HAI assay using guinea pig RBCs. There was no positive control available for the assay using turkey RBCs. Negative controls consisted of serum and RBCs only. Further details of the HAI assay have been described elsewhere (12,24,26).
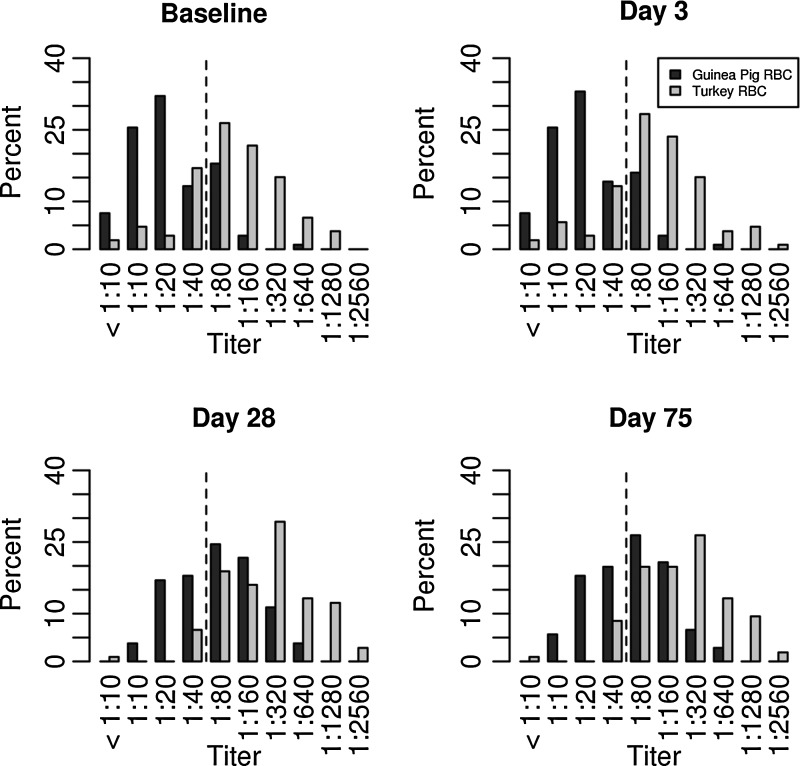
A comparison of the HAI titers determined for serum samples obtained on days 0 (baseline), 3, 28, and 75 post-vaccination with either guinea pig or turkey RBCs is shown in Figure 1 and Table 1. The HAI titers of the two species were statistically compared for each subject at each time point using Wilcoxon signed-rank tests, and a p≤0.0001 was considered significant. As expected, the percentage of subjects achieving seroprotection increased post-vaccination (Fig. 1 and Table 2), regardless of the species of RBCs used. However, the Gaussian distribution appeared to shift consistently to the left when guinea pig RBCs were used.
FIG. 1.
A comparison of hemagglutination inhibition (HAI) titers against the H1N1 influenza vaccine strain obtained with turkey or guinea pig red blood cells (RBCs) in an older adult population (percent). The dotted lines represent the lowest level of seroprotection (1:40 HAI antibody titer).
Table 1.
Distribution of Hemagglutination Inhibition Titers Using Turkey or Guinea Pig Red Blood Cells
| Minimum (HAI titer) | 25th percentile (HAI titer) | Median (HAI titer) | 75th percentile (HAI titer) | Maximum (HAI titer) | |
|---|---|---|---|---|---|
| Turkey RBCs | |||||
| Baseline | 1:5 | 1:40 | 1:80 | 1:320 | 1:1,280 |
| Day 3 | 1:5 | 1:80 | 1:80 | 1:160 | 1:2,560 |
| Day 28 | 1:5 | 1:80 | 1:320 | 1:640 | 1:2,560 |
| Day 75 | 1:5 | 1:80 | 1:320 | 1:320 | 1:2,560 |
| Guinea pig RBCs | |||||
| Baseline | 1:5 | 1:10 | 1:20 | 1:40 | 1:640 |
| Day 3 | 1:5 | 1:10 | 1:20 | 1:40 | 1:640 |
| Day 28 | 1:10 | 1:40 | 1:80 | 1:160 | 1:640 |
| Day 75 | 1:10 | 1:40 | 1:80 | 1:160 | 1:640 |
HAI, hemagglutination inhibition; RBCs, red blood cells.
Table 2.
Rates of Seroprotection (1:40) at Baseline and at Day 28 or Day 75 Using Turkey or Guinea Pig RBCs
| Baseline | ||||
|---|---|---|---|---|
| Turkey RBC | Guinea pig RBC | |||
| <1:40 | ≥1:40 | <1:40 | ≥1:40 | |
| Day 28 | ||||
| <1:40 | 1 | 0 | 21 | 1 |
| ≥1:40 | 9 | 96 | 48 | 36 |
| Day 75 | ||||
| <1:40 | 1 | 0 | 25 | 0 |
| ≥1:40 | 9 | 96 | 44 | 37 |
A fourfold or more increase in HAI titers post-vaccination relative to day 0 indicates individual seroconversion (6,7). On day 3, 106 (100%) and 101 (95.3%) subjects had less than a fourfold increase in HAI titers relative to day 0 using guinea pig and turkey RBCs respectively, as shown in Table 3. However, by day 28, the peak of humoral immunity, differences in HAI titers were found between the two species. For instance, 49 (46.2%) and 37 (34.9%) subjects seroconverted when guinea pig and turkey RBCs, respectively, were used, as shown in Table 3. On day 28, 57 out of 106 subjects (53.8%) had less than a fourfold increase in HAI titers relative to day 0 using guinea pig RBCs, and 69 subjects (65.1%) had less than a fourfold increase in HAI titers to day 0 using turkey RBC (p<0.01), as shown in Table 3. By day 75, 45 subjects (42.5%) seroconverted as determined by HAI assay with guinea pig RBCs, and 32 subjects (30.2%) seroconverted as determined by HAI assay with turkey RBCs were used (p<0.005), as shown in Table 3. We found that turkey RBC-based HAI assays can be more sensitive, but guinea pig RBCs can more accurately measure seroconversion in our study subjects. However, further work is needed to validate these results and to standardize the serological RBC-based HAI assays for influenza vaccine-induced humoral response assessment.
Table 3.
Fold-Change Comparisons of the HAI Titers in Study Subjects at Day 3, Day 28, and Day 75 Using Turkey or Guinea Pig RBCs
| Guinea pig RBC | ||
|---|---|---|
| <Fourfold change | ≥Fourfold change | |
| Day 3 relative to baseline | ||
| Turkey RBC | ||
| <Fourfold change | 101 | 0 |
| ≥Fourfold change | 5 | 0 |
| Day 28 relative to baseline | ||
| Turkey RBC | ||
| <Fourfold change | 54 | 15 |
| ≥Fourfold change | 3 | 34 |
| Day 75 relative to baseline | ||
| Turkey RBC | ||
| <Fourfold change | 54 | 20 |
| ≥Fourfold change | 7 | 25 |
A plausible explanation for this discrepancy of HAI titers between assays is procedural differences, including the passage history of the influenza virus used. For instance, prior to performing the HAI assay, packed guinea pig RBCs (1:20 dilution) were incubated for 1 h with treated serum to eliminate any possible nonspecific hemagglutination that was not inhibited by RDE and/or inactivated by heat. In contrast, prior to carrying out the HAI assay using turkey RBCs, packed turkey RBCs were not preincubated with serum, but were only washed with phosphate-buffered saline (PBS). While RDE treatment and heat inactivation most likely eliminated all nonspecific binding, any nonspecific binding could potentially shift the dose–response curve to the right. Another possible explanation is that the different RBC substrates may provide different HA titers for the same amount of virus. For instance, 1e6 plaque forming units (pfu) of influenza H1N1 virus may give an HA titer of 32 for turkey RBC, but only 16 for guinea pig RBCs. Therefore, virus standardized to 8 HAU on the different RBCs would actually contain different amounts of virions. It is possible that more virus was used on one cell type to obtain an HA titer of 8 than on the other cell type, therefore requiring more antibody to prevent hemagglutination.
Another possible reason is the difference in the ease of detectability of the HAI titration endpoint between the two species. Turkey RBCs are nucleated and quickly settle to the bottom of the V-shaped 96-well plate in the absence of hemagglutination, forming a condensed button (24,26). Guinea pig (and human) RBCs form a halo of cells at the bottom of the well. It is more difficult to distinguish partial hemagglutination from complete hemagglutination when observing “halos” than when observing “buttons.” To improve the distinction and for the better visualization of complete settling of RBCs, the World Health Organization (WHO) recommends using a higher percentage of guinea pig cells (0.75%) and consistent standardization of RBC suspensions (26). While we used a lower percentage of packed guinea pig RBCs (0.6%), we did not observe a noticeable difference between 0.75% and 0.6% RBCs (data not shown).
Though complete hemagglutination occurs in turkey and guinea pig RBCs, along with chicken and human RBCs, higher HAI titers have been reported for turkey RBCs following incubation with pH1N1 and H3N2 influenza strains (14). For instance, the HAI titers were 2,048 HAU and 512 HAU following incubation of a human influenza H1N1 viral strain (A/Puerto Rico/8/34) with turkey or guinea pig RBCs respectively (14). HAI titers were also higher using turkey RBCs (1,024) than guinea pig RBCs (256) with a swine influenza H1N1 isolate (A/Swine/Kor/GC0503/05) (14). In addition, two separate studies reported higher HAI titers using turkey RBCs with different H3N2 isolates (14,21). It is worth noting that the lowest HAI titers reported used horse RBCs, which contains only SAα2,3Gal receptors (14). This may suggest that turkey RBCs have a higher proportion of SAα2,6Gal than guinea pig RBCs and, therefore, hemagglutinate at a higher titer. It may also suggest that the affinity of hemagglutinin is lower for SAα2,6Gal on guinea pig RBCs.
The gold-standard functional serological measurement of humoral immunity against influenza vaccination/infection is microneutralization (11,17). Due to the laborious and costly nature of the assay, HAI assays are generally preferred in larger-scale studies. In a study examining the concordance of HAI and microneutralization antibody titers using RBCs from six different species (human, chicken, turkey, goose, pig, and horse), turkey RBCs produced the most comparable results to human blood group O erythrocytes (geometric mean titer=72) (13).
In conclusion, our study suggests that the use of turkey RBCs for HAI assays provides a higher measure of HAI titers following influenza A/pdH1N1 vaccination than the use of guinea pig RBCs. This study demonstrates the need to standardize HAI protocols, including reference sera, regarding the appropriate species selection of RBCs specific for a particular virus/viral strain, and the potential need to revise WHO guidance on HAI assays. It also suggests that HAI assays utilizing RBCs from difference species may not exhibit the same performance. Thus, the cell choice may vary depending on whether one is looking to detect antibodies present in human serum, or whether one is trying to determine seroconverion rates accurately. These improvements will enhance serodiagnostic accuracy and will serve as a more consistent correlate of vaccine-induced seroprotection.
Acknowledgments
We thank the Mayo Clinic Vaccine Research Group and the subjects who participated in our studies. We thank Matthew J. Phan for his assistance with the HAI assay, Diane E. Grill for her assistance in statistical analysis, and Caroline L. Vitse for her editorial assistance. The authors acknowledge support from NIH grant U01AI089859 and 1U19AI089987-01 for this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
G.A.P. is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. G.A.P. offers consultative advice on vaccine development to Merck & Co., Inc., CSL Biotherapies, Avianax, Sanofi Pasteur, Dynavax, Novartis Vaccines and Therapeutics, PAXVAX, Inc., and Emergent Biosolutions. G.A.P. and I.G.O. hold two patents related to vaccinia and measles peptide research. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
References
- 1.Barr IG, McCauley J, Cox N, et al. . Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 Northern Hemisphere season. Vaccine 2010;28:1156–1167 [DOI] [PubMed] [Google Scholar]
- 2.Baum LG, and Paulson JC. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem Suppl 1990;40:35–38 [PubMed] [Google Scholar]
- 3.Brydak LB, and Machala M. Humoral immune response to influenza vaccination in patients from high risk groups. Drugs 2000;60:35–53 [DOI] [PubMed] [Google Scholar]
- 4.Connor RJ, Kawaoka Y, Webster RG, and Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 1994;205:17–23 [DOI] [PubMed] [Google Scholar]
- 5.Couceiro JN, Paulson JC, and Baum LG. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res 1993;29:155–165 [DOI] [PubMed] [Google Scholar]
- 6.Coudeville L, Bailleux F, Riche B, Megas F, Andre P, and Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 2010;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, and Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73 [PubMed] [Google Scholar]
- 8.Ducatez MF, Cai Z, Peiris M, et al. . Extent of antigenic cross-reactivity among highly pathogenic H5N1 influenza viruses. J Clin Microbiol 2011;49:3531–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai M, and Kawaoka Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr Opin Virol 2012;2:160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, and Kawaoka Y. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology 1997;227:493–499 [DOI] [PubMed] [Google Scholar]
- 11.Katz JM, Hancock K, and Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther 2011;9:669–683 [DOI] [PubMed] [Google Scholar]
- 12.Lowen AC, Steel J, Mubareka S, Carnero E, Garcia-Sastre A, and Palese P. Blocking interhost transmission of influenza virus by vaccination in the guinea pig model. J Virol 2009;83:2803–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makkoch J, Prachayangprecha S, Payungporn S, et al. . Erythrocyte binding preference of human pandemic influenza virus a and its effect on antibody response detection. Ann Lab Med 2012;32:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam JH, Kim EH, Song D, Choi YK, Kim JK, and Poo H. Emergence of mammalian species-infectious and -pathogenic avian influenza H6N5 virus with no evidence of adaptation. J Virol 2011;85:13271–13277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulson JC, and Rogers GN. Resialylated erythrocytes for assessment of the specificity of sialyloligosaccharide binding proteins. Methods Enzymol 1987;138:162–168 [DOI] [PubMed] [Google Scholar]
- 16.Rogers GN, and Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983;127:361–373 [DOI] [PubMed] [Google Scholar]
- 17.Rowe T, Abernathy RA, Hu-Primmer J, et al. . Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999;37:937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan-Poirier KA, and Kawaoka Y. Distinct glycoprotein inhibitors of influenza A virus in different animal sera. J Virol 1991;65:389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skehel JJ, and Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 2000;69:531–569 [DOI] [PubMed] [Google Scholar]
- 20.Stephenson I, Wood JM, Nicholson KG, and Zambon MC. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J Med Virol 2003;70:391–398 [DOI] [PubMed] [Google Scholar]
- 21.Thompson CI, Barclay WS, and Zambon MC. Changes in in vitro susceptibility of influenza A H3N2 viruses to a neuraminidase inhibitor drug during evolution in the human host. J Antimicrob Chemother 2004;53:759–765 [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Taaffe J, Parker C, et al. . Hemagglutinin (HA) proteins from H1 and H3 serotypes of influenza A viruses require different antigen designs for the induction of optimal protective antibody responses as studied by codon-optimized HA DNA vaccines. J Virol 2006;80:11628–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, and Wiley DC. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 1988;333:426–431 [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 2002. WHO Manual on Animal Influenza Diagnosis and Surveillance, pp. 1–99 [Google Scholar]
- 25.World Health Organization. 2009. Influenza (Seasonal). www.who.int/mediacentre/factsheets/fs211/en/ Accessed March20, 2013
- 26.World Health Organization. 2011. WHO Global Influenza Surveillance Network: Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza, pp. 1–139 [Google Scholar]