Abstract
LFA-1/ICAM-1 interaction is essential in support of inflammatory and specific T-cell regulated immune responses by mediating cell adhesion, leukocyte extravasation, migration, antigen presentation, formation of immunological synapse, and augmentation of T-cell receptor signaling. The increase of ICAM-1 expression levels in conjunctival epithelial cells and acinar cells was observed in animal models and patients diagnosed with dry eye. Therefore, it has been hypothesized that small molecule LFA-1/ICAM-1 antagonists could be an effective topical treatment for dry eye. In this letter, we describe the discovery of a potent tetrahydroisoquinoline (THIQ)-derived LFA-1/ICAM-1 antagonist (SAR 1118) and its development as an ophthalmic solution for treating dry eye.
Keywords: LFA-1/ICAM-1 antagonist, tetrahydroisoquinoline derivative, SAR 1118, ophthalmic solution, dry eye
Lymphocyte function-associated antigen-1 [LFA-1, αLβ2, or cluster of differentiation 11a (CD11a)/CD18] is a heterodimeric member of the β2 family of integrins. Its interactions with intercellular adhesion molecule-1 (ICAM-1 or CD54) are not only critical to the adhesion, migration, and proliferation of T-cells at the inflammation sites1−3 but also have influence on T-cell specific immune responses through both adhesive interactions and formations of immunological synapses in conjunction with T-cell receptors and major histocompatibility complex (MHC).4−6 In the past, interest in LFA-1 antagonism of ICAM-1 as an approach to identifying novel anti-inflammatory therapies has focused on psoriasis, atopic dermatitis, rheumatoid arthritis, and asthma as targets for systemic treatments.7,8
Dry eye [keratoconjunctivitis sicca (KCS), keratitis sicca, or xerophthalmia] is an ocular disease caused by either decreased tear production or increased tear film evaporation in humans and some animals.9 The etiology of this chronic disease is multifactorial and complex, but emerging evidence supports the hypothesis that chronic inflammation is an important factor in the pathogenesis of dry eye.10,11 Conjunctival biopsies from patients diagnosed with dry eye have implicated the aberrant presence of CD-3 positive T-cells as the inflammatory basis of the disease12 and provided a rationale for the use of nonspecific immunosuppressant cyclosporine (Restasis) in treating dry eye.13 Also, the increase of ICAM-1 expression levels in conjunctival epithelial cells and acinar cells of animal models and patients diagnosed with dry eye was observed.14 Therefore, we postulated that a small molecule LFA-1/ICAM-1 antagonist could offer a targeted T-cell antagonism with improved patient tolerability for such a chronic ocular disease.
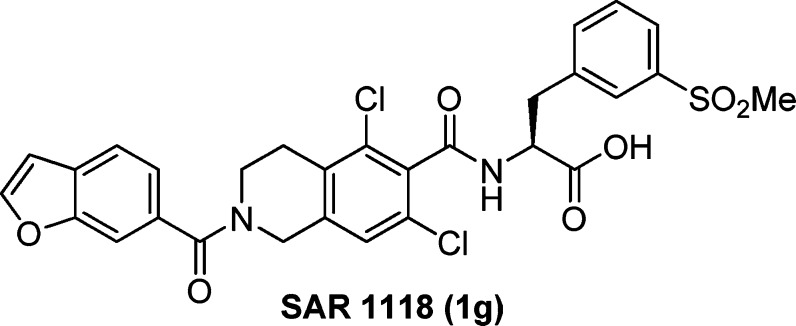
Recently, we have described the discovery of a series of potent tetrahydroisoquinoline (THIQ)-derived LFA-1/ICAM-1 antagonists,15,16 which were shown to bind the I-domain of the CD11a subunit of LFA-1 and serve as direct competitive antagonists of LFA-1 binding to ICAM-1.17,18 To our knowledge, most of the marketed ophthalmic solutions resulted from the repurposing of the drugs developed for nonocular indications. They typically have reasonable oral bioavailability and slow clearance from the systemic circulation.19 Interestingly, Atenolol and Propranolol, two controls that are used to define both para- and trans-cellular uptake of drugs by oral administration, show similar systemic bioavailability when delivered as ophthalmic solutions. Additional studies have demonstrated that molecules as large as monoclonal antibodies can deeply penetrate into periocular tissues and reach the systemic circulation after topical administration as an ophthalmic solution.20 It seems that there is a low barrier to the absorption of a drug from tears into periocular tissues and the systemic circulation. For a drug to be topically delivered as an ophthalmic solution, we thought it would be more advantageous to select a compound that possesses strong in vitro potency against the biological target and good solubility in tears to provide an osmotic gradient for penetration into periocular tissues, while having poor oral bioavailability and fast clearance from the systemic circulation. A compound with such a pharmacokinetic (PK) profile would most likely have low systemic exposure to avoid unexpected side effects after topical application.21 Herein, we report the discovery and the development of a potent direct competitive LFA-1/ICAM-1 antagonist (SAR 1118) (Figure 1) as an ophthalmic solution for treating dry eye.
Figure 1.
Structure of LFA-1/ICAM-1 antagonist SAR 1118 (1g).
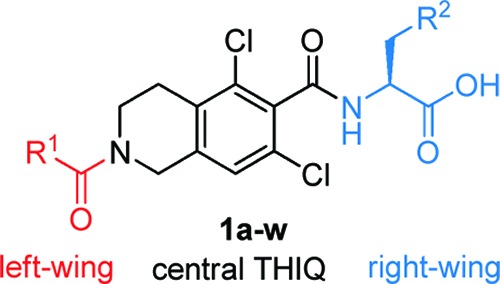
We previously reported that many potent THIQ-derived LFA-1/ICAM-1 antagonists, as exemplified by 1a (Table 1, entry 1), significantly lost their potency in the HuT 78 T-cell adhesion assay in the presence of 10% human serum (HS).15,16 To address this serum shift issue, 1b (entry 2) bearing a 2-methylsulfonylfur-5-yl moiety, was identified. Additionally, a benzofur-6-yl “left-wing” residue (1c, entry 3) was found to be superior to a 4-chlorophenyl moiety (1b, entry 2) in retaining the potency in the presence of HS. Therefore, we decided to incorporate a methylsulfonyl moiety into other “right-wing” amino acids, which previously showed significant serum shift. All of the new “right-wing” amino acids were readily prepared and assembled to the functionalized THIQ scaffold following the disclosed protocols.15,16
Table 1. SAR of the α-Amino Acid Residue of the THIQ-Derived LFA-/ICAM-1 Antagonists (1a–w).
| % at
30 min |
rat
iv PKe |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| entry | compd | R1 | R2 | HuT 78 IC50 (μM)a |
HuT
78 + 10% HSb IC50 (μM) |
HLMc | RLMd | t1/2 (h) | CL (mL/min/kg) | AUC (h·ng/kg) |
| 1 | 1a | 4-ClPh- | 3-i-PrPh- | 0.01 | >10 | f | ||||
| 2 | 1b | 4-ClPh- | 2-MeS(O2)fur-5-yl | 0.006 | 0.24 | 92 | >95 | 0.27 | 74.3 | 1126 |
| 3 | 1c | benzofur-6-yl | 2-MeS(O2)fur-5-yl | 0.0012 | 0.096 | 65 | >95 | 0.43 | 60.0 | 1514 |
| 4 | 1d | 4-ClPh- | 3-MeS(O2)Ph- | 0.003 | 0.20 | |||||
| 5 | 1e | 4-ClPh- | 2-MeS(O2)Ph- | >1.0 | ||||||
| 6 | 1f | 4-ClPh- | 4-MeS(O2)Ph- | 0.25 | ||||||
| 7 | 1g | benzofur-6-yl | 3-MeS(O2)Ph- | 0.009 | 0.074 | 71 | >95 | 0.78 | 139.2 | 705 |
| 8 | 1h | benzofur-6-yl | 2-Cl-3-MeS(O2)Ph- | 0.80 | ||||||
| 9 | 1i | benzofur-6-yl | 4-Cl-3-MeS(O2)Ph- | 0.12 | 2.0 | |||||
| 10 | 1j | benzofur-6-yl | 5-Cl-3-MeS(O2)Ph- | 0.01 | 0.20 | |||||
| 11 | 1k | benzofur-6-yl | 6-Cl-3-MeS(O2)Ph- | 0.12 | ||||||
| 12 | 1l | benzofur-6-yl | 5-F-3-MeS(O2)Ph- | 0.01 | 0.26 | >95 | >95 | 0.65 | 80.5 | 1189 |
| 13 | 1m | benzofur-6-yl | 3-MeS(O2)Pyrid-5-yl | 0.039 | 0.032 | >95 | >95 | 0.62 | 73.5 | 1277 |
| 14 | 1n | benzofur-6-yl | (±)-3-MeS(O)Pyrid-5-yl | 0.057 | 0.050 | >95 | >95 | 0.27 | 121.1 | 753 |
| 15 | 1o | benzofur-6-yl | 3-EtS(O2)Ph- | 0.002 | 0.18 | |||||
| 16 | 1p | benzofur-6-yl | 3-i-PrS(O2)Ph- | 0.20 | >10 | |||||
| 17 | 1q | benzofur-6-yl | 3-NH2S(O2)Ph- | 0.004 | 0.20 | 71 | >95 | 1.03 | 34.4 | 2431 |
| 18 | 1r | benzofur-6-yl | 3-MeNHS(O2)Ph- | 0.013 | 0.64 | |||||
| 19 | 1s | benzofur-6-yl | 3-Me2NS(O2)Ph- | 0.010 | >10 | |||||
| 20 | 1t | benzofur-6-yl | 3-Me2P(O)Ph- | 0.058 | 0.49 | >95 | >95 | 0.37 | 37.0 | 2261 |
| 21 | 1u | benzofur-6-yl | 3-MeC(O)Ph- | 0.012 | 5.6 | |||||
| 22 | 1v | benzofur-6-yl | 3-(R)-MeC(OH)Ph- | 0.010 | 4.0 | |||||
| 23 | 1w | benzofur-6-yl | 3-(S)-MeC(OH)Ph- | 0.014 | 0.94 | |||||
The IC50 value is an average of three titrations with eight concentration points.
Human serum.
Human liver microsomes. Data are shown as % remaining.
Rat liver microsomes. Data are shown as % remaining.
PK experiments were carried out using a group of three male Sprague–Dawley rats administrated intravenously with a single dose of 5 mg/kg of a testing article.
Not determined.
Our immediate attempt was to replace the 3-isopropyl residue in 1a with a 3-methylsulfonyl moiety. To our delight, the resulting 1d (entry 4) showed great potency in the assay with 10% HS. The substitution pattern of the methylsulfonyl moiety is critical to the potency: meta-substitution is optimal, while both ortho- and para-substitutions (1e–f, entries 5–6) result in much diminished potency. As expected, merging a benzofur-6-yl “left-wing” residue with 1d afforded a compound (1g, entry 7) with improved potency in the presence of 10% HS.
Next, a structure–activity relationship (SAR) study around the 3-methylsulfonylphenyl residue was carried out. The introduction of a substituent at the C5-position on the phenyl residue seems to be acceptable (1j and 1l, entries 10 and 12), but with some loss of potency in the presence of 10% HS. Substitutions at the C2-, C4-, and C6-positions (1h,i and 1k, entries 8, 9, and 11) are not well tolerated. The compound bearing a 3-methylsulfonylpyrid-5-yl residue (1m, entry 13) demonstrated comparable potency to 1g. Additionally, a methylsulfoxide residue (1n, entry 14) can substitute the methylsulfonyl moiety without affecting the potency in the presence of 10% HS.
Replacing the methyl (1g, entry 7) with an ethyl (1o, entry 15) is tolerated; however, a bulkier iso-propyl (1p, entry 16) is detrimental to the potency in the assay with or without HS. Moreover, there was a little shift of the potency in the assay without HS when the methyl was substituted by a more polar residue (1q–s, entries 17–19). However, when 10% HS was present, weaker potency was obtained. Assuming the polarity of sulfonyl (S=O) or sulfoxide (S–O) moiety was important to retain a low serum shift, analogues bearing phosphine oxide (P=O) (1t, entry 20), carbonyl (C=O) (1u, entry 21), or alcohol (C–OH) (1v–w, entries 22–23) were also synthesized, respectively. Unfortunately, inferior potency of these analogues to 1g was observed in the assay with 10% HS.
Further biological evaluations demonstrated that 1g has strong inhibition of Jurkat T-cell attachment to ICAM-1 (IC50 = 2.98 nM), lymphocyte activation, and the release of inflammatory cytokines.22 In particular, releases of inflammatory cytokines, such as interferon γ (IFNγ), macrophage inflammatory protein-1α (MIP-1α), interleukin-1α (IL-1α), IL-1β, IL-6, and IL-10, which well correlate with the clinical severity of human KCS when present in tears, are strongly inhibited.23
Subsequently, a number of potent compounds, including 1b–c, 1g, 1l–n, 1q, and 1t, were selected for ADME evaluations. These compounds have good metabolic stability in both human and rat liver microsomes. Rat iv PK studies revealed that these analogues have short half-lives, fast clearances, and low to moderate systemic exposure levels. Among these compounds, 1g has the fastest clearance and lowest exposure level. Moreover, 1g exhibited a good in vitro safety profile, such as being negative in the Ames test and having low potency in both cytochrome P450 (CYP) inhibition (3A4, IC50 >20 μM, and 2C9, IC50 = 3.0 μM) and the human ether-à-go-go-related gene (hERG) (patch clamp, IC50 >20 μM) assays.
A 14C-labeled 1g at the carbonyl of the central THIQ residue was topically administered as an ophthalmic solution to both rats24 and dogs22 to evaluate the corresponding ocular PK profile. Maximal concentrations with good exposure levels were achieved 30 min postdose in all ocular tissues, particularly in bulbar conjunctiva, palpebral conjunctiva, and cornea, of these two species, while having quick initial and slow terminal elimination rates by tears. As expected, low exposure levels were detected in the plasma.
The sodium salt of 1g is freely soluble in phosphate-buffered saline (PBS) to a concentration of ≥200 mg/mL. This solubility allows for formulations with a concentration up to 100 mg/mL (10%), which are isotonic with human tears at approximately 300 mOsmol/L.25 To maintain the ophthalmic solution at physiological pH values in animal and human studies, the highest dosing strength used was up to 50 mg/mL (5%).
To assess the in vivo efficacy and safety, a 1% PBS solution of 1g without ocular lubricating agents26,a was topically administered thrice daily (TID) for 12 weeks for treating dogs diagnosed with idiopathic KCS. Compound 1g was well tolerated and showed significant efficacy with the increase of mean Schirmer's tear test (STT) values from 3.4 mm during week 1 to 5.8 mm at week 12 (p < 0.025). This result compared favorably with the improvement of STT values observed after the treatment of canine KCS with either 1 or 2% topical solution of cyclosporine.27,28
With these encouraging preclinical data, 1g was nominated as SAR 1118 for clinical development. In a randomized, double-masked, placebo-controlled, dose-escalation phase I human clinical study,29 SAR 1118 was topically administered as 0.1, 0.3, 1.0, and 5.0% PBS solutions, respectively. SAR 1118 ophthalmic solution appears to be safe and well tolerated up to 5.0% TID. PK analysis showed maximum plasma concentrations of SAR 1118 were <5 nM and did not affect circulating levels of CD3, CD4, or CD8 T-cells, suggesting a lack of systemic exposure. More importantly, in a recent “proof-of-concept” phase II human clinical trial in treatment of dry eye, SAR 1118 demonstrated dose-dependent significant improvements (p < 0.05) in inferior corneal staining over 12 weeks of treatment. Also, a statistically significant (p < 0.05) increase in tear production determined by STT and an improvement in visual-related functions were observed 2 weeks postdose, demonstrating an early onset of action.30
In summary, we have successfully discovered a potent LFA-1/ICAM-1 antagonist (SAR 1118) and specifically developed it into an ophthalmic solution for treating dry eye. Human clinical data of SAR 1118 have demonstrated that a compound possessing great potency against the biological target, good intrinsic solubility, poor oral bioavailability, and fast clearance can also be developed into a safe and efficacious ophthalmic treatment. Lately, a pivotal phase III human clinical trial of SAR 1118 in treatment of dry eye (OPUS-1) has commenced. Important data generated from this critical clinical trial will be reported in due course.
Acknowledgments
SAR 1118 and its related LFA-1 program are exclusive assets of SARcode Bioscience, Inc. We thank SARcode Bioscience, Inc., for its authorization on this publication.
Supporting Information Available
Synthetic scheme and analytical data, including XRPD, TGA, DSC, FT-IR, ESI-MS, 1H and 13C NMR spectra, elemental analysis, and solubility of SAR 1118, and detailed descriptions of HuT 78 and Jurkat T-cell adhesion assays. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Notes
Author Present Address
§ Presidio Pharmaceuticals, Inc., 1700 Owens Street, Suite 585, San Francisco, California 94158, United States.
Footnotes
Most drugs tested for clinical efficacy in dry eye contain lubricating components to ameliorate patients' clinical symptoms, including the sensation of grittiness or pain with blinking. In many cases, these lubricating components exhibit significant benefit in both clinical symptoms and signs and therefore could mask the real effect of a testing drug. For example, Restasis contains lubricating components, such as glycerin, castor oil, polysorbate 80, and carbomer copolymer type A. Its drug-free version is available as Refresh to provide symptomatic relief to dry eye sufferers. Moreover, it took 6 months of treatment for cyclosporine (0.05%) formulated as an emulsion with lubricating components to show statistically significant improvement over the vehicle alone.26
Supplementary Material
References
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991, 67, 1033–1036. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995, 57, 827–872. [DOI] [PubMed] [Google Scholar]
- Ali H.; Haribabu B.; Richardson R. M.; Snyderman R. Mechanisms of inflammation and tissue destruction. Med. Clin. North Am. 1997, 81, 1–27. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr. Opin. Cell Biol. 1997, 9, 643–650. [DOI] [PubMed] [Google Scholar]
- Bromley S. K.; Dustin M. L. Stimulation of naïve T-cell adhesion and immunological synapse formation by chemokine-dependent and independent mechanisms. Immunology 2002, 106, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman N. C.; Nye J. A.; Groves J. T. Cluster size regulates protein sorting in the immunological synapse. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf-Makagiansar H.; Anderson M. E.; Yakovleva T. V.; Murray J. S.; Siahaan T. J. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 2002, 22, 146–167. [DOI] [PubMed] [Google Scholar]
- Nicolls M. R.; Gill R. G. LFA-1 (CD11a) as a therapeutic target. Am. J. Transplant. 2006, 6, 27–36. [DOI] [PubMed] [Google Scholar]
- Dry Eye Workshop (DEWS) Committee. 2007 Report of the Dry Eye Workshop (DEWS). Ocul. Surf. 2007, 5, 65–204. [Google Scholar]
- Stern M. E.; Pflugfelder S. C. Inflammation in dry eye. Ocul. Surf. 2004, 2, 124–130. [DOI] [PubMed] [Google Scholar]
- Calonge M.; Enríquez-de-Salamanca A.; Diebold Y.; González-García M. J.; Reinoso R.; Herreras J. M.; Corell A. Dry eye disease as an inflammatory disorder. Ocul. Immunol. Inflammation 2010, 18, 244–253. [DOI] [PubMed] [Google Scholar]
- Kunert K. S.; Tisdale A. S.; Stern M. E.; Smith J. A.; Gipson I. K. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: Effect on conjunctival lymphocytes. Arch. Ophthalmol. 2000, 118, 1489–1496. [DOI] [PubMed] [Google Scholar]
- Utine C. A.; Stern M.; Akpek E. K. Clinical review: topical ophthalmic use of cyclosporin A. Ocul. Immunol. Inflammation 2010, 18, 352–361. [DOI] [PubMed] [Google Scholar]
- Gao J.; Morgan G.; Tieu D.; Schwalb T. A.; Luo J. Y.; Wheeler L. A.; Stern M. E. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjögren’s syndrome-like MRL/lpr mice. Exp. Eye Res. 2004, 78, 823–835. [DOI] [PubMed] [Google Scholar]
- Zhong M.; Shen W.; Barr K. J.; Arbitrario J. P.; Arkin M. R.; Bui M.; Chen T.; Cunningham B. C.; Evanchik M. J.; Hanan E. J.; Hoch U.; Huen K.; Hyde J.; Kumer J. L.; Lac T.; Lawrence C. E.; Martell J. R.; Oslob J. D.; Paulvannan K.; Prabhu S.; Silverman J. A.; Wright J.; Yu C. H.; Zhu J.; Flanagan W. M. Discovery of tetrahydroisoquinoline (THIQ) derivatives as potent and orally bioavailable LFA-1/ICAM-1 antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 5269–5273. [DOI] [PubMed] [Google Scholar]
- Zhong M.; Hanan E. J.; Shen W.; Bui M.; Arkin M. R.; Barr K. J.; Evanchik M. J.; Hoch U.; Hyde J.; Martell J. R.; Oslob J. D.; Paulvannan K.; Prabhu S.; Silverman J. A.; Wright J.; Yu C. H.; Zhu J.; Flanagan W. M. Structure-activity relationship (SAR) of the α-amino acid residue of potent tetrahydroisoquinoline (THIQ)-derived LFA-1/ICAM-1 antagonists. Bioorg. Med. Chem. Lett. 2011, 21, 307–310. [DOI] [PubMed] [Google Scholar]
- Gadek T. R.; Burdick D. J.; McDowell R. S.; Stanley M. S.; Marsters J. C. Jr.; Paris K. J.; Pare D. A.; Reynolds M. E.; Ladner C.; Zioncheck K. A.; Lee W. P.; Gribling P.; Dennis M. S.; Skelton N. J.; Yumas D. B.; Clark K. R.; Keatinh S. M.; Beresini M. H.; Tilley J. W.; Presta L. G.; Bodary S. C. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science 2002, 295, 1086–1089. [DOI] [PubMed] [Google Scholar]
- Keating S. M.; Clark K. R.; Stefanich L. D.; Arellano F.; Edwards C. P.; Bodary S. C.; Spencer S. A.; Gadek T. R.; Marsters J. C. Jr.; Beresini M. H. Competition between intercellular adhesion molecule-1 and a small-molecule antagonist for a common binding site on the αl subunit of lymphocyte function-associated antigen-1. Protein Sci. 2006, 15, 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy Y. B.; Prausnitz M. R. The rule of five for non-oral routes of drug delivery: Ophthalmic, inhalation and transdermal. Pharm. Res. 2011, 28, 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y.; Ishimaru N.; Arakaki R.; Tsukumo S.; Fukui H.; Kishihara K.; Shiota H.; Yasutomo K.; Hayashi Y. Effective treatment of a mouse model of Sjögren’s syndrome with eye drop administration of anti-CD4 monoclonal antibody. Arthritis Rheum. 2004, 50, 2903–2910. [DOI] [PubMed] [Google Scholar]
- Bodor N. Retrometabolic drug design concepts in ophthalmic target-specific drug delivery. Adv. Drug Delivery Rev. 1995, 16, 21–38. [Google Scholar]
- Murphy C. J.; Bentley E.; Miller P. E.; McIntyre K.; Leatherberry G.; Dubielzig R.; Giuliano E.; Moore C. P.; Phillips T. E.; Smith P. B.; Prescott E.; Miller J. M.; Thomas P.; Scagliotti R.; Esson D.; Gadek T.; O'Neill C. A. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3174–3180. [DOI] [PubMed] [Google Scholar]
- Lam H.; Bleiden L.; De Paiva C.; Farley W.; Stern M.; Pflugfelder S. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009, 147, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. R.; Prescott E.; Shelke N. B.; Trivedi R.; Thomas P.; Struble C.; Gadek T.; O’Neill C. A.; Kompella U. B. Delivery of SAR 1118 to the retina via ophthalmic drops and its effectiveness in a rat streptozotocin (STZ) model of diabetic retinopathy (DR). Invest. Ophthalmol. Vis. Sci. 2010, 51, 5198–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P.; Simmons P. A.; Patel S.; Tomlinson A. Refractive index and osmolality of human tears. Optom. Vis. Sci. 1995, 72, 718–724. [DOI] [PubMed] [Google Scholar]
- Sall K.; Stevenson O. D.; Mundorf T. K.; Reis B. L. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 study group. Ophthalmology 2000, 107, 631–639. [DOI] [PubMed] [Google Scholar]
- Olivero D.; Davidson M.; English R.; Nasisse M.; Jamieson V.; Gerig T. Clinical evaluation of 1% cyclosporine for topical treatment of keratoconjunctivitis sicca in dogs. J. Am. Vet. Med. Assoc. 1991, 199, 1039–1042. [PubMed] [Google Scholar]
- Morgan R.; Abrams K. Topical administration of cyclosporine for treatment of keratoconjunctivitis sicca in dogs. J. Am. Vet. Med. Assoc. 1991, 199, 1043–1046. [PubMed] [Google Scholar]
- Semba C. P.; Swearingen D.; Smith V. L.; Newman M. S.; O'Neill C. A.; Burnier J. P.; Haughey D. B.; Gadek T. R. Safety and pharmacokinetics of a novel lymphocyte function-associated antigen-1 antagonist ophthalmic solution (SAR 1118) in healthy adults. J. Ocul. Pharmacol. Ther. 2011, 27, 99–104. [DOI] [PubMed] [Google Scholar]
- Semba C. P.; Torkildsen G.; Lonsdale J.; McLaurin E.; Geffin J.; Mundorf T.; Kennedy K.; Ousler G. W.. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am. J. Ophthalmol. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.