Abstract
We report the synthesis of DO3A derivatives of 2,2′-diaminobiphenyl (1a,b) and their Gd complexes of the type [Gd(1)(H2O)]·xH2O (2a,b) for use as new MRI blood-pool contrast agents (BPCAs) that provide strong and prolonged vascular enhancement. Pharmacokinetic inertness of 2 compares well with that of structurally related Dotarem, a DOTA-based MRI CA currently in use. The R1 relaxivity in water reaches 7.3 mM–1 s–1, which is approximately twice as high as that of Dotarem (R1 = 3.9 mM–1 s–1). They show interaction with HSA to give association constants (Ka) in the order of two (∼102), revealing the existence of the blood-pool effect. The in vivo MR images of mice obtained with 2 are coherent, showing strong signal enhancement in both heart, abdominal aorta, and small vessels. Furthermore, the brain tumor is vividly enhanced for an extended period of time.
Keywords: Gd chelates, DO3A, biphenyl, MRI BPCA, brain tumor
Magnetic resonance imaging (MRI) is a powerful technique for noninvasive diagnosis of the human anatomy, physiology, and pathophysiology on the basis of superior spatial resolution and contrast useful in providing anatomical and functional images of the human body.1 At the clinical level, MRI techniques are mostly performed employing Gd(III) chelates (GdL) to enhance the image contrast by increasing the water proton relaxation rate in the body.2 Despite their wide and successful applications in clinics, however, conventional Gd(III)-based low-molecular weight CAs are mostly extracellular fluid (ECF) agents exhibiting rapid extravasation from the vascular space. As a result, the time window for imaging is considerably reduced, thus limiting acquisition of high-resolution images.3−5
To overcome such limitations inherent to ECF CAs, the necessity for the development of blood-pool contrast agents (BPCAs) has risen in the expectation that they reside in the blood vessel for an extended period of time and thus are eliminated much more slowly from the circulation than their ECF counterparts. The BPCAs provide strong and prolonged vascular enhancement. The BPCAs thus far developed may be divided into three classes according to their mechanism of action: (i) the noncovalent binding of low-molecular GdL to HSA to prevent immediate leakage into the interstitial space,6−8 (ii) systems incorporating polymers or liposomes based on an increase in the size of CAs, which slows down leakage through endothelial pores,9−12 and (iii) systems based on nanoparticles, involving a change in the route of elimination.13−15 The most successful approach so far is the class (i) represented by MS-325, a Gd–DTPA derivative containing a cholic acid moiety. These complexes bind strongly and reversibly to HSA, leading to not only high R1 relaxivity at clinical field strengths but also much longer residence times in the blood as compared to ECF agents. The lipophilic component represented by aromatic moieties in the chelate is responsible for the generation of noncovalent interactions between the corresponding GdL and HSA.16,17 The anionic phosphodiester moiety in MS-325 is also known to contribute in a synergistic manner to the noncovalent binding interaction with HSA.
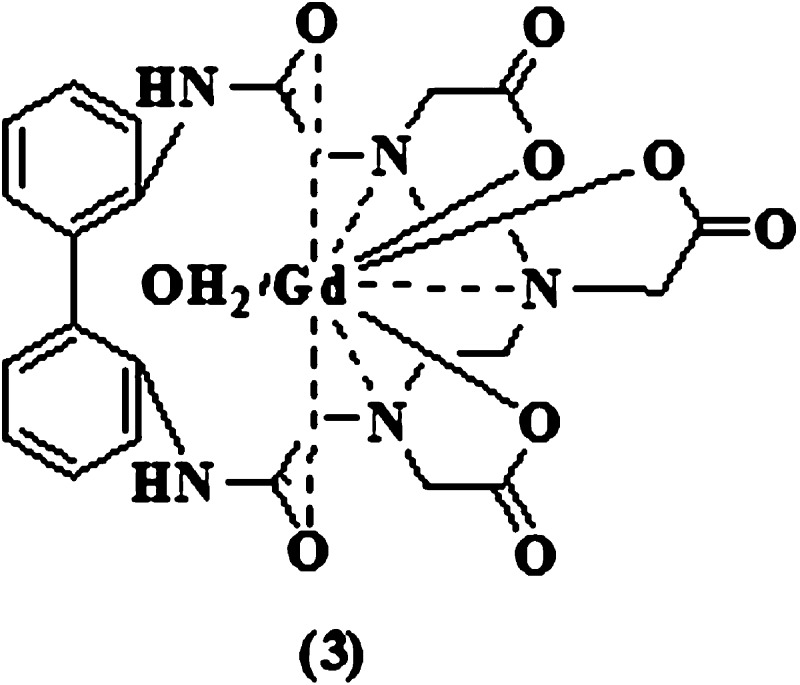
We have also been involved for some time in the design and synthesis of a new series of Gd-based MRI BPCAs.18−21 More recent work includes the synthesis of a series of macrocyclic DTPA-(biphenyl-2,2′-bisamides) (3) (Chart 1).
Chart 1.
They form neutral Gd(III) complexes belonging to class (i) of BPCAs and exhibit very high blood-pool enhancement due to the presence of highly lipophilic biphenyl in the macrocyclic chelate backbone.21 One disadvantage of these imaging probes, however, is much lower thermodynamic and kinetic stabilities as compared with their DO3A analogues, although those stabilities are slightly higher than those of their DTPA counterparts.21 Motivated by these findings and in an effort to increase thermodynamic and kinetic stabilities, we have developed further two new DO3A-(biphenyl-2,2′-bisamide) chelates (1) and their Gd complexes (2) (Chart 2).
Chart 2.
The synthesis of 1 and 2 is straightforward as described in Scheme S1 in the Supporting Information. The synthesis of 2 proceeds in two steps: That is, initial formation of the DO3A-diaminobiphenyl conjugate (1) followed by complexation with GdCl3. For the preparation of the mono-Gd analogue (2b), it is initially required to protect one amino group in 2,2′-bis(amino)biphenyl with di-tert-butyl dicarbonate. The formation of 1 and 2 was confirmed by microanalytical, spectroscopic (NMR and MS), and HPLC methods (cf. ESI).
Table 1 shows the proton relaxivities, R1 and R2, of 2a and 2b along with Gd-DOTA (Dotarem) and MS-325 for comparative purposes. Measurements were made in three different media: water, PBS (pH 7.4), and a PBS solution of HSA. R1 relaxivities of 2a and 2b in water are almost twice as high as that of structurally analogous Dotarem. In PBS, however, the same relaxivities drop to almost half the original values. The observations such as these may be expected when invoking possible displacement of the inner-sphere water molecule in 2 by the phosphate anion of PBS. Related observations such as a R1 drop in the PBS solution of HSA have already been noted and rationalized in terms of the inner-sphere water molecule displaced by a donor atom from a carboxylate on the protein or the phosphate anion of PBS.23R1 relaxivities of 2a and 2b are almost doubled in the PBS solution of HSA, demonstrating the existence of interaction with HSA, although the interactions are not as strong as the one observed with MS-325.
Table 1. Relaxivity Data of 2a, 2b, Dotarem, and MS-325 (64 MHz, 293 K)a.
|
R1 (mM–1 s–1) |
R2 (mM–1 s–1) |
|||||
|---|---|---|---|---|---|---|
| water | PBSb | HSAc | water | PBSb | HSAc | |
| 2a | 7.3 | 4.3 | 8.1 | 7.6 | 5.3 | 15.3 |
| 2b | 7.0 | 3.4 | 6.8 | 7.6 | 4.4 | 11.3 |
| Dotarem | 3.9 | 3.6 | 5.3 | 4.1 | 7.2 | |
| MS-32522 | 5.2 | 19.0 | 5.9 | 37.0 | ||
Concentrations are given in [Gd].
PBS: pH 7.4.
[HSA] = 0.67 mM in PBS.
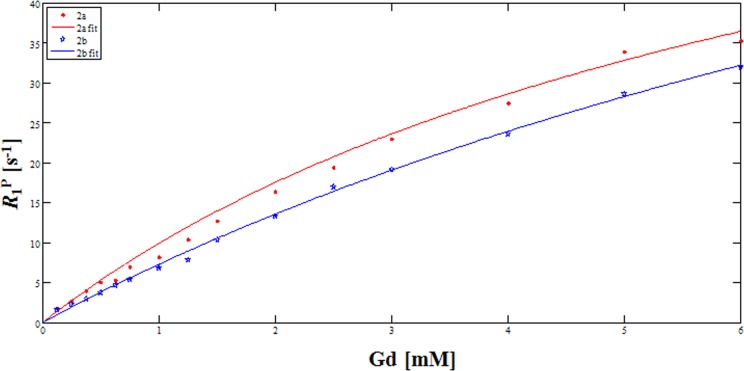
The proton relaxation enhancement (PRE) was measured to obtain more detailed information about such an interaction with protein. The extent of relaxation enhancement, an ε* titration, is as expected in that the relaxation rate increase is much larger in the presence of HSA, and the same rate does not increase linearly with concentration, indicating the presence of protein binding (cf. Figure S1 in the Supporting Information).24 The association constants (Ka) characterizing the interaction between HSA and 2a and 2b are 2.11 × 102 and 1.07 × 102 M–1, respectively. These values can be derived from the so-called M-titration, proton longitudinal relaxation rate as a function of the concentration of the paramagnetic substrate at the fixed concentration of HSA (Figure 1). Fitting of the data was carried out according to the literature method.25 Here, it is worth noting that the MS-325, a well-known BPCA, shows a Ka value of 6.1 × 103 M–1, greater by almost 1 order of magnitude than those of the 2a and 2b.25 As is the case with MS-325, the role of two aromatic units via a lipophilic interaction may be invoked to rationalize the increase in the relaxivity in a PBS solution, although such increases are not so dramatic. Observations such as these in connection with the biphenyl moiety have already been noted in our previous work with the DTPA-biphenyl-2,2′-bis(amide) analogues (3).21 In short, 2a may serve as a novel BPCA in that it carries macrocyclic DOTA units, which would provide further thermodynamic and kinetic stabilities than acyclic DTPA analogues. To the best of our knowledge, 2a is the first example of BPCA possessing DOTA as a chelate.
Figure 1.
Proton longitudinal paramagnetic relaxation rates of 2a and 2b as a function of [Gd] in PBS (pH 7.4) solutions of HSA (0.67 mM) at 64 MHz and 293 K.
Figure 2 shows in vivo coronal images of mice obtained by tail vein injection with 2a. The figure reveals clearly blood-pool enhancement in heart and abdominal aorta, and the image lasts as long as 1 h to be eventually excreted through kidney. Supportive with these observations is the biodistribution data of 2a and the CNR profiles provided in Figures S3 and S4 in the Supporting Information, respectively. Such slow renal clearance as well as high stabilities (both thermodynamic and kinetic) may compensate for the potential risk of nephrogenic systemic fibrosis (NSF), which has often been reported with acyclic DTPA series such as Omniscan.
Figure 2.
In vivo MR T1 weighted spin echo (SE) coronal images of mice obtained by tail vein injection with 2a (0.1 mmol/kg): H, heart; L, liver; K, kidney; B, bladder; and A, abdominal aorta (64 MHz).
An additional feature of 2a is that it is capable of enhancing the brain tumor for as long as an hour, which is rarely observed with an ordinary ECF agent such as Dotarem (cf. Figure 3).
Figure 3.

In vivo MR T1 weighted SE coronal images of brain tumor (C6-glyoma cell) obtained with 2a (0.1 mmol/kg) (64 MHz).
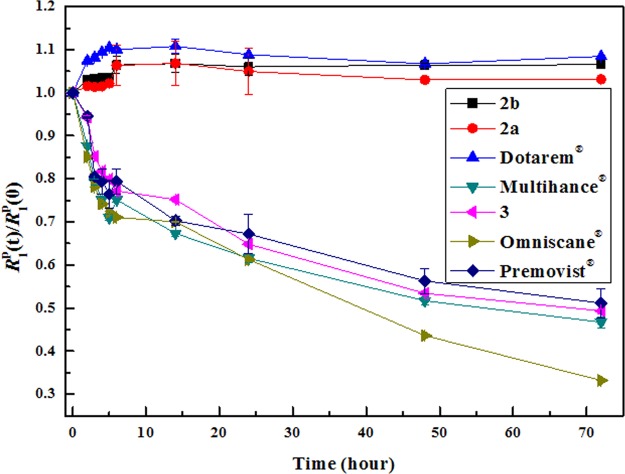
An additional advantage of 2a, when compared with existing MS-325, lies in the fact that the former incorporates DOTA as a chelate backbone rather than DTPA. Thus, greater enhancement in kinetic stabilities would be expected with 2 than their DTPA-based counterparts without a significant loss of the blood-pool effect. Indeed, such an expectation is demonstrated in Figure 4, which shows the plots of R1p(t)/R1p(0) as a function of time for various MRI CAs. The relative value of R1P at any time t, R1P(t)/R1P(0), is therefore a good estimator of the extent of transmetalation by zinc. Among endogenous ions, only Zn2+ can displace a significant amount of Gd because the concentration of the former in blood is relatively high.26 Its evolution over time gives relevant information about the kinetics of the reaction. Quantitative transmetalation kinetic data are provided (Figure S2 and Table S1 in the Supporting Information). A dramatic increase in kinetic stability is observed with 2 as compared with the plots exhibited by DTPA-based CAs including 3, a new type of BPCA recently developed by us.21 In addition, the kinetic stability of 2 compares well with that of Dotarem. Little change in R1 observed in the pH range 1–7 demonstrates that 2 is stable enough under highly acidic conditions (Figure S7 in the Supporting Information).
Figure 4.
Evolution of longitudinal relaxation rates R1p(t)/R1p(0) as a function of time for various MRI CAs {[Gd]0 and [ZnCl2]0 = 2.5 mM in PBS (pH 7.4) at 128 MHz and 293 K}.
The cytotoxicity of the present series compares well with that of Dotarem (Figure 5). The comparison was made by employing IMR-90 human embryonic lung fibroblasts, a normal cell line that is typically used in the nontoxicity test.
Figure 5.

IMR-90 cell viability for 2 and Dotarem at various concentrations.
In conclusion, we have put into a new entry two new Gd complexes (2a and 2b) as novel MRI BPCAs. One of the most characteristic features of 2a is that they not only reveal great signal enhancement in R1 relaxivity in water but also exhibit a certain degree of interaction with HSA solutions with a dramatic increase in kinetic stability as well. The structural uniqueness of 2 lies in that it is neutral in charge and thus makes no resort to electrostatic interaction, supposedly one of the essential factors for the blood-pool effect.
Acknowledgments
We thank M.-K. Kang for her assistance in the biodistribution experiment.
Supporting Information Available
Synthesis and characterization of 2 and other detailed procedures: NMR and HPLC spectra, relaxivity measurements, relaxation enhancement of 2 with HSA, kinetic constants, biodistribution, and CNR profiles. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was partially supported by NRF (Grant Nos. 2010-0024143 and 2011-0015353) and The Advanced Medical Technology Cluster for Diagnosis & Prediction, KNU from MKE (Grant No. RTI04-01-01). The KNU Research Fund (2012) is also gratefully acknowledged.
The authors declare no competing financial interest.
Author Contributions
# These authors contributed equally.
Supplementary Material
References
- Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [DOI] [PubMed] [Google Scholar]
- Caravan P.; Ellison J. J.; McMurry T. J.; Lauffer R. B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [DOI] [PubMed] [Google Scholar]
- Kobayashi H.; Sato N.; Hiraga A.; Saga T.; Nakamoto Y.; Ueda H.; Konishi J.; Togashi K.; Brechbiel M. W. 3D-micro-MR angiography of mice using macromolecular MR contrast agents with polyamidoamine dendrimer core with references to their pharmacokinetic properties. Magn. Reson. Med. 2001, 45, 454–460. [DOI] [PubMed] [Google Scholar]
- Ayyagari A. L.; Zhang X. D.; Ghaghada K. B.; Annapragada A.; Hu X. P.; Bellamkonda R. V. Long-circulating liposomal contrast agents for magnetic resonance imaging. Magn. Reson. Med. 2006, 55, 1023–1029. [DOI] [PubMed] [Google Scholar]
- Schwickert H. C.; Stiskal M.; Vandijke C. F.; Roberts T. P.; Mann J. S.; Demsar F.; Brasch R. C. Tumor Angiography using High Resolution 3D MRI: Comparison of Gd-DTPA and a Macromolecular Blood Pool Contrast Agent. Acad. Radiol. 1995, 2, 851–858. [DOI] [PubMed] [Google Scholar]
- Lauffer R. B.; Parmelee D. J.; Dunham S. U.; Ouellet H. S.; Dolan R. P.; Witte S.; McMurry T. J.; Walovitch R. C. MS-325: Albumin-targeted contrast agent for MR angiography. Radiology 1998, 207, 529–538. [DOI] [PubMed] [Google Scholar]
- Cavagna F. M.; Lorusso V.; Anelli P. L.; Maggioni F.; de Haën C. Preclinical profile and clinical potential of gadocoletic acid trisodium salt (B22956/1), a new intravascular contrast medium for MRI. Acad. Radiol. 2002, 9, S491–S494. [DOI] [PubMed] [Google Scholar]
- Preda A.; Novikov V.; Möglich M.; Turetschek M. K.; Shames D. M.; Brasch R. C.; Cavagna F. M.; Roberts T. P. L. MRI Monitoring of Avastin Antiangiogenesis Therapy Using B22956/1, a New Blood Pool Contrast agent, in an Experimental Model of Human Cancer. J. Magn. Reson. Imaging 2004, 20, 865–873. [DOI] [PubMed] [Google Scholar]
- Merbach A. E.; Tóth E.. The Chemistry of Contrast Agents in Medial Magnetic Resonance Imaging; Wiley-VCH: Chichester, United Kingdom, 2001. [Google Scholar]
- Aime S.; Botta M.; Crich S. G.; Giovenzana G.; Palmisano G.; Sisti M. Novel Paramagnetic Macromolecular Complexes Derived from the Linkage of a Macrocyclic Gd(III) Complex to Polyamino Acids through a Squaric Acid Moiety. Bioconjugate Chem. 1999, 10, 192–199. [DOI] [PubMed] [Google Scholar]
- Curtet C.; Maton F.; Havet T.; Slinkin M.; Mishra A.; Chatal. J. F.; Muller R. N. Polylysine-Gd-DTPAn and polylysine-Gd-DOTAn coupled to anti-CEA F(ab’)2 fragments as potential immunocontrast agents Relaxometry, biodistribution, and magnetic resonance imaging in nude mice grafted with human colorectal carcinoma. Invest. Radiol. 1998, 33, 752–761. [DOI] [PubMed] [Google Scholar]
- Lokling K. E.; Fossheim S. L.; Skurtveit R.; Bjornerud A.; Klaveness J. pH-sensitive paramagnetic liposomes as MRI contrast agents: in vitro feasibility studies. Magn. Reson. Imaging 2001, 19, 731–738. [DOI] [PubMed] [Google Scholar]
- Jung C. W.; Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging 1995, 13, 661–674. [DOI] [PubMed] [Google Scholar]
- Taupitz M.; Schnorr J.; Abramjuk C.; Wagner S.; Pilgrimm H.; Hünigen H.; Hamm B. New generation of monomer-stabilized very small superparamagnetic iron oxide particles (VSOP) as contrast medium for MR angiography: Preclinical results in rats and rabbits. J. Magn. Reson. Imaging 2000, 12, 905–911. [DOI] [PubMed] [Google Scholar]
- Mornet S.; Vasseur S.; Grasset F.; Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar]
- Avedano S.; Tei L.; Lombardi A.; Giovenzana G. B.; Aime S.; Longo D.; Botta M. Maximizing the relaxivity of HSA-bound gadolinium complexes by simultaneous optimization of rotation and water exchange. Chem. Commun. 2007, 45, 4726–4728. [DOI] [PubMed] [Google Scholar]
- Henrotte V.; Elst L. V.; Laurent S.; Muller R. N. Comprehensive investigation of non-covalent binding of MRI contrast agents with human serum albumin. J. Biol. Inorg. Chem. 2007, 12, 929–937. [DOI] [PubMed] [Google Scholar]
- Kim H.-K.; Jung H.-Y.; Park J.-A.; Huh M.-I.; Jung J.-C.; Chang Y.; Kim T.-J. Gold nanoparticles coated with gadolinium-DTPA-bisamide conjugate of penicillamine (Au@GdL) as a T1-weighted blood pool contrast agent. J. Mater. Chem. 2008, 47, 6259–6262. [Google Scholar]
- Kim H.-K.; Park J.-A.; Kim K. M.; Md. N. S.; Kang D.-S.; Lee J.; Chang Y.; Kim T.-J. Gd-complexes of macrocyclic DTPA conjugates of 1,1′-bis(amino)ferrocenes as high relaxivity MRI blood-pool contrast agents (BPCAs). Chem. Commun. 2010, 46, 8442–8444. [DOI] [PubMed] [Google Scholar]
- Lee K.-H.; Chang Y.; Kim T.-J. Blood-Pool and Targeting MRI Contrast Agents: Form Gd-Chelates to Gd-Nanoparticles. Eur. J. Inorg. Chem. 2012, 1942–1933. [Google Scholar]
- Jung K.-H.; Kim H.-K.; Lee K. H.; Kang D.-S.; Park J.-A.; Kim K. M.; Chang Y.; Kim T.-J. Gd complexes of macrcyclic diethylenetriaminepentaacetic acid (DTPA) biphenyl-2,2′-bisamides as strong blood-pool magnetic resonance imaging contrast agents. J. Med. Chem. 2011, 54, 5385–5394. [DOI] [PubMed] [Google Scholar]
- Bremerich J.; Bilecen D.; Reimer P. MR angiography with blood pool contrast agents. Eur. Radiol. 2007, 17, 3017–3024. [DOI] [PubMed] [Google Scholar]
- Anim S.; Gianolio E.; Terreno E.; Giovenzana G. B.; Pagliarin R.; Sisti M.; Palmisano G.; Botta M.; Lowe M. P.; Parker D. Ternary Gd(III)L-HSA adducts: evidence for the replacement of inner-sphere water molecules by coordinating groups of the protein. Implications for the design of contrast agents for MRI. J. Biol. Inorg. Chem. 2000, 5, 488–497. [DOI] [PubMed] [Google Scholar]
- Dwek R. A.Nuclear Magnetic Resonance (N.M.R.) in Biochemistry; Clarendon Press: Oxford, 1973. [Google Scholar]
- Caravan P.; Cloutier N. J.; Greenfield M. T.; McDermid S.; Dunham S.; Bulte J. M.; Amedio J. Jr.; Looby R.; Supkowski R.; Horrocks W. Jr.; McMurry T.; Lauffer R. The interaction of MS-325 with human serum albumin and its effect on proton relaxation rates. J. Am. Chem. Soc. 2002, 124, 3152–3162. [DOI] [PubMed] [Google Scholar]
- Laurent S.; Elst L. V.; Copoix F; Muller R. N. Stability of MRI paramagnetic contrast media. a proton relaxometric protocol for transmetallation assessment. Invest. Radiol. 2001, 36, 115–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.