Abstract
We report the discovery and characterization of natural phenols as G protein-coupled receptor-35 (GPR35) agonists. Pharmacological characterization using label-free dynamic mass redistribution and Tango β-arrestin translocation assays revealed that GPR35-active natural phenols are divergent in their biased agonism.
Keywords: GPR35, natural phenols, ellagic acid, biased agonism
Natural products have been the single most productive source of leads for drug development.1 Almost half of the drugs approved between 1994 and 2007 have been based on natural products,2 and 18 out of the 50 first-in-class small-molecule drugs approved between 1999 and 2008 are originated from natural substances.3 In many drug discovery campaigns, natural products have provided good chemical starting points for lead identification and optimization.4
Here we report the discovery and characterization of certain natural phenols as G protein-coupled receptor-35 (GPR35) agonists. GPR35 is an orphan receptor whose natural agonists have been postulated to be kynurenic acid5 and 2-acyl lysophosphatidic acid.6 GPR35 is expressed with relatively high level in the human pancreas, small intestine, colon, spleen, and immune cells.7 GPR35 is expressed by several human carcinoma cell lines, including HT-29.8,9 Until recently, there have been limited reports of small molecule pharmacological tools5−8,10−15 to further elucidate the role of the receptor and determine its potential as a drug target.
To identify potential natural products as GPR35 agonists, we screened a commercially available 880 natural product library (Prestwick Chemicals) using label-free dynamic mass redistribution (DMR)17,18 and Tango GPR35 β-arrestin translocation assays.8,15 First, we recorded the DMR signals of all natural products, each at 10 μM, in native HT-29 cells. Such a DMR agonism screen showed that out of the 880 natural products, 37 led to a positive DMR signal with amplitude between 100 and 400 pm (picometer, shift in resonant wavelength of the biosensor 10 min poststimulation) (Figure 1). Second, we recorded the DMR signals of 1 μM zaprinast in the cells 1 h after pretreatment with each natural product. Zaprinst is a known full agonist of GPR35.11 Such a DMR desensitization assay8 showed that 48 compounds suppressed the zaprinast DMR by at least 50%. Third, we counter-screened the library using a Tango GPR35 β-arrestin translocation assay in an engineered U2OS cell line (Tango GPR35-bla U2OS, Invitrogen).8,15 Results showed that 25 compounds gave rise to a coumarin to fluorescein ratio greater than 2. Cross-examination of hits showed that 19 were common in all three assays, 13 common to both DMR assays, 5 specific to DMR agonism assay, 16 specific to DMR desensitization assay, and 6 including ethidium bromide specific to the Tango assay.
Figure 1.
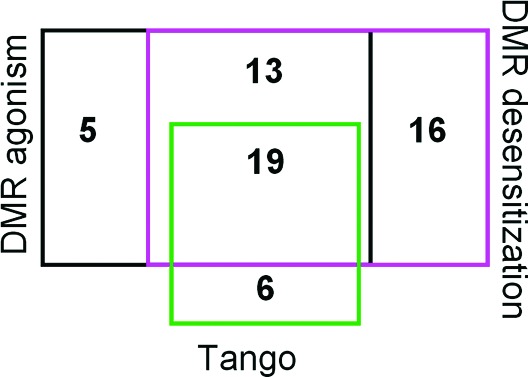
GPR35 agonist hit classifications obtained using three distinct assays: DMR agonism in HT-29 cells, DMR desensitization to repeated stimulation with a known GPR35 agonist zaprinast in HT-29 cells, and Tango β-arrestin translocation in GPR35-bla U2OS cells.
We were primarily focused on 10 natural phenols that were hits common to all three assays for follow-up characterization (Table 1). This is partly because, out of the 19 hits, 15 are natural phenols and partly because DMR or Tango alone may result in false positives. For Tango assays, false positives may be due to the interference of fluorescent molecules—ethidium bromide, the commonly used DNA staining dye, was identified as a false positive. For DMR assays, false positives may be due to the activation of alternative pathways, since these assays are highly sensitive and cover wide pathways.17,18
Table 1. Compounds and Their EC50 Obtained Using DMR and Tango Assays, IC50 of SPB05142 To Block the DMR of Each Agonist at Its EC80, and IC50 of Each Agonist To Desensitize the HT-29 Cells Responding to 1μM Zaprinast, and Their Efficacy (That Is, the Maximal Responses) as Measured Using DMR and Tango Assaysa.
| EC50 (μM) |
IC50 (μM) |
efficacy |
||||
|---|---|---|---|---|---|---|
| compd | DMR | Tango | antagonist | desensitization | DMR (pm) | Tango (FRET ratio) |
| Zaprinast | 0.16 ± 0.02 | 6.10 ± 0.45 | 10.5 ± 0.3 | 1.22 ± 0.10 | 269 ± 11 | 12.8 ± 0.1 |
| YE210 | 0.064 ± 0.004 | 17.6 ± 2.3 | 6.90 ± 0.51 | 0.11 ± 0.01 | 283 ± 21 | 13.9 ± 0.1 |
| Luteolin | 7.24 ± 0.51 | 3.20 ± 0.29 | 5.86 ± 0.32 | 18.6 ± 1.2 | 68 ± 5 | 1.5 ± 0.1 |
| Quercetin | 8.02 ± 0.79 | 5.93 ± 0.47 | 6.69 ± 0.51 | 15.4 ± 2.3 | 40 ± 4 | 2.3 ± 0.1 |
| Niflumic acid | 1.15 ± 0.23 | 40.2 ± 3.7 | 14.2 ± 1.5 | 1.28 ± 0.17 | 260 ± 12 | 3.3 ± 0.1 |
| Baicalein | 10.3 ± 1.9 | 22.7 ± 2.1 | 5.02 ± 0.47 | 28.2 ± 1.9 | 248 ± 9 | 2.1 ± 0.1 |
| Myricetin | 3.02 ± 0.37 | 14.7 ± 1.2 | 10.5 ± 1.3 | 2.12 ± 0.20 | 224 ± 11 | 9.2 ± 0.1 |
| Morin | 1.33 ± 0.21 | 11.2 ± 1.3 | 7.42 ± 0.59 | 1.60 ± 0.15 | 284 ± 17 | 9.8 ± 0.1 |
| Lapachol | 2.84 ± 0.35 | 47.9 ± 3.9 | 28.4 ± 2.1 | 4.66 ± 0.36 | 328 ± 22 | 8.7 ± 0.1 |
| Lobaric acid | 2.39 ± 0.42 | 17.6 ± 3.2 | 13.7 ± 1.2 | 4.54 ± 0.39 | 317 ± 21 | 2.0 ± 0.1 |
| Laccacid acid A | 0.39 ± 0.06 | 51.5 ± 6.7 | 11.9 ± 0.9 | 0.39 ± 0.05 | 263 ± 15 | 3.5 ± 0.2 |
| Hematein | 11.1 ± 1.3 | 4.7 ± 1.1 | 3.64 ± 0.41 | 30.4 ± 3.1 | 391 ± 32 | 2.1 ± 0.1 |
| CHCA | 9.80 ± 1.23 | 70.7 ± 6.7 | 8.96 ± 0.71 | 30.4 ± 2.7 | 331 ± 32 | 4.0 ± 0.1 |
| Ellagic acid | 0.11 ± 0.02 | 2.96 ± 0.21 | 2.12 ± 0.19 | 0.10 ± 0.01 | 319 ± 27 | 3.4 ± 0.1 |
| DCA | 0.054 ± 0.004 | 12.4 ± 0.57 | 3.65 ± 0.21 | 0.11 ± 0.01 | 233 ± 12 | 10.9 ± 0.1 |
The data represents mean ± s.d. from at least 2 independent measurements (n = 4).
Niflumic acid was one of the non-natural phenol hits. Since niflumic acid was recently reported as a GPR35 agonist,13 we first characterized in detail its pharmacology. Niflumic acid triggered a dose-dependent DMR in HT-29 cells (Figure 2a), leading to an EC50 of 1.15 ± 0.23 μM (two independent measurements, n = 4) (Figure 2b). Niflumic acid dose-dependently desensitized HT-29 cells responding to the repeated stimulation with 1 μM zaprinast with an IC50 of 1.18 ± 0.17 μM (2 independent measurements, n = 4) (Figure 2b). SPB05142 (CID2745687), a know GPR35 antagonist,14,15 dose-dependently blocked the DMR of 8 μM niflumic acid with an IC50 of 14.2 ± 1.5 μM (2 independent measurements, n = 4) (Figure 2b). Niflumic acid dose-dependently caused β-arrestin translocation with an EC50 of 40.2 ± 3.7 μM (2 independent measurements, n = 4) (Figure 2c). However, in comparison with YE210 and zaprinast,15 niflumic acid behaved as a full agonist to trigger DMR in HT-29 cells but a partial agonist to cause β-arrestin translocation (Table 1).
Figure 2.
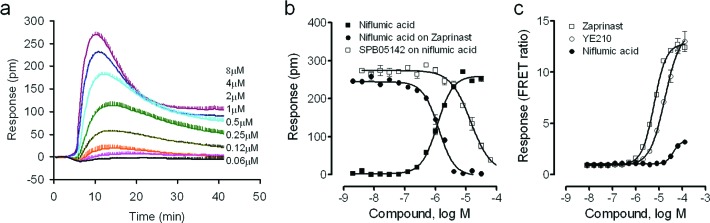
Pharmacological characterization of niflumic acid. (a) Real time kinetic responses of niflumic acid at different doses in HT-29 cells. (b) DMR amplitudes of niflumic acid as a function of its doses, in comparison with the dose-dependent desensitization of the zaprinast DMR by niflumic acid, and the dose-dependent inhibition of the DMR of 8 μM niflumic acid by SPB05142. The DMR amplitudes 10 min poststimulation in HT-29 cells were calculated for all. (c) Dose-dependent responses of niflumic acid, zaprinast, and YE210 as measured by Tango β-arrestin translocation gene reporter assays. The coumarin-to-fluorescein ratios were plotted as a function of ligand doses. The data represents mean ± s.d. from two independent measurements, each in duplicate (n = 4).
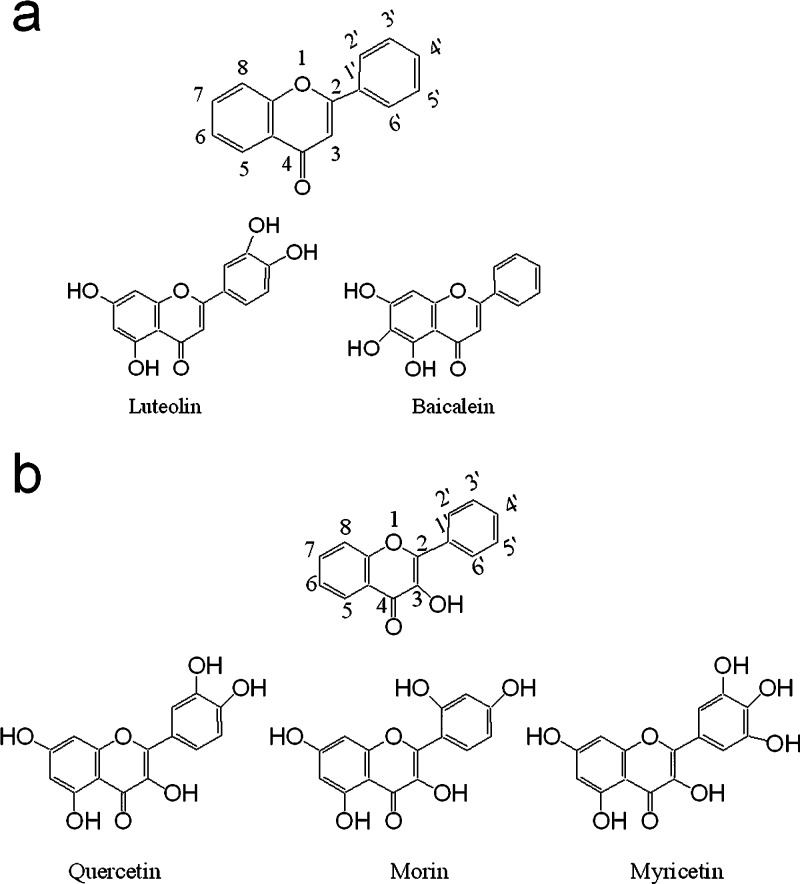
Baicalein, a flavone, was found to be a full agonist to trigger DMR in HT-29 cells but a partial agonist to cause β-arrestin translocation in the GPR35-bla U2OS cells (Figure 3a, Table 1). Structure–activity relationship (SAR) analysis showed that removal of the hydroxyl group(s) at its 6-, 6,7-, and 5,6-positions, converting to chrysin, primuletin, and 7-hydroxyflavone, respectively, all resulted in the loss of its agonist activity at GPR35. Luteolin, another flavone, was found to be a GPR35 partial agonist—it triggered a positive DMR signal but with smaller amplitude, compared to baicalein; and it also resulted in a biphasic dose response in the Tango assay, with a maximal efficacy similar to that of baicalein. SAR analysis showed that removal of its 3′-hydroxyl group (apigenin), converting its 4′-hydroxyl to 4′-methoxyl (diosmetin), or adding a hydroxyl group at its 5′-position (hieracin) all led to the loss of the agonist activity of luteolin. Recently, luteolin was identified to be a GPR35 partial agonist.13
Figure 3.
Structures of certain flavones (a) and flavonols (b) that are GPR35 agonists.
Morin and myricetin were two flavonols, a class of flavonoids that have the 3-hydroxyflavone backbone (Figure 3b). Morin exhibited full agonist activity at GPR35, while myricetin acted as a partial agonist to trigger DMR in HT-29 cells but a full agonist to result in β-arrestin translocation in the engineered cells (Table 1). Morin also displayed slightly higher potency to trigger DMR in HT-29 but similar potency to result in β-arrestin translocation in the engineered cells, compared to myricetin. Further, quercetin acted as a partial agonist, consistent with the recent findings by others.13 Interestingly, SAR studies showed that adding a hydroxyl group or a glucoside group at its 8-position (gossypetin, or gossypin, respectively), or converting all hydroxyl groups to methoxyl groups (quercetin pentamethyl ether), or removal of its hydroxyl group(s) at the 5-position (fisetin), 3′-position (kaempferol), 5,3′,4′-positions (3,7-dihydroxylflavone), or 5,7,3′,4′-positions (3-hydroxyflavone) all led to the loss of the partial agonist activity of quercetin.
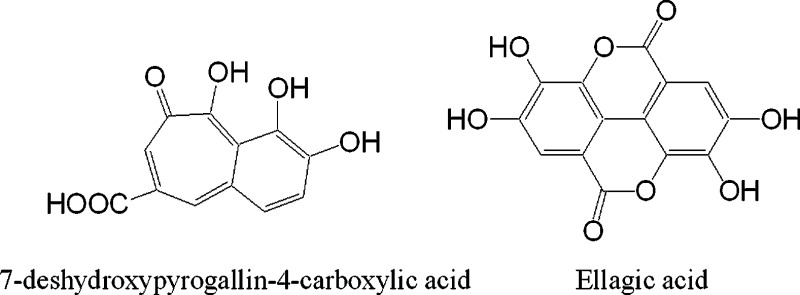
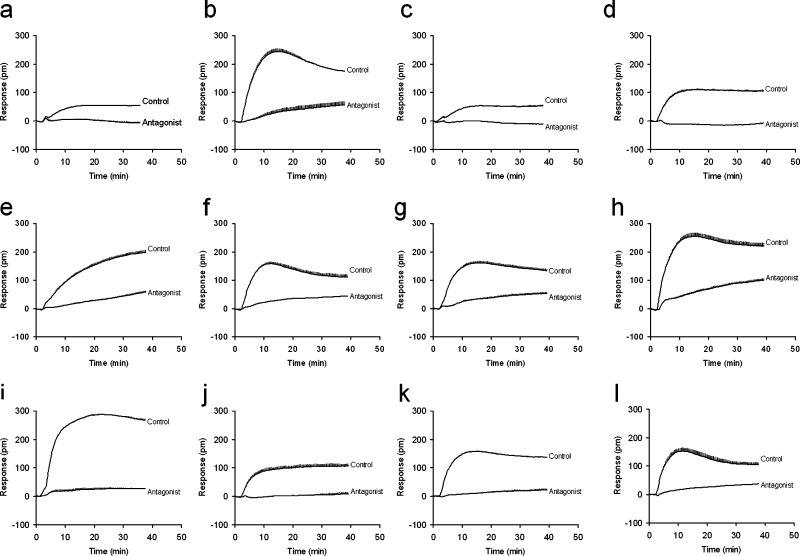
Next, we characterized another seven hits, including lapachol, lobaric acid, laccacid acid A, hematein, α-cyano-4-hydroxycinnamic acid (CHCA), ellagic acid, and 7-deshydroxypyrogallin-4-carboxylic acid (DCA). Results showed that all exhibited agonist activity at the GPR35 but differed greatly in their potency and efficacy (Table 1, Figure 4). Ellagic acid was found to be the most potent agonist in both DMR and Tango assays, leading to an EC50 of 0.11 ± 0.02 and 2.96 ± 0.21 μM (2 independent measurements, each in duplicate, n = 4), respectively. DCA gave rise to the highest potency to result in DMR in HT-29 cells, but relatively low potency to cause β-arrestin translocation in the engineered cells.
Figure 4.
DMR characteristics of natural phenol GPR35 agonists. (a) Luteolin (32 μM), (b) baicalein (32 μM), (c) quercetin (32 μM), (d) morin (8 μM), (e) myricetin (8 μM), (f) lapachol (8 μM), (g) lobaric acid (16 μM), (h) Iaccaic acid A (32 μM), (i) hematein (16 μM), (j) α-cyano-4-hydroxycinnamic acid (CHCA, 8 μM), (k) ellagic acid (2 μM), and (l) 7-deshydroxypyrogallin-4-carboxylic acid (DCA, 4 μM). Each compound induced DMR (control) was compared to its corresponding DMR in the presence of 32 μM SPB05142, the known GPR35 antagonist (antagonist). The data represents mean ± s.d. from two independent measurements, each in four replicates (n = 8).
Since label-free DMR assays mirror the complexity of ligand pharmacology,18 we were interested in the specificity of these agonists to activate GPR35. To determine their specificity, we performed DMR antagonism assays using a known GPR35 antagonist, SPB05142. Here HT-29 cells were pretreated with SPB05142 at different doses for 5 min, followed by stimulation with each agonist at its EC80 or EC100. Results showed that the DMR values of all agonists tested were mostly due to the activation of GPR35 (Figure 4).
Ligand-directed functional selectivity, or biased agonism, is common to GPCRs. Biased agonism describes the ability of distinct ligands to differentially activate one of the vectorial pathways mediated through a receptor.19 Since DMR is a whole cell response and the Tango signal is effectively an end-point measurement (that is, alteration in expression of a gene reporter caused by the GPR35-activation induced β-arrestin translocation), we were interested in examining the biased agonism of the GPR35 agonists identified. Correlation analysis between the maximal responses obtained using DMR and Tango assays suggests that there are two classes of agonists (Figure 5a). The first group of agonists includes quercetin, luteolin, myricetin, morin, DCA, zaprinast, YE210, and lapachol. The relative efficacy of these agonists was well correlated between the two assays. However, other agonists acted as strong partial or full agonists of GPR35 to trigger DMR in native HT-29 cells, but they were only partial agonists to cause β-arrestin-mediated translocation in the engineered cells. Analysis of the relative potency obtained using two different readouts further manifested the biased agonism of these ligands (Figure 5b). Compared to those obtained using Tango assays, the potency obtained using DMR assays was generally left-shifted, except for hematein, baicalein, quercetin, and luteolin. Interestingly, the degree of left-shifted potency obtained using DMR assays is ligand-dependent.
Figure 5.
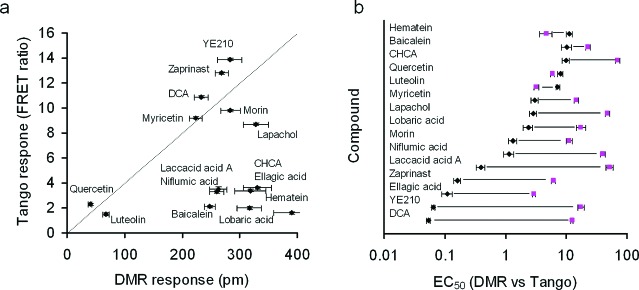
Correlation analyses between DMR and Tango assays. (a) Maximal responses of all agonists. (b) EC50 values (in μM) of all agonists. The pink squares indicate the EC50 obtained using Tango assays, and the black diamonds the EC50 obtained using DMR assays. The data represents mean ± s.d. from two independent measurements, each in duplicate (n = 4).
In summary, combining label-free phenotypic screening with the Tango β-arrestin assay has led to identification of several families of natural phenols as GPR35 agonists, some of which exhibited biased agonism. The most potent agonists were ellagic acid and DCA. Ellagic acid is a natural phenol antioxidant found in numerous fruits and vegetables. In a recent report dealing with a small randomized controlled trial involving 19 patients with carotid artery stenosis, pomegranate juice, which is high in ellagic acid, appeared to reduce blood pressure and carotid artery wall thickness.20 Together with the up-regulated expression of GPR35 in human failing myocardium,21 it is reasonable to speculate that GPR35 may account for the in vivo effect of ellagic acid and represent a druggable target for management of heart diseases.
Glossary
Abbreviations
- CHCA
α-cyano-4-hydroxycinnamic acid
- DCA
7-deshydroxypyrogallin-4-carboxylic acid
- DMR
dynamic mass redistribution
- GPR35
G protein-coupled receptor-35
- YE210
6-bromo-3-methylthieno[3,2-b]thiophene-2-carboxylic acid
Supporting Information Available
Full experimental procedures, structures of seven natural products identified to be GPR35 agonists, antagonist dose inhibition data, and receptor internalization data by myricetin. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare the following competing financial interest(s): HD, HH, SL, AMF, and YF are employees and stock holders of Corning Inc.
Supplementary Material
References
- Newman D. J.; Cragg G. M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [DOI] [PubMed] [Google Scholar]
- Butler M. S. Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008, 25, 475–516. [DOI] [PubMed] [Google Scholar]
- Swinney D. C.; Anthony J. How are new medicines discovered?. Nat. Rev. Drug Discovery 2011, 10, 507–519. [DOI] [PubMed] [Google Scholar]
- Harvey A. L. Natural products in drug discovery. Drug Discovery Today 2008, 13, 894–901. [DOI] [PubMed] [Google Scholar]
- Wang J.; Simonavicius N.; Wu X.; Swaminath G.; Reagan J.; Tian H.; Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [DOI] [PubMed] [Google Scholar]
- Oka S.; Ota R.; Shima M.; Yamashita A.; Sugiura T. GPR35 is a novel lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2010, 395, 232–237. [DOI] [PubMed] [Google Scholar]
- MacKenzie A. E.; Lappin J. E.; Taylor D. L.; Nicklin S. A.; Milligan G. GPR35 as a novel therapeutic target. Front. Endocrinol. 2011, 2, e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H.; Hu H.; Fang Y. Tyrphostin analogs are GPR35 agonists. FEBS Lett. 2011, 585, 1957–1962. [DOI] [PubMed] [Google Scholar]
- Okumura S.; Baba H.; Kumada T.; Nanmoku K.; Nakajima H.; Nakane Y.; Hioki K.; Ikenaka K. Cloning of a G-protein-coupled receptor that shows an activity to transform NIH3T3 cells and is expressed in gastric cancer cells. Cancer Sci. 2004, 95, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Lu J. Y. L.; Wu X.; Summer S.; Whoriskey J.; Saris C.; Reagan J. D. G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology 2010, 86, 1–5. [DOI] [PubMed] [Google Scholar]
- Taniguchia Y.; Tonai-Kachia H.; Shinjo K. Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Lett. 2006, 580, 5003–5008. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y.; Tonai-Kachi H.; Shinjo K. 5-Nitro-2-(3-phenylpropylamino)benzoic acid is a GPR35 agonist. Pharmacology 2008, 82, 245–249. [DOI] [PubMed] [Google Scholar]
- Jenkins L.; Brea J.; Smith N. J.; Hudson B. D.; Reilly G.; Bryant N. J.; Castro M.; Loza M. I.; Milligan G. Identification of novel species-selective agonists of the G-protein-coupled receptor GPR35 that promote recruitment of β-arrestin-2 and activate Gα13. Biochem. J. 2010, 432, 451–459. [DOI] [PubMed] [Google Scholar]
- Zhao P.; Sharir H.; Kapur A.; Cowan A.; Geller E. B.; Adler M. W.; Seltzman H. H.; Reggio P. H.; Heynen-Genel S.; Sauer M.; Chung T. D. Y.; Bai Y.; Chen W.; Caron M. G.; Barak L. S.; Abood M. E. Targeting of the orphan receptor GPR35 by pamoic acid: a potent activator of ERK and β-arrestin2, with antinociceptive activity. Mol. Pharmacol. 2010, 78, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H.; Hu H.; He M.; Hu J.; Niu W.; Ferrie A. M.; Fang Y. 2-(4-methylfuran-2(5H)-ylidene)malononitrile and thieno[3,2-b]thiophene-2-carboxylic acid. J. Med. Chem. 2011, 54, 7385–7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y.; Ferrie A. M.; Fontaine N. H.; Yuen P. K. Characteristics of dynamic mass redistribution of EGF receptor signaling in living cells measured with label free optical biosensors. Anal. Chem. 2005, 77, 5720–5725. [DOI] [PubMed] [Google Scholar]
- Fang Y.; Ferrie A. M.; Fontaine N. H.; Mauro J.; Balakrishnan J. Resonant waveguide grating biosensor for living cell sensing. Biophys. J. 2006, 91, 1925–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y. The development of label-free cellular assays for drug discovery. Exp. Opin. Drug Discovery 2011, 6, 1285–1298. [DOI] [PubMed] [Google Scholar]
- Mailmana R. B. GPCR functional selectivity has therapeutic impact. Trends Pharmacol. Sci. 2007, 8, 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram M.; Rosenblat M.; Gaitini D.; Nitecki S.; Hoffman A.; Dornfeld L.; Volkova N.; Presser D.; Attias J.; Liker H.; Hayek T. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–33. [DOI] [PubMed] [Google Scholar]
- Min K. D.; Asakura M.; Liao Y.; Nakamaru K.; Okazaki H.; Takahashi T.; Fujimoto K.; Ito S.; Takahashi A.; Asanuma H.; Yamazaki S.; Minamino T.; Sanada S.; Seguchi O.; Nakano A.; Ando Y.; Otsuka T.; Furukawa H.; Isomura T.; Takashima S.; Mochizuki N.; Kitakaze M. Identification of genes related to heart failure using global gene expression profiling of human failing myocardium. Biochem. Biophys. Res. Commun. 2010, 393, 55–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.