Abstract
Grounding on our former 3D QSAR studies, a knowledge-based screen of natural bile acids from diverse animal species has led to the identification of avicholic acid as a selective but weak TGR5 agonist. Chemical modifications of this compound resulted in the disclosure of 6α-ethyl-16-epi-avicholic acid that shows enhanced potency at TGR5 and FXR receptors. The synthesis, biological appraisals, and structure–activity relationships of this series of compounds are herein described. Moreover, a thorough physicochemical characterization of 6α-ethyl-16-epi-avicholic acid as compared to naturally occurring bile acids is reported and discussed.
Keywords: bile acid, TGR5, avicholic acid, 16-epi-avicholic acid, FXR, structure−activity relationship, CMC
Bile acid (BA)-activated receptors are attractive targets for drug discovery efforts.1−5 The chief members of this family, namely, farnesoid X receptor (FXR) and TGR5, have been implicated in a number of liver and metabolic diseases, including cholestasis, nonalcoholic steatohepatitis (NASH), obesity, and type II diabetes, to name a few. In the framework of the development of ligands for BA-activated receptors, starting from cholic acid (CA, 1) as a lead compound, we have recently reported the design, synthesis, and pharmacological effects of INT-777 (3, Figure 1) as a potent and selective TGR5 agonist in vivo.6,7 Via TGR5 activation, INT-777 (3) is able to stimulate type 2 iodothyronine deiodinase (D2) activity in brown adipose tissue (BAT) and muscle, as well as induce the release of glucagon-like protein 1 (GLP-1) in enteroendocrine cells. While these results have provided the first proof-of-concept that modulating TGR5 may represent a novel viable strategy for the treatment of metabolic disorders such as type 2 diabetes,7 further aspects of TGR5 activation still remain poorly understood. These include, in particular, the inhibition of hepatic inflammatory responses,8,9 the protection of liver against the production of reactive oxygen species in sinusoidal endothelial cells,10 and, more recently, the release of nitric oxide in enteric neurons leading to the control of intestinal motility.11
Figure 1.
Natural and semisynthetic BA derivatives.
As a continuation of our efforts aimed at providing potent and selective chemical tools with tailored pharmacokinetic properties to probe the functions of BA activated receptors in different tissues,6,12−17 we have been devising further chemical modifications of the BA side chain and nucleus. To this end, we hypothesized that the diverse BA biosynthetic pathways across animal species could be a potential source of novel lead compounds. Starting from our previous results of three-dimensional quantitative structure–activity relationship studies (3D QSAR) that evidenced how different regions around the BA scaffold affect TGR5 activity (Figure 2),18 we thus investigated naturally occurring BAs from 677 vertebrate species19 to identify those that could be endowed with a suitable scaffold in terms of potential activity.
Figure 2.
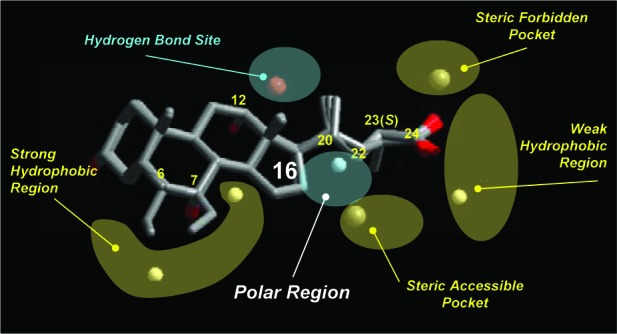
3D QSAR scheme of TGR5 agonists.18
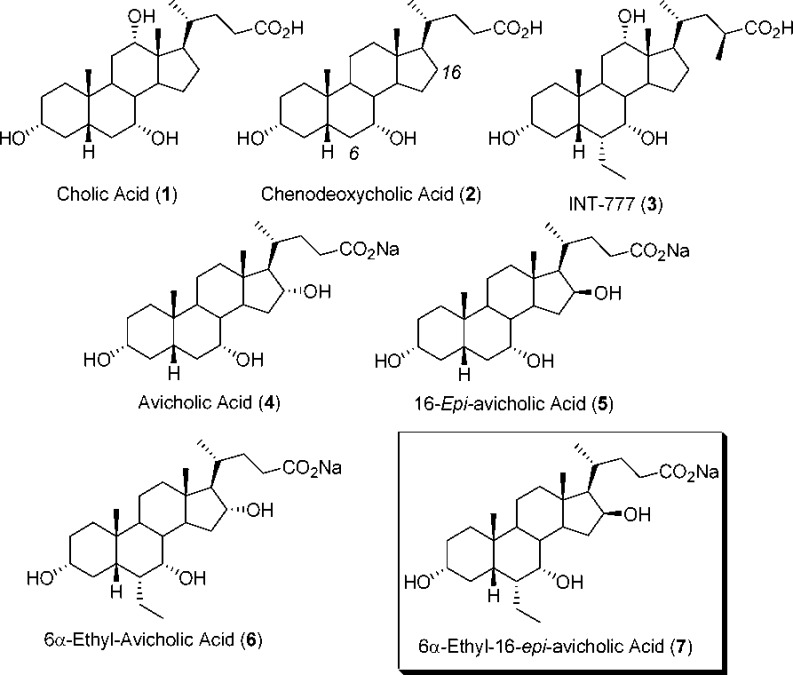
Our attention, in particular, was attracted by C24 BAs bearing a hydroxy group at the C16α-position, as determined in the biliary composition of birds and snakes. Polar groups at this position were indeed suggested by the QSAR model as favoring interaction with TGR5 (Figure 2).18 Accordingly, in this work, we report the synthesis and preliminary evaluation of 3α,7α,16α-trihydroxy-5β-cholan-24-oic acid (avicholic acid, 4), a natural BA first isolated from avian species (Shoebill stork and herons) in 2002,20 and its derivatives 5–7 as TGR5 ligands (Figure 1).
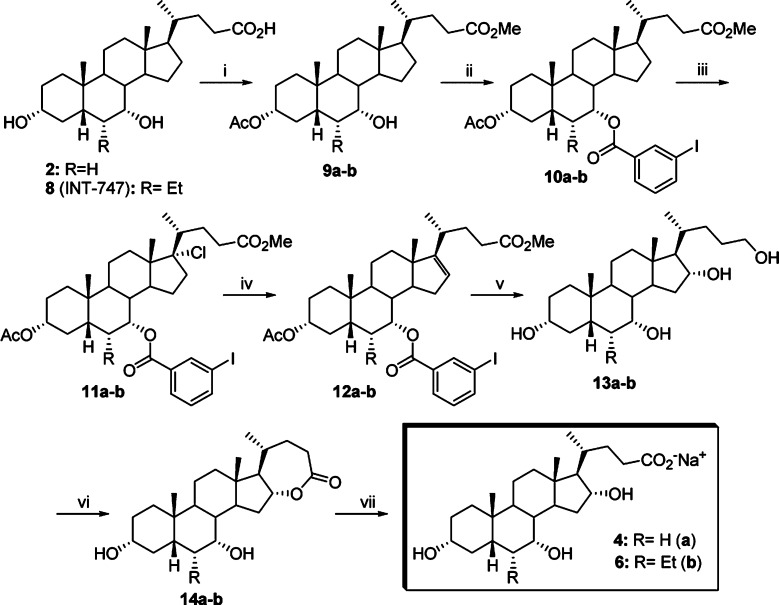
Avicholic acid derivatives 4 and 6 were prepared according to the synthetic approach reported previously by Mukhopadhyay (Scheme 1).21 Thus, CDCA (2) or 6-ECDCA (8)12,17 was treated with p-toluensulfonic acid (pTSA) and MeOH at room temperature to get the corresponding methyl ester analogues, which were selectively protected at the C3 position by reaction with acetic anhydride in the presence of NaHCO3 in refluxing THF (quantitative yield). The 3-acetoxy intermediate 9a,b thus formed was functionalized at the C7 position with a 3-iodo-benzoyl moiety by reaction with 3-iodo-benzoylchloride in the presence of CaH2 and BnEt3N+Cl– in toluene to afford 10a,b in good yield (80 and 74%, respectively).
Scheme 1. Synthesis of Avicholic Acid Derivatives 4 and 6.
Reagents and conditions: (i) (1) MeOH, pTSA; (2) Ac2O, NaHCO3, THF. (ii) 3-I-benzoylchloride, CaH2, BnEt3N+Cl–. (iii) PhICl2, t-BuOH 0.3 M, hυ. (iv) Py, reflux. (v) (1) BH3·THF; (2) NaOH, H2O2; (3) KOH, MeOH. (vi) NCS, TEMPO, Aliquat, CH2Cl2/H2O. (vii) NaOH, MeOH.
Breslow's remote functionalization was achieved by photolysis of PhICl2 in the presence of methyl 3α-acetoxy-7α-m-iodobenzoyloxy-5β-cholan-24-ate 10a,b in 0.3 mM tBuOH/CH2Cl2 solution to yield the 17-Cl derivative 11a,b, in nearly quantitative yield. The corresponding C16–17 olefinic intermediate 12a,b was obtained by refluxing 11a,b in pyridine (81 and 72%). Hydroboration-oxidation of 12a,b by borane–THF complex and alkaline hydrogen peroxide, followed by basic hydrolysis (NaOH/MeOH), furnished the tetrol 13a,b in moderate yield (55 and 47%, respectively). Selective oxidation of the C24 position was achieved by using (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) in CH2Cl2/H2O and Aliquat as a phase transfer catalyst to afford the lactone 14a,b in 37 and 30% yield, respectively. Alkali hydrolysis (NaOH/MeOH) followed by a reverse phase RP-18 chromatography afforded avicholic acid sodium salt (4) and 6α-ethyl-avicholic acid sodium salt (6) in 10 and 6% overall yield, respectively.
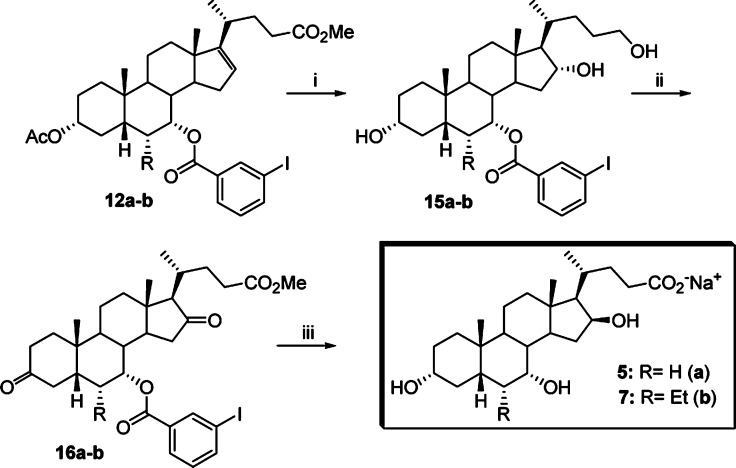
The corresponding C16-epimers 5 and 7 were prepared according to Scheme 2.22 Thus, the C16–17 olefinic BA derivative 12a,b was submitted to hydroboration-oxidation reaction using the previously reported protocol and selectively deprotected at C3 position by using a solution of K2CO3 in MeOH at room temperature. The 7α-m-iodobenzoyloxy derivative 15a,b, obtained in 56 and 50% yield, respectively, was reacted with Jones reagent and esterified at the terminal carboxylic group by treatment with pTSA in MeOH at room temperature to furnish 16a,b, in nearly quantitative yield. Finally, reduction of the keto groups with tert-butylamine–borane complex in CH2Cl2 at reflux, followed by hydrolysis with NaOH in MeOH and purification by medium pressure liquid chromatography, afforded the desired 3α,7α,16β-trihydroxy-5β-cholan-24-oic acid sodium salt (5) and 3α,7α,16β-trihydroxy-6α-ethyl-5β-cholan-24-oic acid sodium salt (7) in 15 and 11% overall yield, respectively. Compounds 4–7 were unstable in the free acid form, spontaneously reacting to give the corresponding lactone derivatives. Therefore, the compounds were prepared and tested as sodium salts.
Scheme 2. Synthesis of 16-epi-Avicholic Acid Derivatives 5 and 7.
Reagents and conditions: (i) (1) BH3·THF; (2) NaOH, H2O2; (3) K2CO3, MeOH. (ii) (1) Jones reagent; (2) MeOH, pTSA. (iii) (1) t-BuNH2BH3, CH2Cl2, reflux; (2) NaOH, MeOH.
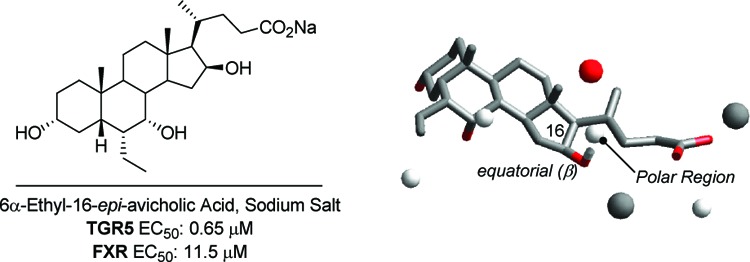
Avicholic acid and its derivatives 4–7 were evaluated, along with CDCA (2) and LCA as reference controls, for their ability to activate TGR5 and FXR using homogeneous time-resolved fluorescence (cell-based) assay and ALPHAscreen assay, respectively (Table 1). The results indicate that avicholic acid (4) is a weak TGR5 agonist, with no activity at FXR. Interestingly, its C16-epimer 5 shows an increased potency by 1 order of magnitude toward TGR5 accompanied by FXR activity, suggesting the preference of the hydroxyl group to form hydrogen bond interactions when placed on the equatorial position of the steroidal nucleus. This finding is also in agreement with the results of the QSAR model,18 indicating that the equatorial position of C16 as favored for polar interactions with TGR5 (Figure 3).
Table 1. TGR5 and FXR Activities of Natural and Semisynthetic BAsa6,15.
| TGR5b |
FXRc |
|||
|---|---|---|---|---|
| BA | EC50 | efficacyd | EC50 | efficacyd |
| CA (1) | 13.6 ± 0.7 | 101 | >100 | 0 |
| CDCA (2) | 6.7 ± 0.3 | 105 | 13.0 ± 0.7 | 62 |
| INT-777 (3) | 0.82 ± 0.04 | 166 | >100 | 18 |
| 4 | 160 ± 8 | 194 | >100 | 0 |
| 5 | 25 ± 1 | 148 | 55 ± 3 | 132 |
| 6 | 87 ± 4 | 198 | 22 ± 1 | 121 |
| 7 | 0.65 ± 0.03 | 120 | 11.5 ± 0.6 | 210 |
Data represent mean values ± SDs of at least three independent experiments.
Units are μM for EC50 and % of 10 μM LCA (EC50: 5 μM) value for efficacy.
Units are μM for EC50 % of 10 μM CDCA (2) value for efficacy.
Efficacy values are here used as a measure of fluorescence change.
Figure 3.
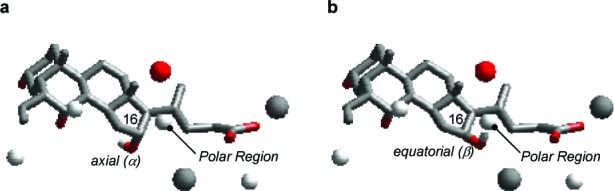
Alignment of 6α-ethyl-avicholic acid (6, panel a) and 6α-ethyl-16-epi-avicholic acid (7, panel b) according to the 3D QSAR scheme. The hydroxyl group of C16 nicely fits the polar region of interaction when located on the equatorial position (β) of the steroidal nucleus rather than the axial (α) position.
The 6α-ethyl derivatives 6 and 7 were synthesized to assess whether the insertion of this alkyl group at the 6α position of the parent derivatives 4 and 5 would have improved the potency toward TGR5 and/or FXR, according to previously reported SARs.6,15 As shown in Table 1, in the case of 6α-ethyl-16-epi-avicholic acid (7), the introduction of the alkyl group markedly increased the potency at TGR5 by about 2 orders of magnitude, while showing a slight improvement of FXR activity. In the case of 6α-ethyl-avicholic acid (6), however, this effect is partially reversed, with enhanced agonistic activity toward FXR rather than TGR5.
Table 2 reports the physicochemical data of 6α-ethyl-16-epi-avicholic acid (7) as compared to INT-777 (3) and natural BAs, including CA (1), CDCA (2), and avicholic acid (4). All of the studied BA presents the same acidity constant with a pKa = 5 like all natural occurring BA,23 and the hydroxyl group on the C16 position does not affect the C24 carboxylic ionization. In addition, it was found that the water solubility (Ws) of 6α-ethyl-16-epi-avicholic acid (7) as a protonated acid is slightly higher than the trihydroxy BA INT-777 (3) and lower than the parent compound 4.
Table 2. Physicochemical Properties of BAs.
| BA | Wsa (μM) | CMCb (mM) | STCMCc (dyn/cm) | Log PAd | albumin binding (%) |
|---|---|---|---|---|---|
| CA (1)6 | 273 | 9.2 | 48 | 1.1 | 50 |
| CDCA (2) | 32 | 3.0 | 46 | 2.2 | 93 |
| INT-777 (3) | 99 | 2.0 | 50 | 1.4 | 62 |
| 4 | 188 | 10 | 50 | 1.1 | 58 |
| 7 | 120 | 5.9 | 53 | 1.6 | 83 |
Ws: water solubility as protonated species in 0.1 M HCl water solution.
CMC: critical micellar concentration determined in 0.15 M NaCl water solution.
STCMC: surface tension at CMC in 0.15 M NaCl water solution.
Log PA (octanol/water partition coefficient of the ionized species): lipophilicity.
The detergent power of BA, that is, the tendency to form micelles, is the main concern for this detergent-like molecule.24 The critical micellar concentration (CMC) and the surface tension at CMC (STCMC) values are indeed correlated with potential (cyto)toxicity of BAs. Molecules with high CMC and STCMC values are poor detergents and therefore poorly toxic to biological membranes. Furthermore, the lipophilicity (Log PA) describes the passive diffusion of BAs across biological membranes, whereas the albumin binding reflects the amount of compound that is protein-bound in the blood. The CMC of 7 is higher than CDCA (2) and 3, while being lower than CA (1) and avicholic acid (4). This relatively high CMC value combines with a high STCMC value, suggesting a low micellar aggregation number and poor detergency for 7 that may favor low toxicity.
Among trihydroxy BAs, compound 7 shows the highest lipophilicity value (Log PA). This is likely ascribed to the presence of the ethyl moiety in the C6α position that combines with the shift of the hydroxyl group from C12α of INT-777 (3) to C16β of 7. Interestingly, the shift of the hydroxyl from C12α of CA (1) to C16α of 4 does not play a role in affecting lipophilicity, as evidenced by the same Log PA values reported in Table 2. The percent albumin binding of 7 is higher than CA (1), INT-777 (3), and avicholic acid (4). Again, this may be due to the presence of the 6α-ethyl moiety and the C16β hydroxyl group. Interestingly, the value of albumin binding of 7 is compatible with a relatively fast hepatic uptake, similar to naturally occurring BAs such as CA (1).25 The physicochemical properties of compound 7, including poor detergency and adequate hydrophilicity, are in agreement with previous structure–property relationship studies carried out on a large number of natural and synthetic BAs.26 The 16β orientation of the hydroxyl group, in particular, confers to the molecule a low detergency that, combined with the relatively high lipophilicity due to the presence of the 6α ethyl group, may further facilitate the intestinal absorption by passive mechanism.
Compound 7 is fully conjugated by the mouse liver with taurine (unpublished data), in contrast with INT-777 (3) that is only partially conjugated with taurine after the C23-methyl isomerization. Akin to naturally occurring taurine-conjugated bile salts,27 the taurine-conjugated compound 7 may be additionally absorbed by the terminal ileum with an active mechanism. This would likely increase its bioavailability and modify its pharmacokinetics and biodistribution in comparison with INT-777 (3).
In conclusion, this work suggests that natural BAs can further provide additional lead compounds on the route to unravel physiological and therapeutic implications of TGR5 modulation. Among these, herein, we have reported avicholic acid (4) as a selective TGR5 modulator, although showing weak potency. Chemical manipulations of 4 have led to the discovery of 6α-ethyl-16-epi-avicholic acid (7) as a potent TGR5 agonist and moderately active FXR ligand, endowed with an interesting physicochemical profile. Further modifications of both the steroidal nucleus and side chain of 7 are currently being investigated with the aim of bestowing TGR5 selectivity over FXR and additional potency to this compound.
Acknowledgments
We thank Erregierre (Bergamo, Italy) for the gift of BAs as starting material.
Glossary
Abbreviations
- BA
bile acid
- FXR
farnesoid X receptor
- SAR
structure–activity relationship
Supporting Information Available
Description of the synthetic procedures, biological methods, and analytical procedures of all target compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by Intercept Pharmaceuticals (New York).
The authors declare no competing financial interest.
Supplementary Material
References
- Thomas C.; Pellicciari R.; Pruzanski M.; Auwerx J.; Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discovery 2008, 7, 678–693. [DOI] [PubMed] [Google Scholar]
- Lefebvre P.; Cariou B.; Lien F.; Kuipers F.; Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009, 89, 147–191. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B.; Zhou H.; Pandak W. M.; Ren S.; Gil G.; Dent P. Bile acids as regulatory molecules. J. Lipid Res. 2009, 50, 1509–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.; Auwerx J.; Schoonjans K. Bile Acids and the membrane bile acid receptor TGR5-connecting nutrition and metabolism. Thyroid 2008, 18, 167–174. [DOI] [PubMed] [Google Scholar]
- Pellicciari R.; Gioiello A.; Costantino G. Potential therapeutic applications of farnesoid X receptor (FXR) modulators. Expert Opin. Ther. Pat. 2006, 16, 333. [Google Scholar]
- Pellicciari R.; Gioiello A.; Macchiarulo A.; Thomas C.; Rosatelli E.; Natalini B.; Sardella R.; Pruzanski M.; Roda A.; Pastorini E.; Schoonjans K.; Auwerx J. Discovery of 6α-ethyl-23(S)-methyl-cholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 2009, 52, 7958–7961. [DOI] [PubMed] [Google Scholar]
- Thomas C.; Gioiello A.; Noriega L.; Strehle A.; Oury J.; Rizzo G.; Macchiarulo A.; Yamamoto H.; Mataki C.; Pruzanski M.; Pellicciari R.; Auwerx J.; Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel V.; Donner M.; Winandy S.; Kubitz R.; Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem. Biophys. Res. Commun. 2008, 372, 78–84. [DOI] [PubMed] [Google Scholar]
- Wang Y. D.; Chen W. D.; Yu D.; Forman B. M.; Huang W. The G-protein coupled bile acid receptor Gpbar1 (TGR5) negatively regulates hepatic inflammatory response through antagonizing Nuclear Factor kappaB. Hepatology 2011, 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel V.; Reinehr R.; Gatsios P.; Rupprecht C.; Gorg B.; Selbach O.; Haussinger D.; Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology 2007, 45, 695–704. [DOI] [PubMed] [Google Scholar]
- Poole D. P.; Godfrey C.; Cattaruzza F.; Cottrell G. S.; Kirkland J. G.; Pelayo J. C.; Bunnett N. W.; Corvera C. U. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol. Motil. 2010, 22, 814–e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicciari R.; Fiorucci S.; Camaioni E.; Clerici C.; Costantino G.; Maloney P. R.; Morelli A.; Parks D. J.; Willson T. M. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 2002, 45, 3569–3572. [DOI] [PubMed] [Google Scholar]
- Pellicciari R.; Costantino G.; Camaioni E.; Sadeghpour B. M.; Entrena A.; Willson T. M.; Fiorucci S.; Clerici C.; Gioiello A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J. Med. Chem. 2004, 47, 4559–4569. [DOI] [PubMed] [Google Scholar]
- Pellicciari R.; Gioiello A.; Costantino G.; Sadeghpour B. M.; Rizzo G.; Meyer U.; Parks D. J.; Entrena-Gaudix A.; Fiorucci S. Back door modulation of the Farnesoid X Receptor: design, synthesis, and biological evaluation of a series of side chain modified chenodeoxycholic acid derivatives. J. Med. Chem. 2006, 49, 4208–4215. [DOI] [PubMed] [Google Scholar]
- Pellicciari R.; Sato H.; Gioiello A.; Costantino G.; Macchiarulo A.; Sadeghpour B. M.; Giorgi G.; Schoonjans K.; Auwerx J. Nongenomic actions of bile acids. Synthesis and preliminary characterization of 23-and 6,23-alkyl-substituted bile acid derivatives as selective modulators for the g-protein coupled receptor TGR5. J. Med. Chem. 2007, 50, 4265–4268. [DOI] [PubMed] [Google Scholar]
- Sato H.; Macchiarulo A.; Thomas C.; Gioiello A.; Une M.; Hofmann A. F.; Saladin R.; Schoonjans K.; Pellicciari R.; Auwerx J. Novel potent and selective bile acid derivatives as TGR5 agonists: Biological screening, structure-activity relationships, and molecular modeling studies. J. Med. Chem. 2008, 51, 1831–1841. [DOI] [PubMed] [Google Scholar]
- Gioiello A.; Macchiarulo A.; Carotti A.; Filipponi P.; Costantino G.; Rizzo G.; Adorini L.; Pellicciari R. Extending SAR of bile acids as FXR ligands: Discovery of 23-N-(carbocinnamyloxy)-3α,7α-dihydroxy-6α-ethyl-24-nor-5β-cholan-23-amine. Bioorg. Med. Chem. 2011, 19, 2650–2658. [DOI] [PubMed] [Google Scholar]
- Macchiarulo A.; Gioiello A.; Thomas C.; Massarotti A.; Nuti R.; Rosatelli E.; Sabbatini P.; Schoonjans K.; Auwerx J.; Pellicciari R. Molecular field analysis and 3D-quantitative structure-activity relationship study (MFA 3D-QSAR) unveil novel features of bile acid recognition at TGR5. J. Chem. Inf. Model 2008, 48, 1792–1801. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F.; Hagey L. R.; Krasowski M. D. Bile salts of vertebrates: structural variation and possible evolutionary significance. J. Lipid Res. 2010, 51, 226–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagey L. R.; Schteingart C. D.; Ton-Nu H.-T.; Hofmann A. F. A novel primary bile acid in the Shoebill stork and herons and its phylogenetic significance. J. Lipid Res. 2002, 43, 685–690. [PubMed] [Google Scholar]
- Mukhopadhyay S.; Maitra U. Facile synthesis, aggregation behavior, and cholesterol solubilization ability of avicholic acid. Org. Lett. 2004, 6, 31–34. [DOI] [PubMed] [Google Scholar]
- Iida T.; Hirosaka M.; Kakiyama G.; Keisuke Shiraishi K.; Schteingart C. D.; Hagey L. R.; Ton-Nu H.-T.; Hofmann A. F.; Mano N.; Goto J.; Nambara T. Potential bile acid metabolites. 25. Synthesis and chemical properties of stereoisomeric 3a,7a,16- and 3a,7a,15-trihydroxy-5b-cholan-24-oic acids. Chem. Pharm. Bull. 2002, 50, 1327–1334. [DOI] [PubMed] [Google Scholar]
- Roda A.; Fini A. Effect of nuclear hydroxy substituents on aqueous solubility and acidic strenght of bile acids. Hepatology 1984, 4, 72–76. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F.; Roda A. Physicochemical properties of bile acids and their relationship to biological properties: An overview of the problem. J. Lipid Res. 1984, 25, 1477–1489. [PubMed] [Google Scholar]
- Aldini R.; Roda A.; Labate A. M.; Cappelleri G.; Roda E.; Barbara L. Hepatic bile acid uptake: Effect of conjugation, hydroxyl and keto groups, and albumin binding. J. Lipid Res. 1982, 23, 1167–1173. [PubMed] [Google Scholar]
- Roda A.; Grigolo B.; Pellicciari R.; Natalini B. Structure-activity relationship studies on natural and synthetic bile acid analogs. Dig. Dis. Sci. 1989, 34, 24S–35S. [DOI] [PubMed] [Google Scholar]
- Aldini R.; Roda A.; Montagnani M.; Roda E. Bile acid structure and intestinal absorption in the animal model. Ital. J. Gastroenterol. 1995, 27, 141–144. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.