Abstract
Objectives. We estimated short-term health care cost savings that would result from oral health professionals performing chronic disease screenings.
Methods. We used population data, estimates of chronic disease prevalence, and rates of medication adherence from the literature to estimate cost savings that would result from screening individuals aged 40 years and older who have seen a dentist but not a physician in the last 12 months. We estimated 1-year savings if patients identified during screening in a dental setting were referred to a physician, completed their referral, and started pharmacological treatment.
Results. We estimated that medical screenings for diabetes, hypertension, and hypercholesterolemia in dental offices could save the health care system from $42.4 million ($13.51 per person screened) to $102.6 million ($32.72 per person screened) over 1 year, dependent on the rate of referral completion from the dental clinic to the physician's office.
Conclusions. Oral health professionals can potentially play a bigger role in detecting chronic disease in the US population. Additional prevention and monitoring activities over the long term could achieve even greater savings and health benefits.
About 133 million Americans, or almost 1 in 2 adults, have at least 1 chronic illness. Chronic conditions account for more than 75% of health care costs and 70% of deaths each year in the United States.1 Chronic diseases cost the United States $153 billion annually in lost productivity, and individuals who are overweight, obese, or have other chronic conditions miss an additional 450 million days from work compared with healthy workers.2 The high prevalence, associated morbidity, and economic impact of chronic diseases, particularly diabetes, hypercholesterolemia (high blood cholesterol), and hypertension, are a serious public health issue in the United States today. According to the Medical Expenditure Panel Survey, about 40% of adults visit the dentist in a given year,3 10% to 20% of whom have not seen a physician in the preceding year.4,5 This presents an opportunity for oral health professionals to be part of an integrated health care team working to combat these chronic diseases.
Screening for undiagnosed medical conditions in the dental office has long been proposed as a potentially valuable public health service.6–8 Widespread adoption of this practice is dependent on determining the efficacy of screening in the dental setting and acceptance by dental care providers and patients. To examine the effectiveness and acceptance of screening programs, several studies have evaluated screening for diabetes, hypercholesterolemia, and hypertension in the dental setting.4,9 These conditions were chosen because of (1) their prevalence in today’s society, (2) the significant morbidity and mortality associated with these conditions, (3) the ability to lessen their burden through early detection, and (4) the availability of well-validated, safe, and easy-to-use screening tools.4,9,10 Additional studies have found that a majority of dentists11 and patients12 believe that it is important for oral health professionals to perform medical screenings for heart disease, diabetes, and hypertension in the dental office.
A study conducted in Sweden concluded that limiting screening to patients older than 40 years of age would increase the percentage of patients who participated in screening and who had hypertension.13 Another study came to a similar conclusion, and also found potential benefits for patients who had been previously diagnosed with hypertension but who did not maintain adequate blood pressure control.14 The utility of screening for diabetes during dental visits has also been evaluated. Among 356 patients with no known history of diabetes who visited an outpatient periodontal clinic in India, diabetes was found in 19.1% of the patients.15
In practice, physicians who detect an abnormal test result for the presence of chronic disease are inclined to provide medication to their patients. The thresholds upon which primary care physicians determine mediation treatment, particularly for diabetes and hypertension, have been lowered since the early 1990s, and newer guidelines encourage the treatment of prediabetes and prehypertension.16 In this analysis, we assumed that people who had undiagnosed diabetes, undiagnosed hypercholesterolemia, or undiagnosed hypertension and were subsequently diagnosed for 1 or more of these conditions by a physician would receive prescription drug treatment per treatment guidelines.
Once patients start medication therapy, it is important that they adhere to the regimen. Medication treatment of cardiovascular disease has been shown to be effective only if patients adhere to their medication.17 Poor medication adherence has been associated with increased hospitalization, increased use of health care resources, and higher overall health care costs.18–20 Poor medication adherence has also been associated with failure to reach treatment target goals, (such as blood pressure control), adverse clinical outcomes, and higher rates of mortality.17,21,22
No previous studies we know of examined the cost implications to the US health care system stemming from chronic disease screenings in a dental office. In the current environment of fiscal constraint and the focus on cost control in health care reform, potential cost savings are important to consider. In this analysis, we estimated 1 component of the overall potential health care cost savings associated with screening for medical conditions in a dental setting. Given that our calculations are based on prevalence rates for undiagnosed disease, in this scenario a positive screening test will always result in a positive diagnosis. This component comprised the 1-year cost savings associated with (1) oral health professionals’ detection of diabetes, hypercholesterolemia, and hypertension in previously undiagnosed patients; (2) their referral of those patients to a physician for diagnosis; and (3) the patients’ initiation of medication therapy. Specifically, we calculated the medical costs and appropriate pharmacy costs associated with medication adherence and nonadherence in a 12-month period after a physician’s diagnosis. Medical costs, as defined by Sokol et al., include the costs of outpatient, inpatient, and emergency room services over a 12-month period.18 Pharmacy costs include all the costs associated with medications dispensed by an outpatient, mail-service, or community-based pharmacy over a 12-month period.18 In our model, “health care savings” means medication health care savings during a 12-month period.
METHODS
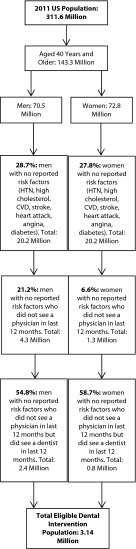
We used data from a number of sources to estimate the cost savings associated with medical screenings in a dental office among adults aged 40 years and older who had undiagnosed diabetes, hypercholesterolemia, or hypertension. These patients had no reported history of coronary heart disease or diabetes, no reported disease-specific risk factors, and no medication use for these conditions, and had not visited a physician in the last 12 months. To estimate the population aged 40 years and older who would be eligible for a medical screening in a dental office on the basis of these criteria, we used data published by Glick and Greenberg, who estimated the percentage of men and women aged 40 years and older who met the criteria outlined and had seen a dentist in the last 12 months.4 We applied these estimates to the total US population in 2011 aged 40 years and older as measured by the US census.23 Figure 1 shows a flowchart for identifying candidates for a screening examination for diabetes, hypercholesterolemia, and hypertension in a dental office. Individuals had hypertension if their systolic blood pressure was 140 millimeters of mercury or above or their diastolic blood pressure was 90 millimeters of mercury or above.24,25 Individuals had hypercholesterolemia if their serum total cholesterol was 240 milligrams per deciliter or above.24,26,27
FIGURE 1—
Determination of intervention population for chronic disease screenings in a dental setting among US adults aged 40 years and older: 2011.
Note. CVD = cardiovascular disease; HTN = hypertension.
Finally, we assumed that individuals had diabetes if their estimated glucose level was 126 milligrams per deciliter or above,24 which has been estimated to be equal to a hemoglobin A1C level of 6.0% or above.28 Applying the screening eligibility algorithm to the 2011 US Census population, 3.14 million individuals, or 2.2% of the US population aged 40 and older, would be eligible for a medical screening for diabetes, hypercholesterolemia, and hypertension in a dental office.
After estimating the total population eligible for a medical screening in a dental office, we estimated the difference in the 12-month medication health care costs between 2 arms: the counterfactual arm (no screening by oral health care professionals) and the treatment arm (screening by oral health care professionals with follow-up to physicians for pharmacological treatment; Figure 2). We assumed that all patients with a chronic disease who complied with their referral and visited a physician would receive pharmacological treatment according to treatment guidelines.16 Using data from the 1999–2004 National Health and Nutrition Examination Survey (NHANES),29 we estimated the prevalence of undiagnosed single-condition and comorbid diabetes, hypercholesterolemia, and hypertension among a population aged 40 and older who had not reported a history of chronic disease or associated risk factors. We applied those rates to the eligible screening population to estimate the percentage of individuals with the following undiagnosed chronic disease: single-condition hypercholesterolemia (7.35%), single-condition hypertension (19.31%), single-condition diabetes (3.70%), hypertension and hypercholesterolemia (4.26%), diabetes and hypercholesterolemia (0.51%), diabetes and hypertension (2.13%), and all 3 conditions (0.51%). We applied these undiagnosed prevalence rates for single and comorbid chronic conditions to the counterfactual and treatment arm.
FIGURE 2—

Flowchart determining cost savings from health screenings in a dental setting among US adults aged 40 years and older: 2011.
Note. Diab = diabetes; HC = hypercholesterolemia; HTN = hypertension.
aData from 1999–2004 National Health and Nutrition Examination Survey.29
bData from Speechley et al.30 and Friedman-Gerlicz and Lilly.31
cData from Jontell and Glick.32
dData from Sokol et al.18
eData from appendix of Roebuck et al.33
Figure 2 shows how we derived total medical costs when oral health professionals performed no chronic disease screenings (counterfactual arm) versus total medication health care costs when there was a dental intervention and a physician referral (treatment arm). To accomplish this, we used data from Sokol et al., who estimated the health care costs associated with medication nonadherence and medication adherence for diabetes, hypercholesterolemia, and hypertension based on a commercially insured population of individuals younger than 65 years.18 The study by Sokol et al. was based on integrated pharmacy and medical claims from 1997 through 1999. Because no patient in the counterfactual arm received medication for chronic disease, we assumed zero pharmacy costs. Sokol et al. estimated medical and pharmacy costs across a range of medication adherence thresholds (1%–19%, 20%–39%, 60%–79%, 80%–100%) over a 12-month period. Adherence, as defined by Sokol et al., is the percentage of days that a patient has medication on hand over an analysis period. Patients with an adherence level at or above 80%, a threshold commonly defined in the adherence literature,19,34–36 were defined as adherent to medication. Because patients with chronic disease in the counterfactual arm received no medication treatment, they were considered nonadherent. To determine the total medical cost of nonadherence, we calculated a weighted average of the total medical costs across the adherence thresholds below 80%. Table 1 summarizes the data from Sokol et al. on the total per-person medical cost of medication nonadherence as well as the total per-person medication health care cost (medical plus pharmacy cost) of medication adherence and nonadherence. For individuals with more than 1 condition, we simply aggregated the single-disease-specific cost estimates across conditions.
TABLE 1—
Disease-Related Health Care Per-Person Cost of Medication Adherence Among Individuals Younger Than 65 Years: United States, 1997–1999
| Chronic Disease | Total Per-Person Medical Cost of Nonadherence, $ | Total Per-Person Cost (Medical + Pharmacy) of Nonadherence, $ | Total Per-Person Cost (Medical + Pharmacy) of Adherence, $ |
| Diabetes | 6543 | 6827 | 4570 |
| Hypercholesterolemia | 4774 | 5176 | 3924 |
| Hypertension | 5149 | 5336 | 4871 |
| Diabetes + hypercholesterolemia | 11 316 | 12 002 | 8494 |
| Diabetes + hypertension | 11 691 | 12 162 | 9441 |
| Hypertension + hypercholesterolemia | 9922 | 10 512 | 8795 |
| All 3 conditions | 16 465 | 17 338 | 13 365 |
Note. The data are based on patients enrolled in a commercial medical and prescription drug plan in the United States from June 1997 through May 1999. We calculated total per-person medical and total cost of nonadherence by taking a weighted average of costs across each adherence threshold below 80%.
Source. Sokol et al.18
In the counterfactual arm, we applied the disease-specific medical costs from Sokol et al. to the estimated population with undiagnosed chronic disease. In the treatment arm, we accounted for the possibility that the screening tests performed by the oral health professional could result in a false negative. Prior literature has suggested that the false-negative rate for a hypertension or hypercholesterolemia screening examination is between 3% and 5%.30,31 We therefore assumed a false-negative rate of 4% for all 3 screening exams that an oral health professional would perform for a patient. We assumed that individuals who had a false-negative test result for diabetes, hypercholesterolemia, or hypertension would not see a physician and would incur the full medical cost of medication nonadherence. After we filtered out individuals with a false-negative test result, 3.10 million individuals remained eligible for a physician’s referral if they tested positive for diabetes, hypercholesterolemia, or hypertension. In a study by Jontell and Glick, which analyzed a group of private dental practices in Sweden, about 83% of patients complied with their dentist’s recommendation and contacted a physician if they were determined to be at increased risk of dying from a severe coronary heart disease event within 10 years.32 In our model, we assumed that 83% of patients who were at risk for diabetes, hypercholesterolemia, or hypertension would comply with their dentist’s referral and visit a physician for treatment. We assumed that the remaining 17% who did not comply with their dentist’s referral and had undiagnosed chronic disease incurred the full medical cost associated with medication nonadherence. As the Jontell and Glick study is the only one that has looked at referral completion rates of referrals from a dentist to a physician, we used these rates for our calculations. Because Sweden has a national health care system, these rates may overestimate what would happen in the US health care model. Hence, we also conducted sensitivity analyses adjusting the referral completion rates (see Results). In our model, we assumed that 83% of screened patients would complete their referral to a primary care physician whether or not they currently had a primary care physician.
We assumed that individuals who had undiagnosed diabetes, hypercholesterolemia, or hypertension (or combinations of these conditions), complied with their dentist’s referral, and visited a physician received medication treatment. Because we used population prevalence rates based on data from the 1999–2004 NHANES,29 any false-positive test results resulting from a physician’s examination would automatically be accounted for in our model. A certain percentage of patients with undiagnosed chronic diseases that received prescription drug treatment would be adherent to their medication regimen. Using a commercially insured population, Roebuck et al. estimated that 50.5% of patients with hypertension, 42.6% of patients with hypercholesterolemia, and 41.2% of patients with diabetes are adherent (adherence level ≥ 80%) to their medication regimen over the course of a year.33 We used these medication adherence rates to estimate the number of individuals with chronic disease who would be adherent or nonadherent to their medication regimen. We then applied the total cost of medication adherence and nonadherence (Table 1) from Sokol et al.18 to the adherent and nonadherent chronic disease population (Figure 2). These costs included pharmacy and medical expenditures. We inflated all costs from 1999 to 2011 dollars using the gross domestic product deflator.37,38
We calculated the total savings before labor as the total cost in the counterfactual arm minus the total cost in the treatment arm (Figure 2). The total cost in the treatment arm was the sum of the total medical cost from false-negative test results, the total medical cost from patients not complying with their dentist referral, and the total medication health care cost (pharmacy and medical) from patients complying with their dentist referral and receiving medication for diabetes, hypercholesterolemia, or hypertension. To account for labor costs, we used data from the American Dental Association Survey of Dental Practice39 to estimate the hourly wage for a general practice dentist and dental assistant. On the basis of our experience, we estimated that it would take approximately 12 minutes to complete a diabetes, hypercholesterolemia, and hypertension screening in a dental office. We expected that it would take approximately 7 minutes for a dental assistant to take a blood sample, measure blood pressure, and test the blood for A1C and lipid levels and 5 minutes for a general practice dentist to read the results and report the findings back to the patient. On the basis of this assumption, we estimated that labor costs were $11.90. We divided total savings before labor by the eligible intervention population to generate savings per intervention. To generate our final savings estimates, we subtracted labor costs from the estimate of before-labor savings per intervention.
We performed a number of sensitivity analyses around the referral completion rate. Prior studies have shown that physicians’ referral completion rates range from 63% to 83%.40–43 The upper bound of this range is similar to what Jontell and Glick estimated.32 We applied this range to our model to check the sensitivity of our savings estimates to various referral completion rates, holding all other parameters fixed. We conducted all analyses with Microsoft Excel Office 2010 (Microsoft Corporation, Redmond, WA).
RESULTS
We estimated that, before labor costs were factored into our model and using an 83% referral completion rate, diabetes, hypercholesterolemia, and hypertension screenings in dental offices would save the health care system $102.6 million over 1 year, or $32.72 per person screened. With labor costs factored in, diabetes, hypercholesterolemia, and hypertension screenings in the dental office would save the health care system $65.3 million, or $20.82 per person screened.
At a 77% completion referral rate, before labor costs are factored in, we estimated that diabetes, hypercholesterolemia, and hypertension screenings in the dental office would save the health care system $83.8 million, or $26.74 per person screened over 1 year. With labor costs factored in, the health care system over 1 year would save $46.5 million, or $14.84 per person screened. At a referral completion rate of 63%, before labor costs are factored in, we estimated that medical screenings would save the health care system $42.4 million, or $13.51 per person screened, over 1 year. With labor costs factored in, the health care system would save $5.1 million, or $1.61 per person screened, over 1 year.
DISCUSSION
We used the existing literature on medication adherence, chronic disease prevalence, and behavior health to generate a unique and transparent cost–benefit model to calculate the short-term health care cost savings gained from performing medical screenings in a dental setting. We found that medical screenings for diabetes, hypercholesterolemia, and hypertension in a dental office can reduce health care costs, particularly if patients who screen positive complete the dentist’s referral to the physician and adhere to the physician-prescribed medication regimen. The calculated savings range from $102.6 million ($32.72 per person screened) to $42.4 million ($13.51 per person screened) before factoring in labor costs. Once labor costs are factored in, there are net savings when the general practice dentist and dental assistant collaborate to screen the patient and report the findings back to the patient. Using our undiagnosed prevalence rates, we found that a 62% completion referral rate was the lower bound at which there are short-term savings associated with medication treatment. Given that the screening examinations for diabetes, hypercholesterolemia, and hypertension are easy to perform, we believe that dental hygienists and assistants would be able to conduct these exams and could easily be trained to do so. The calculated savings represent a small slice of the total potential savings to the health care system, specifically the short-term health care savings associated with medication use among individuals with the chronic diseases of interest.
Our estimated model savings are dependent on a number of assumptions based on estimated undiagnosed chronic disease prevalence,29 the referral completion rates based on the literature,32,40–43 and our assumed false-negative rates for the screening examinations.30,31 From 1999 to 2010, the percentage of those aged 45 years and older with multiple chronic diseases, such as hypertension and diabetes, increased.44 On the basis of these data, one can assume that the prevalence of multiple undiagnosed conditions also increased during that time, particularly the prevalence of diabetes along with comorbid chronic diseases. Accordingly, we would anticipate higher estimated cost savings with our model. The estimated savings in our model are highly dependent on capturing people with diabetes or multiple chronic conditions comorbid with diabetes. People with diabetes who complete their referral, start medication therapy, and adhere to their medication will achieve more cost savings than people with single-condition hypercholesterolemia or hypertension. The health care cost savings from adhering to diabetes medication over 1 year is greater than the savings generated from remaining adherent to medication for hypercholesterolemia or hypertension. Currently, according to guidelines from the American Diabetes Association (ADA), people with an A1C level of 6.5% or higher have diabetes.45 If one estimates undiagnosed prevalence rates for the single and multiple chronic conditions measured in our model using the diagnostic criteria for diabetes outlined by the ADA, there would be no cost savings at the estimated referral completion rates. In our model, we used the diagnostic criteria for diabetes defined by the CDC,24 which assumes that individuals with a glucose level above 126 milligrams per deciliter have diabetes. In fact, it has been shown that the prevalence of undiagnosed diabetes in adults aged 45 years and older is greater when the glucose diagnostic criteria is used than when the A1C diagnostic definition is used.46 Higher referral completion rates and lower false-negative rates for the screening exams lead to higher health care savings.
Adding strength to these estimated savings are data showing that medication therapy can lead to better health care outcomes. Drug trials of hypertension medication have shown that a 10-millimeters of mercury drop in systolic blood pressure is associated with a 32% reduction in stroke risk and a 14% reduction in ischemic heart disease risk. A 1-millimole per liter reduction in total cholesterol by statins has been associated with a 21% relative risk reduction in ischemic heart disease and a 17% reduction in the risk of stroke.47 Type 2 diabetes medication reduced hemoglobin A1C levels by 1% to 2% on average.48 A reduction of 1% in A1C levels in individuals with type 2 diabetes was associated with a 21% reduction in the risk of death and a 14% risk reduction for myocardial infarction.49
In addition to economic advantages, there are other benefits to identifying individuals with chronic conditions or increased risk of developing these conditions in the dental setting. There is evidence of a bidirectional relationship between diabetes and periodontal disease.50,51 Oral fungal infections have also been associated with diabetes.52 Hence, identifying patients with diabetes or prediabetes could help oral health professionals to better treat their patients. The American Heart Association recently documented the association between periodontal disease and atherosclerosis,53 which rationalizes the need for oral health professionals to screen for hypercholesterolemia and hypertension; however, no causative relationship to date has been established between heart disease and periodontal health. These associations between oral health and overall health lend support to the argument that oral health professionals can perform a valuable public health function by screening for diabetes, hypercholesterolemia, and hypertension.
Although we did not explicitly determine the cost savings that could occur through prevention and screening over a longer time span, there are likely to be added cost and health benefits that could accrue from oral health care professionals engaging in medical screenings for early identification of individuals at increased risk of developing disease. The ADA and the American Heart Association have promoted lifestyle changes through diet and exercise in people with diabetes, moderate to high levels of cholesterol, or prehypertension.54,55 Oral health professionals could play a bigger role in helping patients achieve better health and prevent the onset of chronic disease.
The dental community could serve as a beneficial resource for helping individuals unaware of their disease status to engage with the primary health care system. To ensure that chairside medical screening in a dental setting is a cost-beneficial strategy that also improves patients’ outcomes, attention to developing formal referral mechanisms between the dentist and the physician and identifying optimum approaches to ensuring referral completion is warranted. The health care savings that we captured in our model are realized within 1 year. Our model did not include potential savings associated with disease prevention or disease control, which are likely to be much larger. These chronic diseases of interest develop over a long period, such that cost savings associated with preventing disease onset would most likely be seen over a longer time span. We also did not look at the role dentists can play in monitoring disease control among patients who have been diagnosed and may already be on medication treatment. Some researchers claim that greater coordination and synergy among health care providers could enhance medication adherence,56 suggesting that additional contact between patients and dentists may encourage more desirable outcomes and in turn translate into additional health care cost savings and better health.
Acknowledgments
We thank Helen Ristic for providing assistance in the review of the literature and Shuying Jiang for collection of NHANES data.
Human Participant Protection
Institutional review board approval was not required because no human participants were involved in the study.
References
- 1.Centers for Disease Control and Prevention. Chronic diseases. The power to prevent, the call to control: at a glance 2009. December 17, 2009. Available at: http://www.cdc.gov/chronicdisease/resources/publications/aag/chronic.htm. Accessed May 9, 2013. [Google Scholar]
- 2.Witers D, Agrawal S. Unhealthy US workers’ absenteeism costs $153 billion. Gallup. October 17, 2011. Available at: http://www.gallup.com/poll/150026/unhealthy-workers-absenteeism-costs-153-billion.aspx. Accessed May 13, 2013. [Google Scholar]
- 3.Vujicic M, Nasseh K, Wall T. Dental care utilization declined for adults, increased for children during the past decade in the United States. Health Policy Resources Center Research Brief. American Dental Association. February 2013. Available at: http://www.ada.org/sections/professionalResources/pdfs/HPRCBrief_0213_2.pdf. Accessed May 29, 2013. [Google Scholar]
- 4.Glick M, Greenberg BL. The potential role of dentists in identifying patients’ risk of experiencing coronary heart disease events. J Am Dent Assoc. 2005;136(11):1541–1546. doi: 10.14219/jada.archive.2005.0084. [DOI] [PubMed] [Google Scholar]
- 5.Pollack HA, Metsch LR, Abel S. Dental examinations as an untapped opportunity to provide HIV testing for high-risk individuals. Am J Public Health. 2010;100(1):88–89. doi: 10.2105/AJPH.2008.157230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbey LM, Kenner LH. A resurvey of hypertensive patients detected in a dental office screening program. J Public Health Dent. 1976;36(4):244–249. doi: 10.1111/j.1752-7325.1976.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 7.Berman CL, Guarino MA, Giovannoli SM. High blood pressure detection by dentists. J Am Dent Assoc. 1973;87(2):359–363. doi: 10.14219/jada.archive.1973.0392. [DOI] [PubMed] [Google Scholar]
- 8.Glick M. Screening for traditional risk factors for cardiovascular disease: a review for oral health care providers. J Am Dent Assoc. 2002;133(3):291–300. doi: 10.14219/jada.archive.2002.0168. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg BL, Glick M, Goodchild J, Duda PW, Conte NR, Conte M. Screening for cardiovascular risk factors in a dental setting. J Am Dent Assoc. 2007;138(6):798–804. doi: 10.14219/jada.archive.2007.0268. [DOI] [PubMed] [Google Scholar]
- 10.Douglass CW, Shanmugham JR. Primary care, the dental profession, and the prevalence of chronic diseases in the United States. Dent Clin North Am. 2012;56(4):699–730. doi: 10.1016/j.cden.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg BL, Glick M, Frantsve-Hawley J, Kantor ML. Dentists’ attitudes toward chairside screening for medical conditions. J Am Dent Assoc. 2010;141(1):52–62. doi: 10.14219/jada.archive.2010.0021. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg BL, Kantor ML, Jiang SS, Glick M. Patients’ attitudes toward screening for medical conditions in a dental setting. J Public Health Dent. 2012;72(1):28–35. doi: 10.1111/j.1752-7325.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- 13.Engström S, Berne C, Gahnberg L, Svärdsudd K. Efficacy of screening for high blood pressure in dental health care. BMC Public Health. 2011;11:194. doi: 10.1186/1471-2458-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández-Feijoo J, Núñez-Orjales JL, Limeres-Posse J, Pérez-Serrano E, Tomás-Carmona I. Screening for hypertension in a primary care dental clinic. Med Oral Patol Oral Cir Bucal. 2010;15(3):e467–e472. doi: 10.4317/medoral.15.e467. [DOI] [PubMed] [Google Scholar]
- 15.Shetty S, Kohad R, Yeltiwar R, Shetty K. Gingival blood glucose estimation with reagent test strips: a method to detect diabetes in a periodontal population. J Periodontol. 2011;82(11):1548–1555. doi: 10.1902/jop.2011.110009. [DOI] [PubMed] [Google Scholar]
- 16.Hunt LM, Kreiner M, Brody H. The changing face of chronic illness management in primary care: a qualitative study of underlying influences and unintended outcomes. Ann Fam Med. 2012;10(5):452–460. doi: 10.1370/afm.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frishman WH. Importance of medication adherence in cardiovascular disease and the value of once-daily treatment regimens. Cardiol Rev. 2007;15(5):257–263. doi: 10.1097/CRD.0b013e3180cabbe7. [DOI] [PubMed] [Google Scholar]
- 18.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 19.Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood) 2011;30(1):91–99. doi: 10.1377/hlthaff.2009.1087. [DOI] [PubMed] [Google Scholar]
- 20.Stuart BC, Simoni-Wastila L, Zhao L, Lloyd JT, Doshi JA. Increased persistency in medication use by US Medicare beneficiaries with diabetes is associated with lower hospitalization rates and cost savings. Diabetes Care. 2009;32(4):647–649. doi: 10.2337/dc08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott MM, Schmitt B, Wallner E. Impact of medication nonadherence on coronary heart disease outcomes: a critical review. Arch Intern Med. 1997;157(17):1921–1929. [PubMed] [Google Scholar]
- 22.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: A meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 23.US Census Bureau, Population Division. Table 1: annual estimates of the population for the United States, regions, states, and Puerto Rico: April 1, 2010 to July 1, 2011. Available at: http://www.census.gov/popest/data/state/totals/2011/tables/NST-EST2011-01.xls. Accessed April 29, 2013. [Google Scholar]
- 24.Fryar CD, Hirsch R, Eberhardt MS, Yoon SS, Wright JD. Hypertension, High Serum Total Cholesterol, and Diabetes: Racial and Ethnic Prevalence Differences in US Adults, 1999–2006. Hyattsville, MD: National Center for Health Statistics; 2010. NCHS Data Brief no. 36. [PubMed] [Google Scholar]
- 25.Mayo Clinic Staff. High blood pressure (hypertension). Tests and diagnosis. Mayo Clinic. 2012 Available at: http://www.mayoclinic.com/health/high-blood-pressure/DS00100/DSECTION=tests-and-diagnosis. Accessed June 4, 2013. [Google Scholar]
- 26.Mayo Clinic Staff. Cholesterol levels: what numbers should you aim for? Mayo Clinic. 2012 Available at: http://www.mayoclinic.com/health/cholesterol-levels/CL00001. Accessed June 4, 2013. [Google Scholar]
- 27.Roger VL, Go AS, Lloyd-Jones DM et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan DM, Kuenen J, Borg R et al. Translating the A1c assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Health and Nutrition Examination Survey. Questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention. 2012 Available at: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed June 4, 2013. [Google Scholar]
- 30.Speechley M, McNair S, Leffley A, Bass M. Identifying patients with hypercholesterolemia. Can Fam Physician. 1995;41:240–245. [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman-Gerlicz C, Lilly I. Misclassification rates in hypertension diagnosis due to measurement errors. Society for Industrial and Applied Mathematics. 2009 Available at: http://www.siam.org/students/siuro/vol2issue2/S01031.pdf. Accessed April 30, 2013. [Google Scholar]
- 32.Jontell M, Glick M. Oral health care professionals’ identification of cardiovascular disease risk among patients in private dental offices in Sweden. J Am Dent Assoc. 2009;140(11):1385–1391. doi: 10.14219/jada.archive.2009.0075. [DOI] [PubMed] [Google Scholar]
- 33.Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood) 2011;30(1):91–99. doi: 10.1377/hlthaff.2009.1087. Appendix. Available at: http://content.healthaffairs.org/content/suppl/2011/01/05/30.1.91.DC1/2009-1087_Roebuck_Appendix.pdf. Accessed May 1, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 35.Peterson AM, Nau DP, Cramer JA, Benner J, Gwardry-Srdihar F, Nichol F. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 36.Andrade S, Kahler K, Frech F, Chan K. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 37.Agency for Healthcare Research and Quality. Using appropriate price indices for analyses of healthcare expenditures or income across multiple years. 2013 Available at: http://meps.ahrq.gov/mepsweb/about_meps/Price_Index.shtml. Accessed April 30, 2013. [Google Scholar]
- 38.Bureau of Economic Analysis. National income and product accounts tables. Section 1: domestic product and income, Table 1.1.4. Price indexes for gross domestic products. 2013 Available at: http://www.bea.gov/iTable/iTable.cfm?ReqID=9&step=1#reqid=9&step=3&isuri=1&903=4. Accessed April 30, 2013. [Google Scholar]
- 39.2012 Survey of Dental Practice. Data Year 2011. Chicago, IL: American Dental Association; 2011. [Google Scholar]
- 40.Forrest CB, Shadmi E, Nutting PA, Starfield B. Specialty referral completion among primary care patients: results from the ASPN Referral Study. Ann Fam Med. 2007;5(4):361–367. doi: 10.1370/afm.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrd JC, Moskowitz MA. Outpatient consultation: interaction between the general internist and the specialist. J Gen Intern Med. 1987;2(2):93–98. doi: 10.1007/BF02596304. [DOI] [PubMed] [Google Scholar]
- 42.Bourguet C, Gilchrist V, McCord G. The consultation and referral process. A report from NEON. Northeastern Ohio Network Research Group. J Fam Pract. 1998;46(1):47–53. [PubMed] [Google Scholar]
- 43.Hacker KA, Weintraub TA, Fried LE, Ashba J. Role of school-based health centers in referral completion. J Adolesc Health. 1997;21(5):328–334. doi: 10.1016/S1054-139X(97)00045-1. [DOI] [PubMed] [Google Scholar]
- 44.Freid VM, Bernstein AB, Bush MA. Multiple Chronic Conditions Among Adults Aged 45 and Over: Trends Over the Past 10 Years. Hyattsville, MD: National Center for Health Statistics; 2012. NCHS Data Brief no. 100. [PubMed] [Google Scholar]
- 45.American Diabetes Association. Diabetes basics. Diagnosing diabetes and learning about prediabetes. 2013 Available at: http://www.diabetes.org/diabetes-basics/diagnosis. Accessed August 12, 2013. [Google Scholar]
- 46.Centers for Disease Control and Prevention. 2011 National Diabetes Fact Sheet, Table 2. March 15, 2013. Available at: http://www.cdc.gov/diabetes/pubs/factsheet11/tables1_2.htm. Accessed August 12, 2013. [Google Scholar]
- 47.Rodgers A, Lawes CMM, Gaziano T, Vos T. The growing burden of risk from high blood pressure, cholesterol, and bodyweight. In: Jamison DT, Breman JG, Measham AR, editors. Disease Control Priorities in Developing Countries. 2nd ed. Washington, DC: World Bank; 2006. chap 45. [PubMed] [Google Scholar]
- 48.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287(3):360–372. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 49.Stratton IM, Adler AI, Neil HA et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control, and complications. Oral Dis. 2008;14(3):191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 51.Simpson TC, Needleman I, Wild SH, Moles DR, Mills EJ. Treatment of periodontal disease for glycemic control in people with diabetes. Cochrane Database Syst Rev. 2010;(5):CD004714. doi: 10.1002/14651858.CD004714.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Lamster IB, Eaves K. A model for dental practice in the 21st century. Am J Public Health. 2011;101(10):1825–1830. doi: 10.2105/AJPH.2011.300234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lockhart PB, Bolger AF, Papapanou PN et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 2012;125(20):2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 54.American Diabetes Association. Diabetes basics. Prevention. 2013 Available at: http://www.diabetes.org/diabetes-basics/prevention. Accessed August 12, 2013. [Google Scholar]
- 55.American Heart Association Nutrition Committee. Lichtenstein AH, Appel LJ et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 56.Marcum ZA, Sevick MA, Handler SM. Medication nonadherence: a diagnosable and treatable medical condition. JAMA. 2013;309(20):2105–2106. doi: 10.1001/jama.2013.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]