Abstract
Objectives. This study investigated if the type of drinking water source (treated municipal, untreated municipal, and private well water) modifies the effect of hydrology on childhood (aged < 5 years) gastrointestinal illness.
Methods. We conducted a time series study to assess the relationship between hydrologic and weather conditions with childhood gastrointestinal illness from 1991 to 2010. The Central and Northern Wisconsin study area includes households using all 3 types of drinking water systems. Separate time series models were created for each system and half-year period (winter/spring, summer/fall).
Results. More precipitation (summer/fall) systematically increased childhood gastrointestinal illness in municipalities accessing untreated water. The relative risk of contracting gastrointestinal illness was 1.4 in weeks with 3 centimeters of precipitation and 2.4 in very wet weeks with 12 centimeters of precipitation. By contrast, gastrointestinal illness in private well and treated municipal areas was not influenced by hydrologic conditions, although warmer winter temperatures slightly increased incidence.
Conclusions. Our study suggests that improved drinking water protection, treatment, and delivery infrastructure may improve public health by specifically identifying municipal water systems lacking water treatment that may transmit waterborne disease.
Contaminated drinking water is responsible for a widespread disease burden in developed countries.1 Exposure analysis and field studies in the United States estimate gastrointestinal illness (GI) attributable to drinking water in the range of 2 to 19 million cases per year.2,3 Measuring this disease burden is difficult because GI produces a broad spectrum of symptoms, specific tests are rarely conducted to determine etiology, and most infected people do not seek medical treatment. Compared with the general population, children are most commonly infected with enteric pathogens and may suffer more severe health consequences.
In the United States, regulations and treatment practices differ by drinking water source (surface, ground) and by water system type (public municipal, private well). The federal Safe Drinking Water Act and Groundwater Rule mandate municipal ground and surface water monitoring and surface water treatment. Community municipal water systems without water treatment tend to have higher rates of waterborne disease.4,5 Water treatment refers to multiple methods (coagulation, flocculation, sedimentation, filtration, primary and secondary disinfection) that may be combined to remove pathogens.6,7 Treatment sanitizes, filters, or inactivates most pathogens. However, pathogens that survive treatment or infiltrate finished water distribution systems cause a sizeable GI burden.3,8,9 There are no federal regulations for private well water quality. Some states require water quality testing when houses are being bought or sold.
Roughly 104 million people in the United States rely on groundwater.10 Groundwater accounted for 61% of documented US drinking water–borne disease outbreaks during 2007 and 2008.11 There is an increasing recognition that groundwater can be contaminated by human viruses.12 Soil layers incompletely “filter” miniscule (25–90 nm) viruses. The waterborne disease burden may be widespread but is difficult to identify with existing public health surveillance systems.3
Enhanced knowledge of processes that degrade drinking water quality may augment surveillance activities. Hydrologic events may transport pathogens to drinking water sources or overwhelm sewage or drinking water infrastructure. Most documented North American waterborne disease outbreaks are preceded by extreme rainfall events.13,14 In fact, communities that are primarily served by minimally treated drinking water may record elevated GI incidence during these hydrologic events.15,16 Snowmelt or extreme precipitation may cause combined sewer overflow events, which also increase GI rates.17 Assuming drinking water is the primary exposure route to waterborne pathogens, GI rates are likely insensitive to hydrologic changes when drinking water infrastructure and treatments function properly. Without treatment, precipitation in the spring/summer/fall and snowmelt in the winter/spring may flush a larger pollutant and pathogen load into drinking water systems and increase GI rates.
A temporal lag between water contamination and seeking medical attention for GI is related to environmental transport, the disease system, and health-seeking behaviors. Environmental transport is influenced by hydrology, season of the year, and geographic context (urban vs rural). Depending on the hydrology and soil, precipitation events flush pathogens into groundwater over the course of a day to weeks.18 The disease system includes variable pathogen incubation times, and virulence may produce clinical manifestations in hours to week(s) after infection.19 Finally, health-seeking behaviors influence the lag between hydrologic events and cases. In urban Milwaukee, Wisconsin, families typically waited 2 days after experiencing symptoms to visit a clinic.20 Living farther away from a health care facility and inclement weather may also delay seeking health care.21,22 Most previous studies have focused on urban environments and few studies have examined how these factors relate in a more rural context.
The study investigates if the type of drinking water source (treated municipal, untreated municipal, and private well water) modifies the effect of hydrology on childhood (aged < 5 years) GI health care visitations in Central and Northern Wisconsin. This study is a novel application of established geographic and temporal techniques to identify drinking water that may require better protection, treatment, or delivery.
METHODS
The Marshfield Epidemiologic Study Area (MESA) is a geographically defined region encompassing 24 zip codes in Central and Northern Wisconsin. The primary land uses are beef, milk cattle, and agriculture production (e.g., corn).23 MESA clinics and hospitals provide health care services to 97% of this area’s 90 000 residents including 4800 children.24 More than 90% of outpatient visits and 95% of inpatient stays are captured by the electronic medical record system. The system records all patient encounters and demographic information such as age, gender, type of health insurance, and household address.
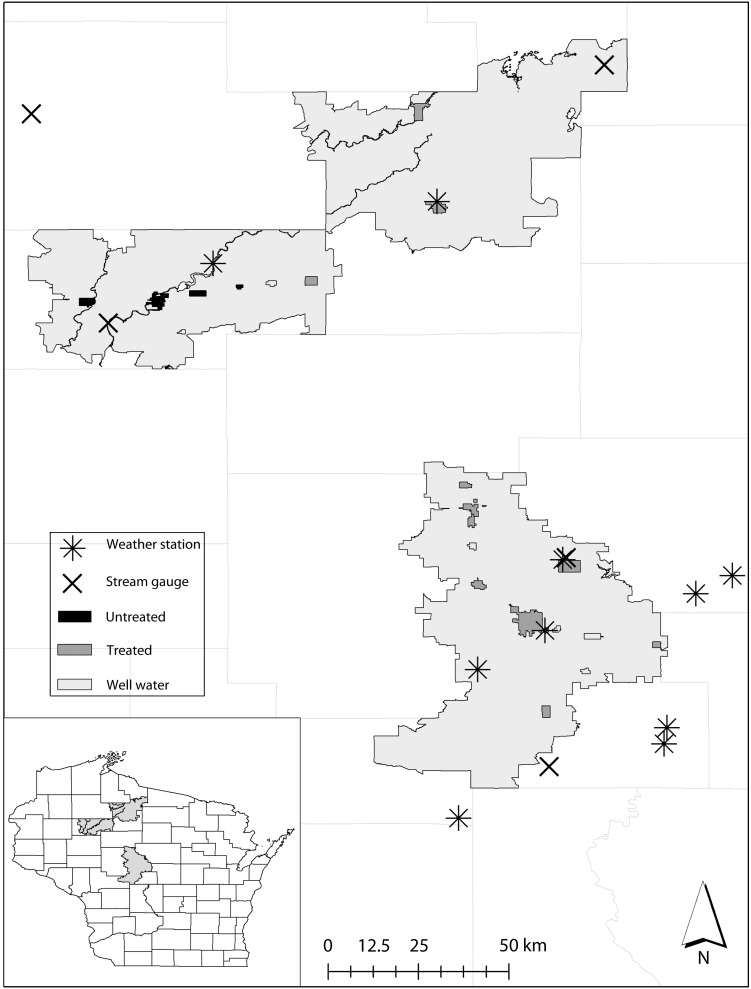
The present study analyzed health care visitations (clinic, emergency department, and hospitalization) from December 1, 1991, through December 31, 2010. The MESA population-based cohort monitors the population at risk by continuously tracking migration, births, and deaths. Figure 1 maps the zip codes that delineate MESA, drinking water source of cities and villages, and locations of weather and hydrology stations. The US Census (2000) estimated the proportion of children living in the MESA served by municipal treated water (39%), private well water (54%), and untreated municipal water (6%).25 In the study area, chlorine is the only form of municipal treatment except for the City of Marshfield, which has a more comprehensive treatment plant. The untreated municipalities do not use chlorination or other forms of treatment.
FIGURE 1—
The Marshfield Epidemiologic Study Area in Central and Northern Wisconsin: 1991–2010.
Note. The study area services households accessing municipal treated, municipal untreated, and private well water. The map identifies the locations of hydrology and weather stations used in the analysis.
The International Classification of Diseases, Ninth Revision (ICD-9) categorized MESA health care visits into billing codes.26 Waterborne diseases can be attributed to a specific disease-causing agent or classified according to common symptoms. We considered a relatively broad definition because GI is often not ascribed to a specific pathogen: specified gastrointestinal infections (ICD-9 codes 001–009.9; excluding 003.2, 005.0–005.3), unspecified gastroenteritis (558.9), and diarrhea (787.91).27,28 For comparability with previous studies, we included vibrio cases (005.4, 005.81) and nonspecific food poisoning, which may plausibly be waterborne. We excluded other symptoms involving the digestive system and dehydration to increase the specificity of the case definition. The inclusion of certain waterborne helminthes may be justified. However, helminthes constituted a small percentage of the total cases (0.5%), and their inclusion was unlikely to change the study results. Cases were identified from the GI billing code in the first-second position for outpatient visits and hospital discharge diagnosis. The study is restricted to children (aged < 5 years) and descriptive statistics summarize GI by age categories.
If a patient had more than 1 GI visitation in a 30-day period, we conservatively counted only the first encounter, similar to an earlier MESA study relating GI to suspected environmental sources.29 Patients who filled or were prescribed an antibiotic prescription in the 7 days preceding health care visits were excluded. The case definition did not exclude patients with chronic gastrointestinal conditions (e.g., irritable bowel syndrome or inflammatory bowel disease). Quality assurance validated the sample cases and the coding accuracy was 95%. GI data were aggregated at the level of week and reported as weekly cumulative incidence. Funding limitations prevented us from extracting all cases in MESA. We randomly extracted 50% of the first visits for childhood GI stratified by year, which was representative of the GI disease burden over time. This translated into a total of 4951 cases. Therefore sampled GI incidence rates reported here are underestimated, at least, by a factor of 2. The study results and discussion will distinguish between the sampled or population estimated GI counts.
Drinking Water Source, Hydrology, and Weather Information
The most likely household drinking water source (treated municipal, untreated municipal, or private well) was inferred from geographic relationships. Household addresses were assigned latitudes and longitudes (geocoded) using ArcGIS versions 10.0 and 9.3 (ESRI, Redlands, CA), 2010 TIGER US Census data, and county government street data. The address matching accuracy was 95.7% (85% sensitivity or higher). A municipality’s drinking water source was assigned to households located within its boundaries.30 The Wisconsin Department of Natural Resources and water managers provided information on municipal water treatment practices.31 Households situated in unincorporated areas or townships (political subdivision) were presumed to access private well water.
Table 1 outlines the sources of hydrology and weather information and how daily information was aggregated to weekly periods.32,33 We generated representative environmental conditions for the regions served by each drinking water source. Municipal treated and private well conditions were recorded by monitoring stations located within 25 kilometers of the study area. The locations of municipalities accessing untreated water are clustered in the northwestern portion of the MESA. One weather station and 2 stream gauges provided hydrology and weather conditions for this water source.
TABLE 1—
Environmental Independent Variables That May Influence the Timing and Magnitude of Childhood Gastrointestinal Illness Incidence: Marshfield Epidemiologic Study Area, Central and Northern Wisconsin, 1991–2010
| Independent Variable | Aggregation Period (Weekly) | Source | No. Stations |
| Precipitation, cm | Sum | GHCN, USGS | 15 |
| Stream discharge change, % | Change (week0 – week-1)a | USGS | 3 |
| Average temperature, °C | Average | GHCN | 8 |
| Precipitation (cm) × average temperature (°C) | Not applicable | GHCN | 8 |
Note. GHCN = Global Historical Climatology Network (US National Climatic Data Center); USGS = US Geologic Survey.
Week0 refers to the current week and week-1 to the previous week.
Stream discharge reflects water from natural precipitation and anthropogenic sources like irrigation. We briefly describe how the stream discharge change metric was created. First, we converted each station’s weekly stream discharge into percentiles, which we calculated over the entire study period. Second, we averaged stream discharge percentiles for each drinking water source. Discharge seasonally increases in the spring and decreases throughout the fall. Finally, we calculated stream discharge change by subtracting the current from the previous week’s average percentiles, which removed seasonality.
In the winter/spring, hydrologic conditions were represented by precipitation during nonfreezing periods. Snowfall is unlikely to lead to groundwater contamination if the ground is frozen. Nonfreezing temperatures were designated by an indicator variable of average weekly temperature above freezing (0°C). The interaction between precipitation and the nonfreezing-indicator variable captured precipitation that was more likely to percolate into groundwater.
Statistical Analysis
We used generalized additive models (GAMs) to evaluate the association between hydrologic conditions and weather to childhood GI incidence, taking care to control for repeated measurements over time.34 Childhood GI incidence was estimated from the subset of health care records. GAMs expand upon linear regression models by considering nonlinear environment and GI relationships. A linear relationship is characterized by a proportional risk factor and GI association such as a doubling of precipitation correlated with a 75% GI increase. In a nonlinear relationship, the risk factor and GI-association changes based on the level of the risk factor. The relationship is graphically summarized by a smooth relative-risk function of the number of fitted cases at different levels of the independent variable.
In treated municipal or private well-water areas, GAMs (Poisson family) associate weather and hydrology against weekly GI counts. The smaller number of untreated municipal water cases required a more flexible quasipoisson GAM to account for overdispersion. We used a variable screening procedure to identify the best-fitting hydrologic/temperature metric and temporal lag using Akaike Information Criterion or the Un-Biased Risk Estimator. Each metric was regressed against GI cases as we controlled for seasonality and secular trends. Temporal lags of 0 to 3 weeks for hydrologic conditions and 0 to 1 week for temperature were considered. Next, we compared 3 time series models: (1) the best fitting hydrologic model, (2) temperature model, and 3) the combination of hydrology and temperature (Equation 1). Weather patterns jointly influence temperature and hydrology. Including temperature in the analysis may control for potential seasonal confounding.35
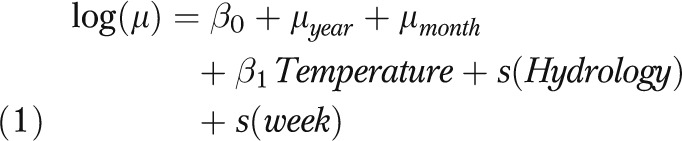 |
In Equation 1, μ is the expectation of weekly childhood GI counts. The terms μyear, μmonth are factors that adjust for secular trends and monthly variability. The screening process determines the best fitting temperature and hydrology metric and lag. The notation s(x) is the nonlinear smoothing terms for hydrologic conditions and time (study week) using penalized cubic regression splines.36 GAM results are generally robust to the choice of smoothing spline.37 The spline degrees of freedom were determined by knowledge of the processes and range of conditions: precipitation (1 df; 2.54 cm), stream discharge percentiles (1df; 10%), and sampling week (3 df; 6 months). The degrees of freedom balanced controlling autocorrelation versus overfitting.
Regression assumptions of independent and identically distributed residuals and homoscedasticity were verified by autocorrelation and partial autocorrelation functions and a McLeod-Li test of the residuals.38 The analysis was conducted in R (version 2.15.1) using the MGCV package.34 We report the best-fitting model stratified by season and drinking water source.
RESULTS
Most cases were identified during clinic and outpatient visits (78%). Seven percent of the cases were admitted to the hospital directly or through the emergency department. For these cases, the median hospitalization was 2 days (SD = 10 days) with a range of 0 to 69 days. A sizeable number of patients only received care from the emergency department (14%). Younger children had higher rates of GI, with infants (< 1 year old) representing one third of the cases (34%) and patients aged 1 to younger than 2 years accounting for another third (31%). The remaining cases occurred in children aged 2 to younger than 3 years (17%), 3 to younger than 4 years (10%), and 4 to younger than 5 years (8%). There were slightly more cases among boys (55%) than among girls. The proportion of cases accessing treated water (48%; 2240 children) was elevated, but not significantly, over the proportion of the population living in these areas (39%). Correspondingly, children assigned to private wells constitute 54% of the population and 46% of the cases (2422 children). The proportion of cases from untreated municipal areas (6%, 289 cases) was equivalent to the proportion of the study population living in these areas
The GI disease burden fell disproportionately on lower socioeconomic status (SES) households. About 7% of MESA residents access Medicaid, but these households accounted for half of the cases. This suggests that lower SES households have an elevated GI disease burden but the small sample size limits formal testing. The nontreated municipal water households had an even higher proportion of cases using Medicaid (58%). After the initial visit, many cases (∼24%) returned to the clinic for a GI-related visit within a month.
The population annual GI incidence, estimated from the study sample, was 1076 per 10 000 children (SD of mean annual incidence = 110 per 10 000 children) from 1992 to 2006. Although not directly comparable, a national private insurance GI rate was 1561 per 10 000 children.39 MESA GI rates significantly (P = .02) decreased from 1992 to 2006 at a rate of 15.3 per year. The decline was further hastened to 865 per 10 000 children (SD of mean annual incidence = 131 per 10 000 children) per year from 2007 to 2010, which is the period when the second-generation rotavirus vaccines were introduced. This translates into a 20% decrease in childhood GI rates in 2007 to 2010 compared with 1992 to 2006.
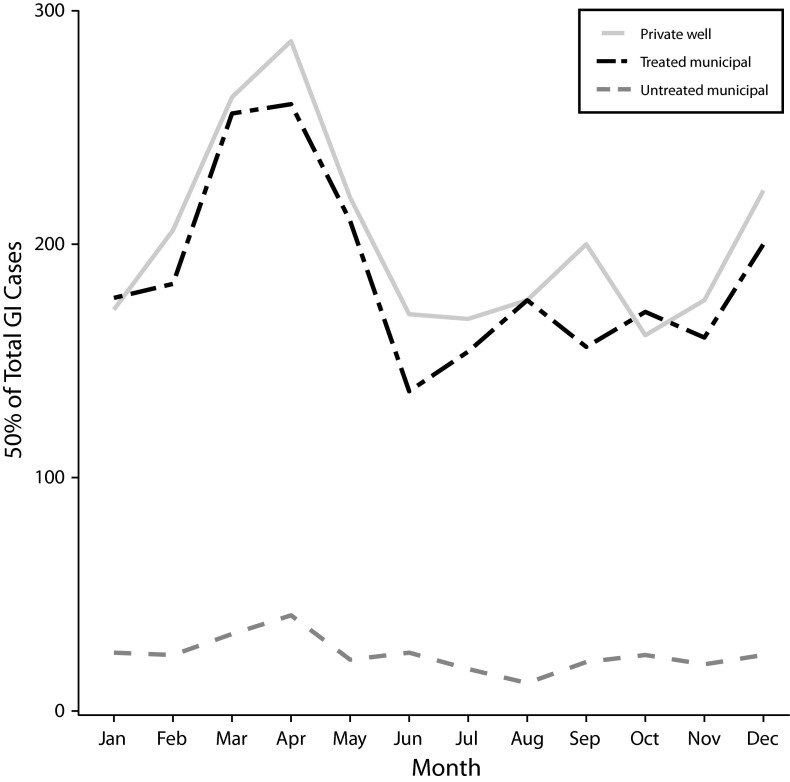
The plot of the number of GI cases (50% sample) per month over the entire study period exhibited clear seasonal patterns for private well and untreated municipal systems (Figure 2). Winter/spring GI cases peaked in April across all drinking water systems. The private well and the untreated municipal areas exhibited a smaller December peak in cases. In the same areas, GI cases reached a nadir during the summer and gradually increase throughout the fall.
FIGURE 2—
Total number of childhood gastrointestinal illness (GI) cases (50% sample) for each drinking water source by month: Marshfield Epidemiologic Study Area, Central and Northern Wisconsin, 1991–2010.
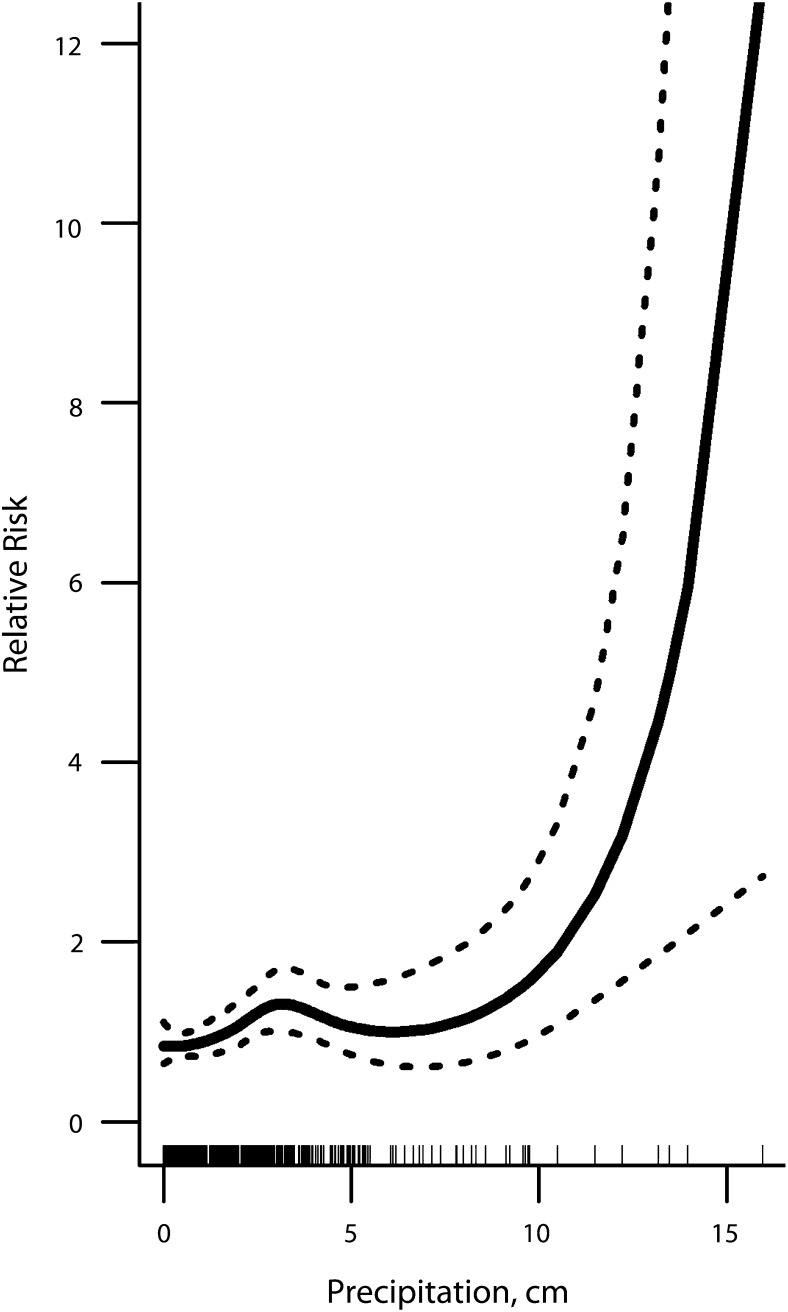
During the summer/fall, more precipitation and higher temperatures were associated with increased GI incidence in untreated municipal drinking water communities (adjusted R2 = 20%). We first discuss the relationship between precipitation (concurrent week) and fitted weekly cumulative GI incidence (P = .003; Figure 3). The solid curve (3.5 df) illustrates the GI relative risk for the range of observed precipitation conditions. The relative risk was adjusted for temperature, secular trends, seasonality, and temporal autocorrelation. The dashed lines are the 95% relative risk confidence intervals. The density of hash marks along the x-axis indicates the frequency of weeks with each precipitation amount. On average, the relative risk of contracting GI increased from 1 to 1.4 in weeks with 3.3 centimeters of precipitation compared with weeks without precipitation. Risk remained elevated with similarly high levels during moderate to wet weeks (3.4–9 cm). There were 14 very wet weeks with more than 9 centimeters of precipitation during the 19-year analysis period. During these periods, children were at least 2.4 times as likely to contract GI compared with weeks with no precipitation. In untreated municipal water communities, weekly temperatures (concurrent week) were also positively associated (B = 0.07; 95% CI = 0.02, 0.12) with GI cases. GI was not systematically related to hydrology or weather conditions in treated municipal or private well communities (data available as a supplement to the online version of this article at http://www.ajph.org).
FIGURE 3—
Association between childhood gastrointestinal illness and weekly summer/fall hydrologic conditions adjusted for seasonality and secular trends in untreated municipal areas: Marshfield Epidemiologic Study Area, Central and Northern Wisconsin, 1991–2010.
Note. Precipitation is positively and nonlinearly associated with gastrointestinal illness. The solid line represents the estimate spline curve, and the dashed lines represent the 95% confidence intervals. The density of hash marks along the x-axis indicates the frequency of weeks with each precipitation observation.
In the winter/spring, hydrology was unrelated to GI across all communities. Warmer winter temperatures (previous week) increased GI rates in treated water municipalities (B = 0.02; 95% CI = 0.01, 0.04) and private well areas (B = 0.01; 95% CI = 0.00, 0.03; supplemental data). Cold temperatures and snow- or ice-covered roads may decrease access to health care.22 Winter temperatures were not associated with GI relative risk in untreated municipalities (supplemental data).
DISCUSSION
In our study region, the link between hydrologic events and childhood GI was absent in those municipalities with treated drinking water. By contrast, GI in municipalities without drinking water treatment increased in weeks with more precipitation compared with dry weeks in the summer/fall. The relationship between hydrologic events and GI is likely to be place-specific. The study methodology can help identify other areas where water treatment or improving water delivery infrastructure may decrease waterborne disease.
Analogous studies provide evidence of endemic waterborne disease in developed nations. On average, rainy days recorded 11% more emergency department GI visits than nonrainy days in Milwaukee.15 Very high (> 90th percentile) water volumes (combined effect of snowmelt and rainfall) increased weekly GI clinic visits 30% compared with moderate or dry weeks in an Inuit community.40 In Northwestern England, wet conditions in the previous week (75th percentile) increased weekly cryptosporidiosis rates 27%.41 Similarly, the proportion of UK private wells above an Escherichia coli standard increased with the amount of precipitation.16
Hydrologic events likely transport pathogens into groundwater or the drinking water distribution system.42,43 Contaminated water is subsequently transported to households and ingested by children. This mechanism suggests that the entire population and not just children may contract disease from their drinking water. There are multiple potential health benefits of reducing drinking waterborne GI. Previous gastrointestinal infections may lead to long-term negative health consequences (e.g., irritable bowel syndrome, reactive arthritis) that accrue significant public health costs.44
Public water systems accessing groundwater face challenges across the United States. Small municipal groundwater systems deliver nondisinfected water to 20 million people.6 Improved drinking water monitoring and corrective actions in these communities would prevent about 32 000 GI cases per year.10 Most untreated systems serve small populations who may be reluctant to pay for water treatment and underestimate the risks of contaminated water.45 Water treatment is associated with a reduction in GI outbreaks, physician visits, self-reported illness, and water quality indicators.46–52 A study of severe waterborne disease suggests that Melbourne, Australia, may be an exception where treatment did not reduce GI cases.53
US public water systems that primarily access groundwater must conduct sanitary surveys and monitor source water.6 Monitoring is required for systems that do not adequately filter or inactivate viruses. Water quality sampling is more frequent in systems that serve larger numbers of people and rely on surface water.54 For example, small municipal groundwater systems (< 1000 people) may monitor as infrequently as 1 sample per 3 months. Each state designates corrective actions (e.g., eliminate contamination, treatment) when a sample fails a microbial test, and the range of actions available to state regulators can vary considerably. In Wisconsin, the state where we conducted our study, the legislature recently passed Wisconsin Act 19 (2011) explicitly preventing state-level regulations requiring municipal disinfection.55 The law perpetuates a 2-tiered municipal drinking water system where municipalities without treatment may continue to contract GI from drinking water.
Two short-term strategies for untreated municipal water systems may immediately improve public health during wet weeks. First, routine microbial groundwater surveillance should be targeted to these periods with the greatest enteric waterborne disease risk. Second, government agencies may consider issuing boiling water advisories as these would be particularly beneficial to vulnerable populations such as the immunocompromised, the elderly, pregnant mothers, and children. Long-term strategies include eliminating contamination sources or installing municipal or point-of-use water treatment.
In our study, hydrologic events did not directly increase GI in localities served by private wells. Private wells are often assumed more susceptible to waterborne pathogen contamination than municipal wells, but this is not necessarily true. Twenty-seven percent of public water supply wells in the United States are expected to be contaminated with human enteric viruses.56 In a recent study of 6 deep municipal wells supplying drinking water for Madison, Wisconsin, 100% were all virus positive.57 By contrast, Borchardt et al. performed quarterly testing of 50 private wells in Wisconsin and found only 8% were virus positive.58 The difference in contamination rates is believed to be related to the size of potential fecal-contamination sources. The zone of contribution for private well water is usually small, encompassing only several septic systems serving a handful of people. High-capacity municipal wells, on the other hand, have a very large zone of contribution that can include areas beneath a city’s sanitary sewer system, which leak to the subsurface and can contain fecal wastes from thousands of people.59
Importantly, the study design cannot infer waterborne disease caused by constant or nonhydrologic contamination sources. For example, a continuous leaking sanitary sewer or septic system may still cause GI in private well or treated municipal communities. The study suggests where improvements are helpful but does not provide enough evidence to prove that a drinking water system is properly functioning. It is unclear why hydrology was not associated with GI in untreated drinking water municipalities in the winter/spring. In previous studies, winter/spring GI is dominated by rotavirus, which may be more likely to be spread via direct transmission or fomites.
The population-based time series approach limits the strength of the study results. Potential waterborne cases were inferred from the proportion of GI that systematically coincided with hydrologic events.46 Time series analysis compares a population against itself and implicitly controls for individual-level confounders that change relatively slowly over time. The primary limitation is that statistics cannot distinguish between competing hydrologic pathways that may cause GI. For example, the risk of contracting GI from swimming in surface water may also increase during rainy weeks.60 The study results are also limited by the sample size of the untreated municipal cases. A small amount of drinking water source misclassification was created by not accounting for towns that are served by neighboring municipal-water systems.
Separate time series models were created for the 3 drinking water sources and half-yearly periods of winter/spring (December-May) and summer/fall (June-November). Stratifying by half-yearly periods accounted for potentially different mechanisms and temporal lags between hydrology and diagnosis of human cases. The most important GI pathogens, hydrologic processes, and human behaviors change over the course of the year. For example, childhood winter/spring GI cases are thought to be primarily caused by rotavirus and norovirus.61,62 Summer and fall GI cases are related to a broader suite of enteric pathogens such as enterovirus, cryptosporidium, and giardia.63–65 In the summer/fall, children may swim more frequently and potentially increase exposure to recreational waterborne pathogens. Families may also alter cooking behaviors (e.g., grilling outdoors) that promote foodborne infections like Salmonella.36 Freezing temperatures may limit bacteria survival or regrowth, although warmer-than-average temperatures increase reproduction rates.
This study provides further insights on how climate change interacts with existing societal vulnerabilities to exacerbate public health problems. From 1895 to 2006, Wisconsin mean annual precipitation increased by 5.58 centimeters (2.2 in).66 Plausible future precipitation changes are highly uncertain. Region-specific climate projections suggest that mean annual precipitation will modestly increase by less than 10% by 2041 to 2070.67 Precipitation may increase in the winter, spring, and fall but stay constant during the summer. Winter temperatures are also projected to increase. For untreated municipalities in the study region, climate change may increase precipitation and waterborne disease risk, particularly in the fall season.
Acknowledgments
This work was supported by the Centers for Disease Control and Prevention (grant 1U01EH000428-01), the National Center for Atmospheric Research/Centers for Disease Control and Prevention Fellowship Program, and the National PERISHIP Dissertation Fellowship funded by The National Science Foundation, University of Colorado Natural Hazards Center, Swiss Re, and the Public Entity Risk Institute.
We thank Sharon C. Long, Stephen Vavrus, Richard Keller, and Miles Kirby for providing comments on previous versions of the article. We thank anonymous reviewers whose comments improved the article. Finally, we recognize Brian Butterworth for geocoding household addresses.
Human Participant Protection
The study was reviewed and approved by the Marshfield Clinic Institutional Review Board (#873) on April 26, 2011.
References
- 1.Roy SL, Scallan E, Beach MJ. The rate of acute gastrointestinal illness in developed countries. J Water Health. 2006;4(suppl 2):31–69. doi: 10.2166/wh.2006.017. [DOI] [PubMed] [Google Scholar]
- 2.Messner M, Shaw S, Regli S, Rotert K, Blank V, Soller J. An approach for developing a national estimate of waterborne disease due to drinking water and a national estimate model application. J Water Health. 2006;4(suppl 2):201–240. doi: 10.2166/wh.2006.024. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds KA, Mena KD, Gerba CP. Risk of waterborne illness via drinking water in the United States. Rev Environ Contam Toxicol. 2008;192:117–158. doi: 10.1007/978-0-387-71724-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Environmental Protection Agency. National primary drinking water regulations: Ground Water Rule, final rule. Fed Regist. 2006;71(216):65574–65660. [Google Scholar]
- 5.Naumova EN, Chen JT, Griffiths JK, Matyas BT, Estes-Smargiassi SA, Morris RD. Use of passive surveillance data to study temporal and spatial variation in the incidence of giardiasis and cryptosporidiosis. Public Health Rep. 2000;115(5):436–447. doi: 10.1093/phr/115.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratby J. Coagulation and Flocculation in Water and Wastewater Treatment. 2nd ed. London, UK: International Water Assn; 2006. [Google Scholar]
- 7.Leiknes T. The effect of coupling coagulation and flocculation with membrane filtration in water treatment: A review. J Environ Sci (China) 2009;21(1):8–12. doi: 10.1016/s1001-0742(09)60003-6. [DOI] [PubMed] [Google Scholar]
- 8.Lambertini E, Borchardt MA, Kieke BA, Jr, Spencer SK, Loge FJ. Risk of viral acute gastrointestinal illness from nondisinfected drinking water distribution systems. Environ Sci Technol. 2012;46(17):9299–9307. doi: 10.1021/es3015925. [DOI] [PubMed] [Google Scholar]
- 9.Mann AG, Tam CC, Higgins CD, Rodrigues LC. The association between drinking water turbidity and gastrointestinal illness: a systematic review. BMC Public Health. 2007;7:256–262. doi: 10.1186/1471-2458-7-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Environmental Protection Agency. Economic Analysis for the Final Ground Water Rule. EPA-815-R-06-014. Washington, DC: US Environmental Protection Agency, Office of Water; 2006.
- 11.Brunkard JM, Ailes E, Roberts VA, et al. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2007–2008. MMWR Surveill Summ. 2011;60(12):38–68. [PubMed] [Google Scholar]
- 12.Fout GS, Martinson BC, Moyer MWN, Dahling DR. A multiplex reverse transcription-PCR method for detection of human enteric viruses in groundwater. Appl Environ Microbiol. 2003;69(6):3158–3164. doi: 10.1128/AEM.69.6.3158-3164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curriero FC, Patz JA, Rose JB, Lele S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948-1994. Am J Public Health. 2001;91(8):1194–1199. doi: 10.2105/ajph.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas KM, Charron DF, Waltner-Toews D, Schuster C, Maarouf AR, Holt JD. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975–2001. Int J Environ Health Res. 2006;16(3):167–180. doi: 10.1080/09603120600641326. [DOI] [PubMed] [Google Scholar]
- 15.Drayna P, McLellan SL, Simpson P, Li SH, Gorelick MH. Association between rainfall and pediatric emergency department visits for acute gastrointestinal illness. Environ Health Perspect. 2010;118(10):1439–1443. doi: 10.1289/ehp.0901671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson HY, Nichols G, Lane C, Lake IR, Hunter PR. Microbiological surveillance of private water supplies in England—the impact of environmental and climate factors on water quality. Water Res. 2009;43(8):2159–2168. doi: 10.1016/j.watres.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Redman RL, Nenn CA, Eastwood D, Gorelick MH. Pediatric emergency department visits for diarrheal illness increased after release of undertreated sewage. Pediatrics. 2007;120(6):e1472. doi: 10.1542/peds.2007-0283. [DOI] [PubMed] [Google Scholar]
- 18.Bradbury KR, Borchardt MA, Gotkowitz MB, Spencer SK. Human viruses as tracers of wastewater pathways into deep municipal wells. 2010 Open File Report 2010-04A. [Google Scholar]
- 19. Centers for Disease Control and Prevention. Compendium of acute foodborne and waterborne diseases. Available at: http://www.cdc.gov/eis/casestudies/xoswego.401.303.compendium.pdf. Accessed September 14, 2012.
- 20.Gorelick MH, McLellan SL, Wagner D, Klein J. Water use and acute diarrhoeal illness in children in a United States metropolitan area. Epidemiol Infect. 2011;139(2):295–301. doi: 10.1017/S0950268810000828. [DOI] [PubMed] [Google Scholar]
- 21.Winters CA, Cudney SA, Sullivan T, Thuesen A. The rural context and women’s self-management of chronic health conditions. Chronic Illn. 2006;2(4):273–289. doi: 10.1177/17423953060020040801. [DOI] [PubMed] [Google Scholar]
- 22.Wong ST, Regan S. Patient perspectives on primary health care in rural communities: effects of geography on access, continuity and efficiency. Rural Remote Health. 2009;9:1142–1153. [PubMed] [Google Scholar]
- 23. Census of agriculture [Internet]. Washington, D.C.: United States Department of Agriculture National Agricultural Statistical Service; 2007; cited May 25, 2013]. Available at: http://www.agcensus.usda.gov/index.php. Accessed February 9, 2012.
- 24.DeStefano F, Eaker ED, Broste SK, et al. Epidemiologic research in an integrated regional medical care system: The Marshfield Epidemiologic Study Area. J Clin Epidemiol. 1996;49(6):643–652. doi: 10.1016/0895-4356(96)00008-x. [DOI] [PubMed] [Google Scholar]
- 25. US Census Bureau. 2000. Decennial Census. Available at: http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed February 9, 2012.
- 26. International Classification of Diseases, Ninth Revision. Geneva, Switzerland: World Health Organization; 1975.
- 27.Gangarosa RE, Glass RI, Lew JF, Boring JR. Hospitalizations involving gastroenteritis in the United States, 1985: The special burden of the disease among the elderly. Am J Epidemiol. 1992;135(3):281–290. doi: 10.1093/oxfordjournals.aje.a116282. [DOI] [PubMed] [Google Scholar]
- 28.Hoxie NJ, Davis JP, Vergeront JM, Nashold RD, Blair KA. Cryptosporidiosis-associated mortality following a massive waterborne outbreak in Milwaukee, Wisconsin. Am J Public Health. 1997;87(12):2032–2035. doi: 10.2105/ajph.87.12.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borchardt MA, Chyou PH, DeVries EO, Belongia EA. Septic system density and infectious diarrhea in a defined population of children. Environ Health Perspect. 2003;111(5):742–748. doi: 10.1289/ehp.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. U.S. Census Bureau. 2000. 2012. Census 2000: Incorporated Places/Census Designated Places Cartographic Boundary Files. Available at: https://www.census.gov/geo/www/cob/pl2000.html. Accessed February 9, 2012.
- 31. Wisconsin Department of Natural Resources. 2012. Learn If Your Municipal Water System Disinfects Its Water. Available at: http://dnr.wi.gov/topic/DrinkingWater/documents/MunicipalDisinfectList.pdf. Accessed March 6, 2012.
- 32.Daily Documentation for GHCN (Global Historical Climatology Network) Asheville, NC: National Climatic Data Center; 2011. [Google Scholar]
- 33. United States Geological Survey. Water data for the nation. Available at: http://waterdata.usgs.gov/nwis. Updated 2012. Accessed September 4, 2011.
- 34. Wood SN. Generalized Additive Models: An Introduction With R. Vol 66. Boca Raton, FL: CRC Press; 2006.
- 35.Naumova EN, Jagai JS, Matyas B, DeMaria A, MacNeill I, Griffiths JK. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol Infect. 2007;135(2):281–292. doi: 10.1017/S0950268806006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovats RS, Edwards SJ, Hajat S, Armstrong BG, Ebi KL, Menne B. The effect of temperature on food poisoning: a time-series analysis of Salmonellosis in ten European countries. Epidemiol Infect. 2004;132(3):443–453. doi: 10.1017/s0950268804001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng RD, Dominici F, Louis TA. Model choice in time series studies of air pollution and mortality. J Roy Stat Soc Ser A Stat Soc. 2006;169(2):179–203. [Google Scholar]
- 38.McLeod AI, Li WK. Diagnostic checking ARMA time series models using squar—residual autocorrelations. J Time Ser Anal. 1983;4(4):269–273. [Google Scholar]
- 39.Cortes JE, Curns AT, Tate JE, Parashar UD. Trends in healthcare utilization for diarrhea and rotavirus disease in privately insured US children < 5 years of age, 2001-2006. Pediatr Infect Dis J. 2009;28(10):874–878. doi: 10.1097/INF.0b013e3181a653cd. [DOI] [PubMed] [Google Scholar]
- 40.Harper SL, Edge VL, Schuster-Wallace CJ, Berke O, McEwen SA. Weather, water quality and infectious gastrointestinal illness in two Inuit communities in Nunatsiavut, Canada: potential implications for climate change. EcoHealth. 2011;8(1):93–108. doi: 10.1007/s10393-011-0690-1. [DOI] [PubMed] [Google Scholar]
- 41.Naumova EN, Christodouleas J, Hunter PR, Syed Q. Effect of precipitation on seasonal variability in Cryptosporidiosis recorded by the North West England surveillance system in 1990–1999. J Water Health. 2005;3:185–196. [PubMed] [Google Scholar]
- 42.National Research Council. Drinking Water Distribution Systems Assessing and Reducing Risks. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 43.Borchardt MA, Spencer SK, Kieke BA, Lambertini E, Loge FJ. Viruses in nondisinfected drinking water from municipal wells and community incidence of acute gastrointestinal illness. Environ Health Perspect. 2012;120(9):1272–1279. doi: 10.1289/ehp.1104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moorin RE, Heyworth JS, Forbes GM, Riley TV. Long-term health risks for children and young adults after infective gastroenteritis. Emerg Infect Dis. 2010;16(9):1440–1447. doi: 10.3201/eid1609.081665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Severtson DJ, Baumann LC, Brown RL. Applying the common sense model to measure representations of arsenic contaminated well water. J Health Commun. 2008;13(6):538–554. doi: 10.1080/10810730802281627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craun GF, Calderon RL. Observational epidemiologic studies of endemic waterborne risks: cohort, case-control, time-series, and ecologic studies. J Water Health. 2006;4(suppl 2):101–119. doi: 10.2166/wh.2006.020. [DOI] [PubMed] [Google Scholar]
- 47.Kuusi M, Aavitsland P, Gondrosen B, Kapperud G. Incidence of gastroenteritis in Norway–a population-based survey. Epidemiol Infect. 2003;131(1):591–597. doi: 10.1017/s0950268803008744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teschke K, Bellack N, Shen H et al. Water and sewage systems, socio-demographics, and duration of residence associated with endemic intestinal infectious diseases: a cohort study. BMC Public Health. 2010;10:767–779. doi: 10.1186/1471-2458-10-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tulchinsky TH, Burla E, Clayman M, Sadik C, Brown A, Goldberger S. Safety of community drinking-water and outbreaks of waterborne enteric disease: Israel, 1976-97. Bull World Health Organ. 2000;78(12):1466–1473. [PMC free article] [PubMed] [Google Scholar]
- 50.Beaudeau P, Valdes D, Mouly D, Stempfelet M, Seux R. Natural and technical factors in faecal contamination incidents of drinking water in small distribution networks, France, 2003-2004: A geographical study. J Water Health. 2010;8(1):20–34. doi: 10.2166/wh.2009.043. [DOI] [PubMed] [Google Scholar]
- 51.Birkhead G, Vogt RL. Epidemiologic surveillance for endemic Giardia lamblia infection in Vermont. The roles of waterborne and person-to-person transmission. Am J Epidemiol. 1989;129(4):762–768. doi: 10.1093/oxfordjournals.aje.a115191. [DOI] [PubMed] [Google Scholar]
- 52.Fraser GG, Cooke KR. Endemic giardiasis and municipal water supply. Am J Public Health. 1991;81(6):760–762. doi: 10.2105/ajph.81.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellard ME, Sinclair MI, Dharmage SC, Bailey MJ, Fairley CK. The rate of gastroenteritis in a large city before and after chlorination. Int J Environ Health Res. 2002;12(4):355–360. doi: 10.1080/0960312021000056384. [DOI] [PubMed] [Google Scholar]
- 54. US Environmental Protection Agency. Drinking water; national primary drinking water regulations; Total Coliforms Final Rule. 1989;40 CFR Parts 141 and 142:27544–27568.
- 55. Wisconsin Legislature. Wisconsin Act 19. 2011. Available at: http://docs.legis.wisconsin.gov/2011/related/acts/19. Accessed November 15, 2011.
- 56. US Environmental Protection Agency. Occurrence and Monitoring Document for the Final Ground Water Rule. EPA-815-R-06–012. Washington, DC: US Environmental Protection Agency, Office of Water; 2006.
- 57.Bradbury KR, Borchardt MA, Gotkowitz M, Spencer SK, Zhu J, Hunt RJ. Source and transport of human enteric viruses in deep municipal water supply wells. Environ Sci Technol. 2013;47(9):4096–4103. doi: 10.1021/es400509b. [DOI] [PubMed] [Google Scholar]
- 58.Borchardt MA, Bertz PD, Spencer SK, Battigelli DA. Incidence of enteric viruses in groundwater from household wells in Wisconsin. Appl Environ Microbiol. 2003;69(2):1172–1180. doi: 10.1128/AEM.69.2.1172-1180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rutsch M, Rieckermann J, Cullmann J, Ellis JB, Vollertsen J, Krebs P. Towards a better understanding of sewer exfiltration. Water Res. 2008;42(10-11):2385–2394. doi: 10.1016/j.watres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 60.Uejio CK, Peters TW, Patz JA. Inland lake indicator bacteria: Long-term impervious surface and weather influences and a predictive Bayesian model. Lake Reservior Manage. 2012;28(3):232–244. [Google Scholar]
- 61.Cook SM, Glass RI, LeBaron CW, Ho MS. Global seasonality of rotavirus infections. Bull World Health Organ. 1990;68(2):171–177. [PMC free article] [PubMed] [Google Scholar]
- 62.Rohayem J. Norovirus seasonality and the potential impact of climate change. Clin Microbiol Infect. 2009;15(6):524–527. doi: 10.1111/j.1469-0691.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- 63.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MACenters for Disease Control and Prevention. Enterovirus surveillance–United States, 1970-2005 MMWR Surveill Summ 20065581–20. [PubMed] [Google Scholar]
- 64.Jagai JS, Castronovo DA, Monchak J, Naumova EN. Seasonality of cryptosporidiosis: a meta-analysis approach. Environ Res. 2009;109(4):465–478. doi: 10.1016/j.envres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lal A, Hales S, French N, Baker MG. Seasonality in human zoonotic enteric diseases: a systematic review. PLoS ONE. 2012;7(4):e31883. doi: 10.1371/journal.pone.0031883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Midwest Regional Climate Center. Climate change and variability in the midwest. Available at: http://mrcc.sws.uiuc.edu/climate_midwest/mwclimate_change.htm. Accessed October 28, 2011.
- 67.Vavrus SJ, Behnke RJ. A comparison of projected future precipitation in Wisconsin using global and downscaled climate model simulations: implications for public health. Int J Climatol. In press. [Google Scholar]