Abstract
An efficient synthesis of aryl substituted cyclic sulfonimidamides designed as chiral nonplanar heterocyclic carboxylic acid bioisosteres is described. The cyclic sulfonimidamide ring system could be prepared in two steps from a trifluoroacetyl protected sulfinamide and methyl ester protected amino acids. By varying the amino acid, a range of different C-3 substituted sulfonimidamides could be prepared. The compounds could be further derivatized in the aryl ring using standard cross-coupling reactions to yield highly substituted cyclic sulfonimidamides in excellent yields. The physicochemical properties of the final compounds were examined and compared to those of the corresponding carboxylic acid and tetrazole derivatives. The unique nonplanar shape in combination with the relatively strong acidity (pKa 5–6) and the ease of modifying the chemical structure to fine-tune the physicochemical properties suggest that this heterocycle can be a valuable addition to the range of available carboxylic acid isosteres.
Keywords: Carboxylic acid bioisostere, sulfonimidamide, bioisostere, acidic heterocycle, chiral heterocycle
The use of bioisosteres is a very important aspect of modern medicinal chemistry and drug discovery.1 Introduction of a suitable bioisostere allows the medicinal chemist to make relatively small structural modifications of a lead structure to improve the compound's overall properties without losing biological activity. Carboxylic acid bioisosteres are one of the most frequently studied bioisosteric replacements in drug discovery. This can be due to carboxylic acids often having poor ADME properties, but also due to the fact that carboxylic acids can be metabolized to reactive species such as acyl glucuronides or CoA esters that can give rise to toxicity effects.2,3 Some of the most commonly applied carboxylic acid bioisosteres are heterocyclic motifs such as tetrazoles4 and isoxazolones.5
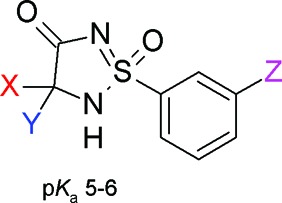
In an ongoing medicinal chemistry program, we wanted to explore new potential carboxylic acid isosteres attached directly to an aromatic ring. One compound class that attracted our attention was the five membered cyclic sulfonimidamide ring system (Figure 1). This heterocycle has an acidic proton that can be situated on either of the tautomeric nitrogens N-2 or N-5 or on the oxygens at S-1 or C-4 (Figure 1a). Calculations suggested that the lowest energy tautomer was the one with the proton situated at N-2.6 Another important feature that drew our attention to this heterocycle is that it contains a stereogenic tetrahedral sulfur atom and a sp3-hybridized carbon, which results in a twist of the heterocyclic ring (Figure 1b), thus disrupting the molecular planarity compared to that of other commonly used carboxylic acid isosteres, such as the corresponding tetrazole and isoxazolone (see Figure 1b). This structural differentiation can be beneficial compared to that of more planar motifs when optimizing drug–receptor interactions. It has also been shown that disrupting the molecular planarity is beneficial for aqueous solubility.7 In addition, the possibility to modify and alternate the stereochemistry at S-1 and C-3 might allow optimization to minimize off-target interactions, thus achieving greater selectivity. All these properties suggested that this heterocycle could be an attractive acid bioisostere, and we set out to synthesize this relatively unexplored ring-system.
Figure 1.
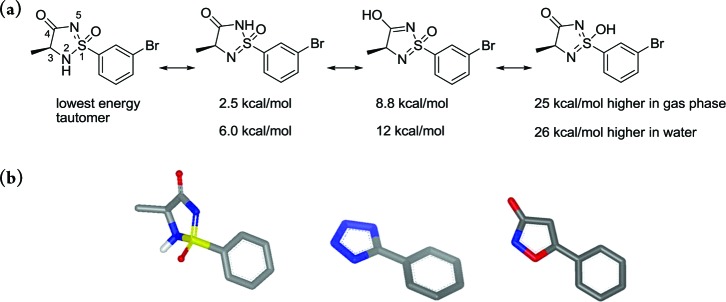
(a) Four different tautomeric forms can be adopted by the cyclic sulfonimidamide ring-system. Calculations show that the tautomer with the acidic proton situated on N2 (left molecule depicted above) has the lowest energy. (b) Calculated structure of the 1S,3S stereoisomer of the cyclic sulfonimidamide depicted in part a and also the corresponding tetrazole and isoxazol-3-one.6
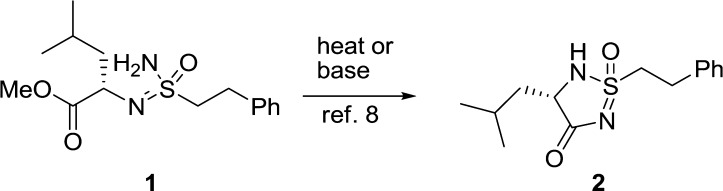
To date, only one report of a five membered cyclic sulfonimidamide ring has been published.8 Cathers et al. obtained compound 2 when they attempted to crystallize sulfonimidamide 1 (Scheme 1), which had been prepared in six steps starting from 2-phenylethanethiol. Even though the literature on cyclic sulfonimidamides is scarce, there are some reported preparations of noncyclic sulfonimidamides.9−18 These have mainly been explored as catalysts in asymmetric synthesis13,14 but a few reports have also applied this functionality in bioactive molecules,16,17 and recently, Arvidsson and co-workers reported the use of noncyclic sulfonimidamides as a bioisostere for sulfonamides.18 Sulfonimidamides are most commonly prepared from N-protected sulfinamides by doing an oxidative chlorination to generate a sulfonimidoylchloride, which can be further reacted with an amine to generate the noncyclic sulfonimidamide. We envisioned that we could apply a similar strategy to prepare the desired aryl substituted cyclic sulfonimidamides—hence, by first preparing a suitable N-protected sulfinamide and then performing the key oxidative chlorination/amination step to introduce an ester protected amino acid, which upon deprotection of the nitrogen can ring-close to the desired five membered heterocycle in the same way as observed by Cathers et al.8
Scheme 1.
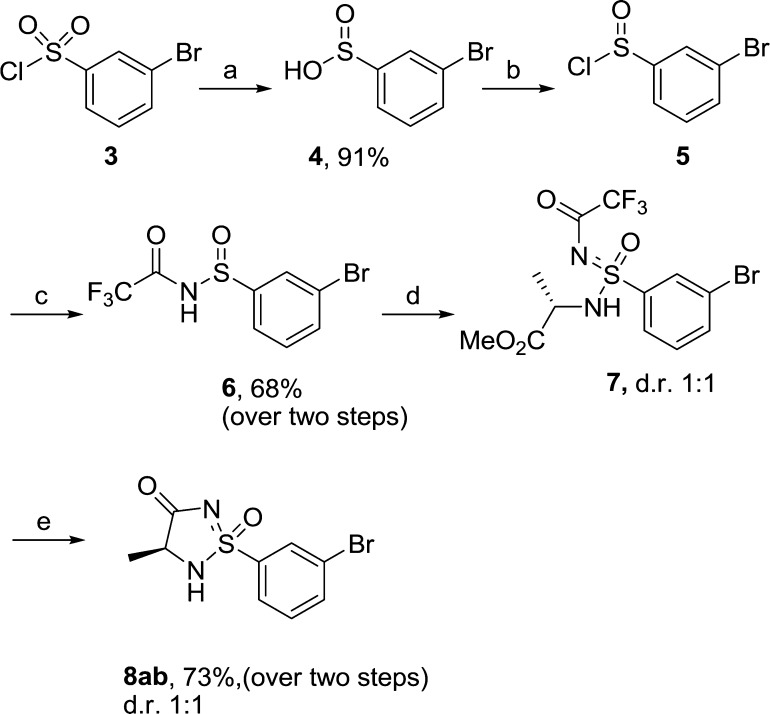
We first set out to prepare the N-protected aryl substituted sulfinamide 6, which also contains an aryl bromide that can function as a handle to enable further functionalization of the aromatic ring (Scheme 2). The synthesis of intermediate 6 was relatively straightforward and could be prepared in three steps starting from the commercially available sulfonyl chloride 3. The sulfonyl chloride 3 was first reduced to sulfinic acid 4 using sodium sulfite, and 4 was then converted to the sulfinyl chloride 5 using thionyl chloride in DCM. Reacting 5 with trifluoro acetamide in the presence of 2 equiv of n-BuLi yielded trifluoroacetyl protected sulfinamide 6 in good yield.19 To introduce the amino acid moiety and transform sulfinamide 6 to sulfonimidamide 7, an oxidative chlorination/amination step was performed. This transformation was done by first generating a sulfonimidoylchloride, which is then reacted with a nucleophilic amine. The most commonly applied reagent to perform this transformation is the explosive tert-butylhypochlorite. However, recently Bolm and co-workers showed that N-chlorosuccinimide can be used as a milder and more convenient alternative.20
Scheme 2.
Initial attempts thus involved reacting 6 with N-chlorosuccinimide in MeCN. The reaction was monitored by 1H NMR, and after 10 min at −10 °C, no remaining starting material could be observed. A solution of l-alanine methyl ester hydrochloride that had been stirred together with a tertiary amine base (DIPEA or TEA) in acetonitrile to obtain the free base was then added to this mixture. Initial attempts gave the desired noncyclic sulfonimidamide 7 but also several byproducts and resulted in low isolated yields. However, with further fine-tuning of the reaction conditions by varying the amount of amino acid and amine base, the formation of byproduct could be avoided, and using 2 equiv of amino acid and 2 equiv of base proved to be the optimal conditions. The crude sulfonimidamide 7 was then deprotected and ring-closed using NaOMe in refluxing dry MeOH to yield the desired cyclic sulfonimidamide 8ab as a diastereomeric mixture (ratio 1:1) in 73% over two steps. The diastereomers could be separated using chiral HPLC, and the relative stereochemistry was determined by single crystal X-ray analysis of diastereomer 8a (Figure 2). The hydrogen bond pattern seen in the crystal structure is consistent with the most stable tautomer based on calculations (see Figure 1). It was discovered that the quality of NaOMe was important for the outcome, since some batches of the commercially available reagent gave hydrolysis of the ester and the generated acid did not ring-close to the desired sulfonimidamide. This could be circumvented by instead using commercially available sodium amylate in MeOH, and this also proved more convenient from a practical point of view.
Figure 2.
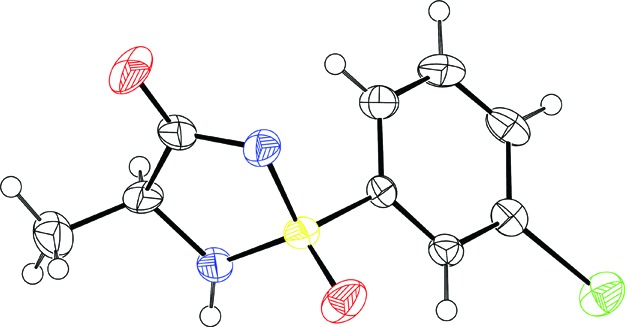
X-ray crystal structure of 8a.21
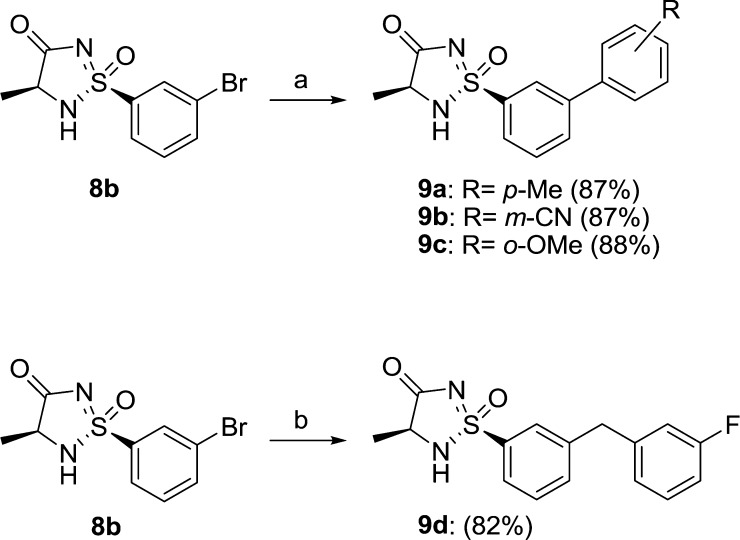
With this methodology in hand, a small series of close analogues were prepared. Using different commercially available ester-protected amino acids, C-3 substituted analogues 8c–f were readily synthesized in moderate to good yields (40–79%), starting from sulfinamide 6 (Table 1). We were now interested to investigate the possibility to further derivatize the compounds. As mentioned earlier, an aryl bromide had been incorporated in the starting sulfonylchloride 3 to enable further chemistry in this part of the molecule, and we were mainly interested in performing cross-coupling chemistry to further functionalize the scaffold. By applying standard Suzuki conditions (PdCl2(dtbpf), K2CO3, MeCN/H2O), cyclic sulfonimidamide 8b could be coupled with aryl boronic acids to give biaryls 9a–c (Scheme 3). The reaction worked well using both electron rich and electron poor boronic acids, and 9a–c were isolated in excellent yields (87–88%). To introduce a benzyl substituent, a Negishi reaction using (3-fluorobenzyl)zinc bromide was carried out. This more basic organometallic reagent was also well tolerated and 9d was prepared in an efficient manner using 2 equiv of the zinc reagent (Scheme 3).
Table 1. Synthesis of Cyclic Sulfonimidamides 8c–f from Sulfinamide 6a.
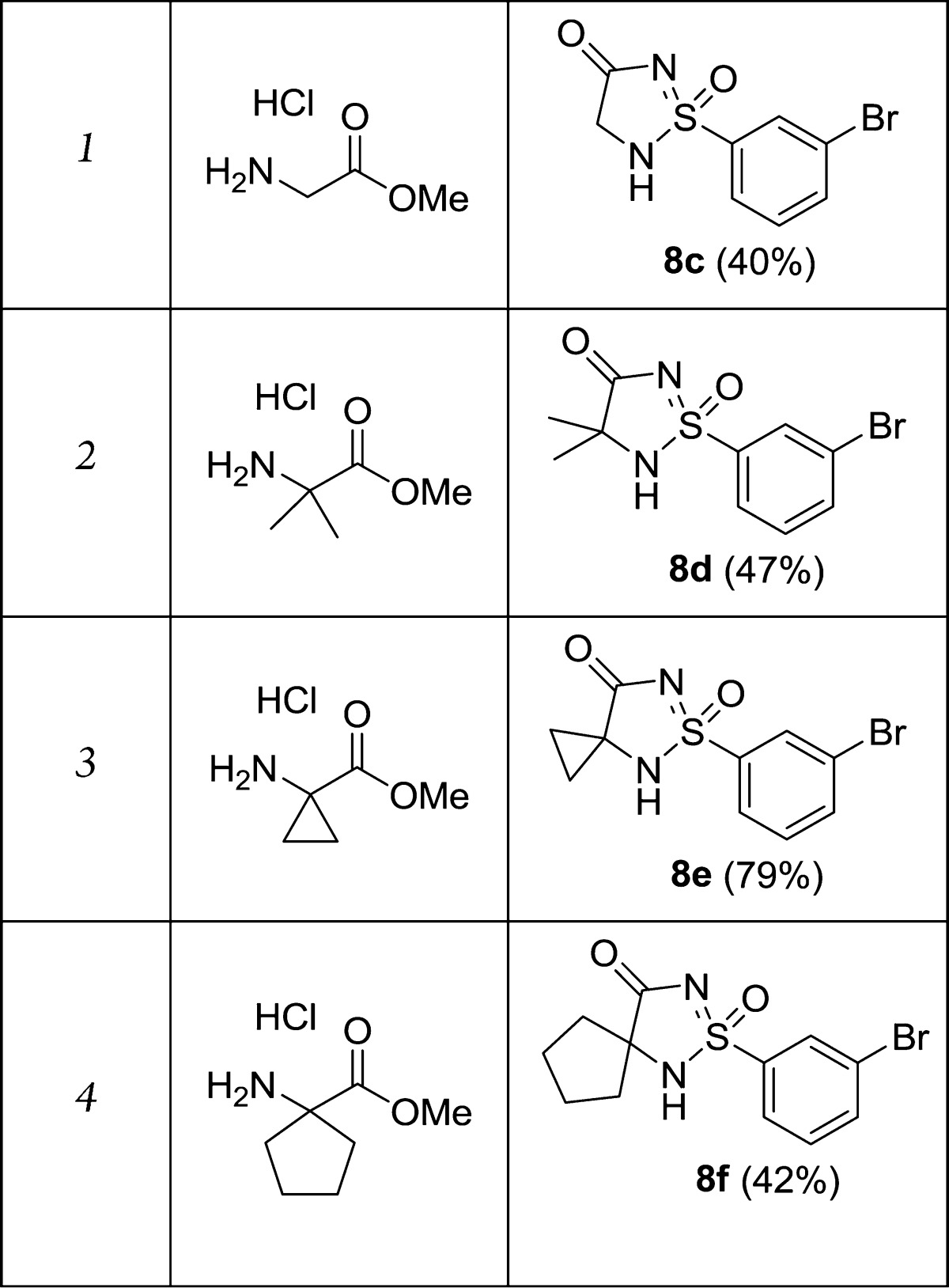
Typical conditions: (1) 6, MeCN, N-chlorosuccinimide, 15 min at 0 °C, then amino acid reagent and DIPEA, rt; (2) sodium amylate, MeOH, reflux.
Isolated yields over two steps given in parentheses.
Scheme 3.
Since our main interest in cyclic sulfonimidamides revolved around using this heterocycle as a potential carboxylic acid bioisostere, some physicochemical properties (log D, pKa, Papp A-B, efflux ratio) were measured on compounds 9a and 9b, and to be able to compare the data, the corresponding carboxylic acids 10a–b and tetrazoles 10c–d were also prepared and evaluated22 (Table 2). The pKa's of cyclic sulfonimidamides 9a and 9b were measured and found to be 6.0 and 5.8, respectively. This was about two log units higher than those of the corresponding acid 10a and tetrazoles 10c and 10d. The calculated clogP for the cyclic sulfonimidamides was one unit lower than the values for the corresponding matched pair carboxylic acids and tetrazoles but the measured log D values (at pH 7.4) were similar those of to the analogous carboxylic acid and tetrazole. One of the reasons for using isosteric replacements for carboxylic acids is to improve permeability, and this prompted us to investigate and compare the permability of these two acidic heterocycles. Permeability was measured in a Caco-2 membrane assay at pH 7.4, and it was found that the cyclic sulfonimidamides 9a and 9b had considerably higher permeability and lower efflux ratio than the matched-pair tetrazoles 10c and 10d.
Table 2. Physicochemical Properties (log D, pKa, Papp A-B, Efflux Ratio) for 9a, 9b, and 10a–d.
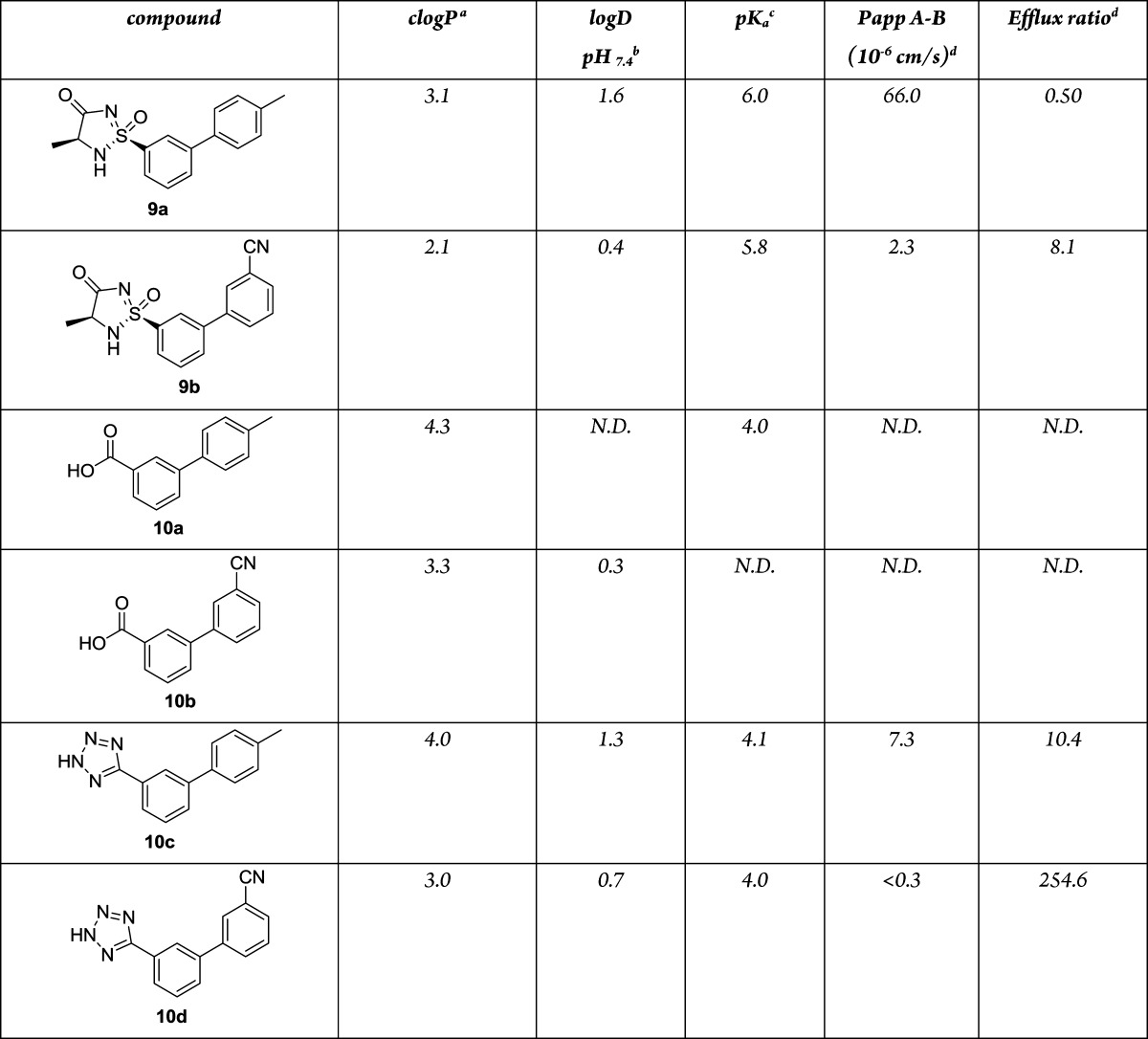
Biobyte clogP.
Experimental log D; see the Supporting Information for further details.
pKa measured using pressure-assisted capillary electrophoresis described in ref (23); see the Supporting Information for further details.
Papp A-B is the permeability when applied to the apical side, while Papp B-A is the permeability when applied to the basolateral side in a Caco-2 membrane assay (10–6 cm/s). The ratio of these two values is a measure of the efflux ratio (Papp B-A/Papp A-B), while the Papp A-B value is a measure of permeability. The experimental protocol is described in the Supporting Information.
In conclusion, we have developed an efficient synthetic pathway to five membered aryl substituted cyclic sulfonimidamides 8a–f. The heterocyclic ring-system was constructed starting from trifluoroacetyl protected sulfinamide 6, which was first transformed to a sulfonimidamide by using an oxidative chlorination/amination sequence using methyl ester protected amino acids as the amine nucleophile. This was followed by deprotection and ring-closure to the desired heterocycle under basic conditions. The cyclic sulfonimidamides could be further substituted on the aryl ring using standard Suzuki and Negishi transition metal-catalyzed cross-coupling reactions. This heterocyclic core was designed as a potential carboxylic acid isostere. It is less acidic (pKa – 6) than the corresponding carboxylic acid and tetrazole but has a similar log D at physiological pH. Compared to the matched-pair tetrazoles, this heterocycle has higher permeability and lower efflux ratio. The substitution pattern of the sulfonimidamide ring can easily be modified by starting from different amino acids. This gives a straightforward way to decorate the scaffold and vary the substitution pattern to tune physicochemical properties, as well as giving access to different stereoisomers. It has also been shown that this heterocycle has a nonplanar geometry which is different from those of many of the frequently applied carboxylic acid isosteres, such as tetrazoles and isoxazolones. All this suggests that the cyclic sulfonimidamide can be a useful addition to the range of available carboxylic acid isosteres, and it is currently being further studied and evaluated in our laboratories.
Acknowledgments
The authors wish to thank Eva-Lotte Lindstedt (AstraZeneca R&D CVGI iMed) and Magnus Johansson (AstraZeneca R&D CVGI iMed) for contributions and fruitful discussions.
Supporting Information Available
Experimental protocols and 1H NMR, 13C NMR spectra of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Meanwell N. A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. [DOI] [PubMed] [Google Scholar]
- Li C.; Benet L. Z.; Grillo M. P. Studies on the Chemical Reactivity of 2-Phenylpropionic Acid 1-O-Acyl Glucuronide and S-Acyl-CoA Thioester Metabolites. Chem. Res. Toxicol. 2002, 15, 1309–1317. [DOI] [PubMed] [Google Scholar]
- Regan S. L.; Maggs J. L.; Hammond T. G.; Lambert C.; Williams D. P.; Park B. K. Acyl Glucuronides: The Good, The Bad and The Ugly. Biopharm. Drug Dispos. 2010, 31, 367–395. [DOI] [PubMed] [Google Scholar]
- Herr J. 5-Substituted-1H-tetrazoles as Carboxylic Acid Isosteres: Medicinal Chemistry and Synthetic Methods. Bioorg. Med. Chem. 2002, 10, 3379–3393. [DOI] [PubMed] [Google Scholar]
- Bang-Andersen B.; Lenz S. M.; Skjaerbaek N.; Soby K. K.; Hansen H. O.; Ebert B.; Bogeso K. P.; Krogsgaard-Larsen P. Heteroaryl Analogues of AMPA. Synthesis and Quantitative Structure-Activity Relationships. J. Med. Chem. 1997, 40, 2831–2842. [DOI] [PubMed] [Google Scholar]
- Geometry optimization of the system in gasphase and single point energy calculation in water (Poisson-Poisson Boltzmann Finite element method) using B3LYP/6-31+G** using the Jaguar software from Schrödinger (Jaguar, version 7.8; Schrödinger, LLC: New York, NY, 2011).
- Ishikawa M.; Hashimoto Y. Improvement in Aqueous Solubility in Small Molecule Drug Discovery Programs by Disruption of Molecular Planarity and Symmetry. J. Med. Chem. 2011, 54, 1539–1554. [DOI] [PubMed] [Google Scholar]
- Cathers B. E.; Schloss J. V. The Sulfonimidamide as a Novel Transition State Analog for Aspartic Acid and Metallo Proteases. Bioorg. Med. Chem. Lett. 1999, 9, 1527–1532. [DOI] [PubMed] [Google Scholar]
- Johnson C. R.; Wambsgans A. Sulfonimidoyl Chlorides by Oxidation of Sulfinamides with tert-Butyl Hypochlorite. J. Org. Chem. 1979, 44, 2278–2280. [Google Scholar]
- Worch C.; Atodiresei I.; Raabe G.; Bolm C. Synthesis of Enantiopure Sulfonimidamides and Elucidation of Their Absolute Configuration by Comparison of Measured and Calculated CD Spectra and X-Ray Crystal Structure Determination. Chem.—Eur. J. 2010, 16, 677–683. [DOI] [PubMed] [Google Scholar]
- Worch C.; Bolm C. Efficient Synthesis of Sulfonimidoylguanidines by Coupling of Sulfonimidamides with Uronium Reagents. Synthesis 2007, 9, 1355–1358. [Google Scholar]
- Leca D.; Toussaint A.; Mareau C.; Fensterbank L.; Lacote E.; Malacria M. Efficient Copper-Mediated Reactions of Nitrenes Derived from Sulfonimidamides. Org. Lett. 2004, 6, 3573–3575. [DOI] [PubMed] [Google Scholar]
- Liang C.; Robert-Peillard F.; Fruit C.; Mueller P.; Dodd R. H.; Dauban P. Efficient Diastereoselective Intermolecular Rhodium-Catalyzed C-H Amination. Angew. Chem., Int. Ed. 2006, 45, 4641–4644. [DOI] [PubMed] [Google Scholar]
- Steurer M.; Bolm C. Synthesis of Amino-Functionalized Sulfonimidamides and Their Application in the Enantioselective Henry Reaction. J. Org. Chem. 2010, 75, 3301–3310. [DOI] [PubMed] [Google Scholar]
- Azzaro S.; Murr M.; Fensterbank L.; Lacote E.; Malacria M. Copper-Catalyzed N-Arylation of Sulfonimidamides. Synlett 2011, 849–851. [Google Scholar]
- Gnamm C.; Jeanguenat A.; Dutton A. C.; Grimm C.; Kloer D. P.; Crossthwaite J. Novel Diamide Insecticides: Sulfoximines, Sulfonimidamides and Other New Sulfonimidoyl Derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3800–3806. [DOI] [PubMed] [Google Scholar]
- Toth J. E.; Grindey G. B.; Ehlhardt W. J.; Ray J. E.; Boder G. B.; Bewley J. R.; Klingerman K. K.; Gates S. B.; Rinzel S. M.; Schultz R. M.; Weir L. C.; Worzalla J. F. Sulfonimidamide Analogs of Oncolytic Sulfonylureas. J. Med. Chem. 1997, 40, 1018–1025. [DOI] [PubMed] [Google Scholar]
- Sehgelmeble F.; Janson J.; Ray C.; Rosqvist S.; Gustavsson S.; Nilsson L. I.; Minidis A.; Holenz J.; Rotticci D.; Lundkvist J.; Arvidsson P. I. Sulfonimidamides as Sulfonamides Bioisosteres: Rational Evaluation through Synthetic, in Vitro, and in Vivo Studies with γ-Secretase Inhibitors. ChemMedChem 2012, 7, 396–399. [DOI] [PubMed] [Google Scholar]
- This material proved to be unstable and decomposed slowly at rt and even faster in the presence of chlorinated solvents. However, by storing it in the freezer and avoiding chlorinated solvents, this issue could be circumvented.
- Mancheno O. G.; Bolm C.. Synthesis of Sulfonimidamides from Sulfinamides by Oxidation with N-Chorosuccinimide. Beilstein J. Org. Chem. 2007, 3, No. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Full crystallographic data have been deposited to the Cambridge Crystallographic Data Center (CCDC reference number 874773). Copies of the data can be obtained free of charge via the internet at http://www.ccdc.cam.ac.uk.
- The compounds were prepared from the corresponding aryl bromides using standard Suzuki coupling conditions. See Supporting Information for experimental details.
- Wan H.; Holmen A. G.; Wang Y.; Lindberg W.; Englund M.; Nagard M. B.; Thompson R. A. High-throughput Screening of pKa Values of Pharmaceuticals by Pressure-Assisted Capillary Electrophoresis and Mass Spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2639–2648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.