Abstract
Treating insulin resistance and type 2 diabetes in rodents, currently known retinoid X receptor (RXR) agonists induce significant adverse effects. Here we introduce a novel RXR partial agonist CBt-PMN (11b), which shows a potent glucose-lowering effect and improvements of insulin secretion and glucose tolerance without the serious adverse effects caused by RXR full agonists. We suggest that RXR partial agonists may be a new class of antitype 2 diabetes drug candidates.
Keywords: Nuclear receptors, RXR, partial agonists, type 2 diabetes
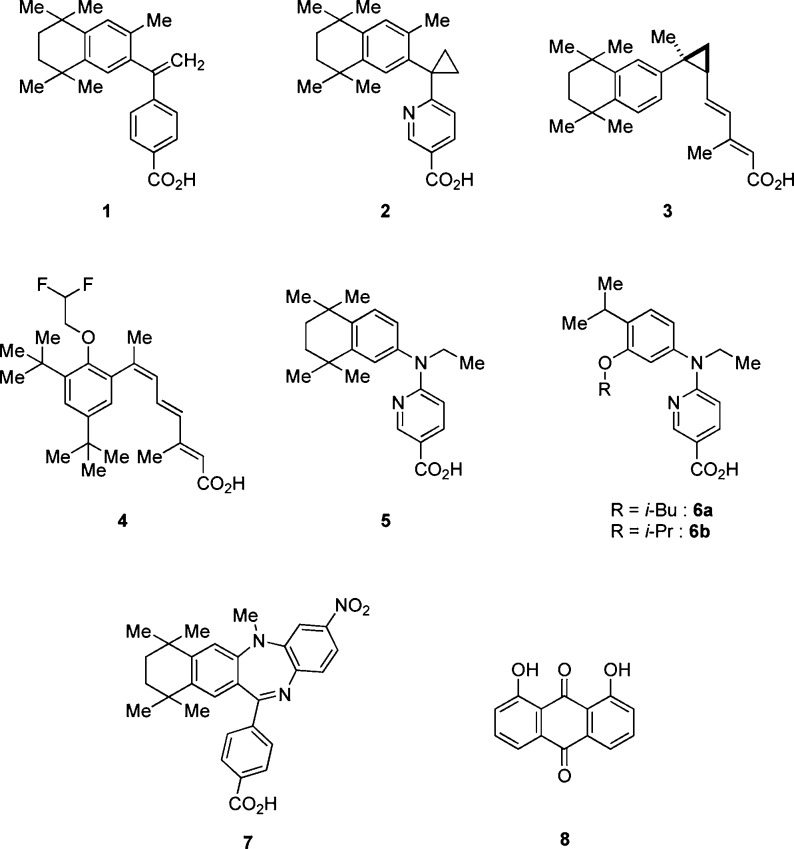
Retinoid X receptors (RXRs) are of interest as nuclear receptors that serve to regulate transcription of genes relevant to diabetes. They function either as homodimers or as heterodimers, for example with peroxisome proliferator-activated receptors (such as PPARγ, the well-known target of thiazolidinedione-type agents to improve insulin resistance) or liver X receptors (LXRs, whose activation induces glucose metabolism and improves glucose tolerance).1−5 In addition, LXR activation is reported to induce glucokinase expression, which is coregulated with insulin,6 and to promote insulin secretion via stimulation of pancreatic islets.7 So-called permissive RXR-heterodimers, such as PPAR/RXR and LXR/RXR, can be activated by RXR agonists alone.8 Therefore, RXR agonists seem to be promising candidates for improving both insulin resistance and glucose tolerance. Several RXR agonists 1–4 (Figure 1) have been evaluated for the treatment of insulin resistance and type 2 diabetes in rodents.9 However, all of them induce significant adverse effects, such as blood triglyceride (TG) elevation,10 weight gain,11 hepatomegaly,12 and hypothyroidism.13
Figure 1.
Chemical structures of known RXR ligands.
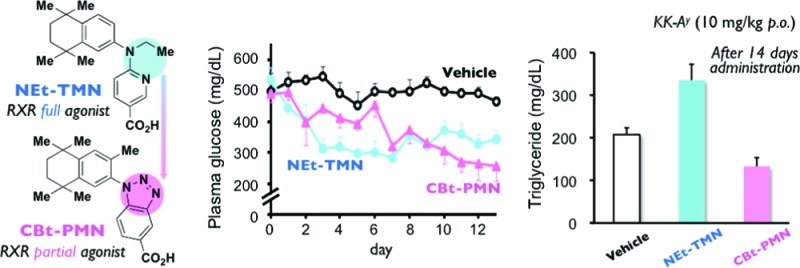
On the basis of a report that structurally different RXR agonists show different patterns of activation of RXR-heterodimers,14 we previously examined the feasibility of separating the blood glucose-lowering action of RXR agonists from the adverse effects. For this purpose, we administered RXR agonists NEt-TMN (5),15 NEt-3IB (6a), and NEt-3IP (6b)16 (Figure 1), which similarly activate RXR, but differently activate PPAR/RXR and LXR/RXR,17 to KK-Ay type 2 diabetes model mice, and we examined the relationship of the RXR-heterodimeric activation pattern to the antihyperglycemic effect and adverse effects such as hepatomegaly and TG elevation.18 We found that 6a has a less potent TG-elevating activity and hepatomegaly than the other RXR agonists examined, though all of them showed similar blood glucose-lowering action on repeated administration. This result suggested that it might be possible to separate the therapeutic effects from the side effects of RXR agonists. However, even 6a shows significant TG-elevation and hepatomegaly. Since all RXR agonists that have been reported to show serious side effects are RXR full agonists that potently activate RXR, we hypothesized that there is a threshold difference between the therapeutic effects and adverse effects of RXR activation. Therefore, we decided to focus on RXR partial agonists, which would activate RXR only moderately or whose transcriptional efficacy would be limited. We further considered that this idea might be consistent with reports that RXR antagonists HX531 (7)19 and danthron (8)20 (Figure 1) show blood glucose-lowering effects in type 2 diabetes model mice, because partial antagonists are also thought to behave as partial agonists.
To test our hypothesis, we aimed to synthesize RXR partial agonists by modifying the representative RXR agonist structure, focusing on restriction of the molecular flexibility by linking the hydrophobic or acidic domain and the linking domains to form a new ring moiety (Figure 2). Screening of the synthesized compounds identified CBt-PMN (11b) as a novel RXR partial agonist. Studies in mice and rats showed that 11b did not induce the side effects typically caused by RXR full agonists, while oral administration to KK-Ay mice, a widely used model of type 2 diabetes, resulted in a potent glucose-lowering effect and improved insulin secretion and glucose tolerance. These results indicate that RXR partial agonists may be a new class of antitype 2 diabetes drug candidates without the serious adverse effects shown by RXR full agonists. In this article, we describe the molecular design strategy and the results of the in vitro and in vivo experiments, and we discuss the mechanism of action of 11b.
Figure 2.
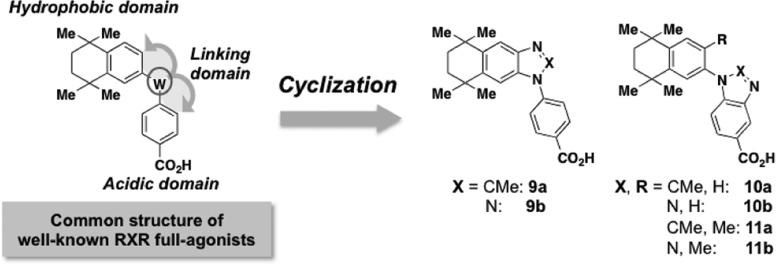
Molecular design strategy for creating RXR partial agonists.
In general, representative RXR agonists consist of a hydrophobic domain including 1,1,4,4-tetramethyltetralin, an acidic domain bearing benzoic acid, nicotinic acid, or pyrimidinecarboxylic acid, and a linking domain connecting these domains, as shown in Figure 2. Our design strategy to obtain RXR partial agonistic activity was to form a ring between the hydrophobic or acidic domain and the linking domain to decrease the molecular flexibility.
Compounds were synthesized as illustrated in Schemes S1 and S2 (see Supporting Information) and evaluated by measuring their RXR-agonistic activities in reporter-gene assays. Although the compounds possessing ring structures between the hydrophobic and the linking domains did not show RXR agonistic activities, compounds 11a and 11b, in which the ring was formed between the acidic and the linking domains, did show agonistic activity (Supporting Information Table S2). Interestingly, while 11a, possessing a 2-methylimidazole structure, showed full agonistic activity, 11b, possessing a triazole structure, showed only partial agonistic activity toward RXRα (EC50 = 143 nM, Emax = 75%) (Figure 3a and Supporting Information Table S2). The RXR partial agonist activity of 11b is thought to be due to not only the closed ring structure but also other factors, including the polarity/positions of the nitrogen atoms. Moreover, 11b behaved similarly toward other RXR subtypes (Figure 3b,c). The RXR agonistic activity of the full agonist LGD1069 (1) at 1 μM was reduced in the presence of increasing concentrations of 11b, indicating that 11b acts as a partial antagonist (Supporting Information Figure S1). In addition, 11b showed moderate RAR activation (Supporting Information Figure S2). These results support the idea that 11b acts as a RXR partial agonist.
Figure 3.
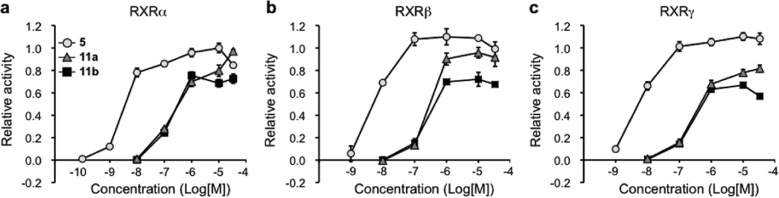
Results of reporter gene assays of 5, 11a, and 11b. COS-1 cells were transfected with three kinds of vectors consisting of a RXR receptor subtype, a luciferase reporter gene under the control of the appropriate RXR response element (CRBPII-tk-Luc), and secreted alkaline phosphatase (SEAP) gene as a background. (a) RXRα, (b) RXRβ, and (c) RXRγ, based on the luciferase activity of 1 μM 1 (RXR full agonist) taken as 1.0. Circles, triangles, and squares indicate 5, 11a, and 11b, respectively. The data (n = 3) represent the mean ± sem. Data for NEt-TMN were taken from ref (18), because these experiments were performed at the same time.
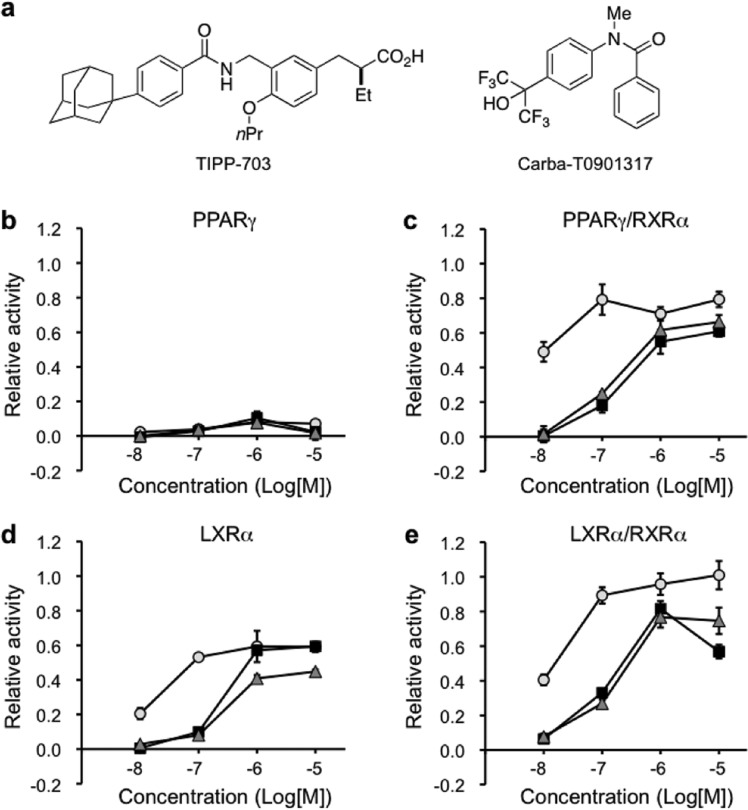
The activities of 11a and 11b toward PPARγ/RXRα and LXRα/RXRα were next examined, with RXR full agonist 5 as a positive control.18 None of the compounds showed PPARγ activity, but compounds 11a and 11b showed similar PPARγ/RXRα activation, though they were both less potent than 5 (Figure 4b,c). Compounds 11a and 11b also activated both LXRα and LXRα/RXRα less potently than 5 (Figure 4d,e). Since these compounds showed both PPARγ/RXRα and LXRα/RXRα agonistic activities in vitro, we next examined their in vivo activities.
Figure 4.
Relative transactivation activities of 5, 11a, and 11b toward PPARγ, PPARγ/RXRα, LXRα, and LXRα/RXRα. COS-1 cells were transfected with four kinds of vectors, consisting of RXRα, a partner receptor (PPARγ or LXRα), the partner response element (tk-PPREx3-Luc for PPARγ or tk-rBARx3-Luc for LXRα), and secreted alkaline phosphatase (SEAP) gene as a background. (a) Chemical structures of TIPP70321 (PPAR pan-agonist) and carba-T090131722 (LXR pan-agonist). (b) Relative transactivation data for PPARγ, based on the luciferase activity of 1 μM TIPP703 taken as 1.0. (c) Relative transactivation data for PPARγ/RXRα, based on the luciferase activity of 1 μM TIPP703 taken as 1.0. (d) Relative transactivation data for LXRα, based on the luciferase activity of 1 μM carba-T0901317 (LXR pan-agonist) taken as 1.0. (e) Relative transactivation data for LXRα/RXRα, based on the luciferase activity of 1 μM carba-T0901317 taken as 1.0. TIPP703 or carba-T0901317 at 1 μM give each Emax value. Circles, triangles, and squares indicate 5, 11a, and 11b, respectively. The data (n = 3–6) represent the mean ± sem.
First, it was confirmed that oral single administration of each compound at 30 mg/kg resulted in a serum concentration over 1 μM in ICR male mice (Supporting Information Figure S3). Then, changes in body weight, hepatomegaly, and serum triglyceride (TG) elevation were assessed when each compound was administered orally to ICR mice at 30 mg/kg/day for 7 days (Supporting Information Figure S4). The mice given the RXR full agonists 5 and 11a showed greater body weight gain than did the vehicle control group, and the increase was significant in the case of 5. In contrast, the group treated with 11b showed similar body weight change to the control group (Supporting Information Figure S4a). Liver weight was increased significantly by both 5 and 11a, but not by 11b, compared to the control group (Supporting Information Figure S4b). Compound 11b also did not increase serum TG and total cholesterol values compared to the control group, whereas 5 significantly increased the TG level (Supporting Information Figure S4c,d). Further, when 11b was administered orally to male and female SD rats at 30 mg/kg/day for 28 days, no significant difference in body weight change, water intake, or food intake was observed, compared with the vehicle control (Supporting Information Figure S5). The testes of animals treated with 11b were slightly enlarged, but the other agents had no such effect (Supporting Information Table S4). As for serum constituents, although some significant differences from the vehicle were observed, the data were within the ranges considered normal by the suppliers (Charles River, Ltd.) (Supporting Information Table S5). Thus, it seems unlikely that the RXR partial agonist 11b would cause the side effects associated with RXR full agonists.
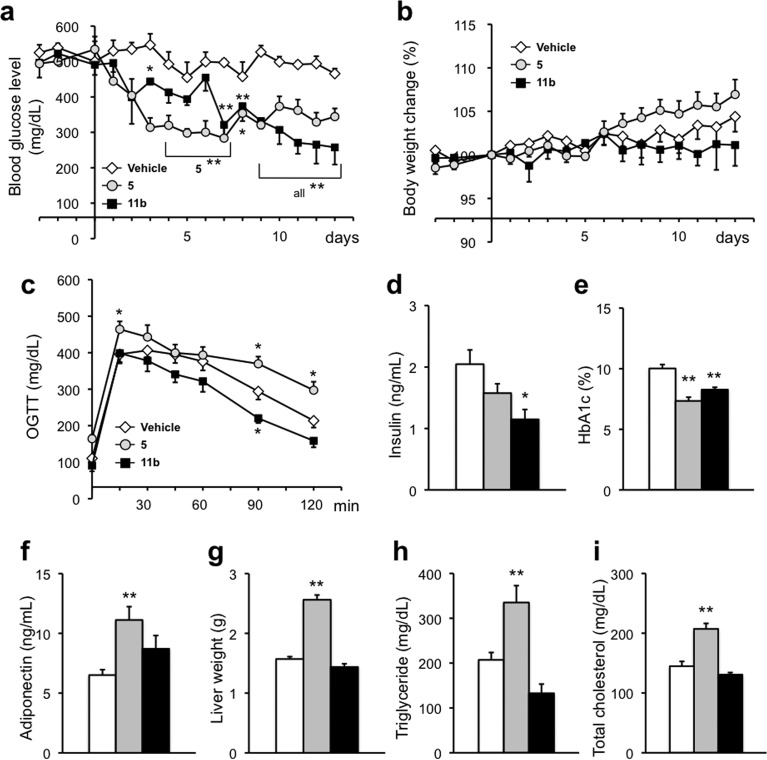
Next, we examined the antitype 2 diabetes activity in male KK-Ay mice. Compound 5 or 11b was orally administered at 10 mg/kg/day for 14 days. The average blood glucose level in vehicle-treated mice was about 500 mg/dL, while the level in 5-treated mice was reduced to about 300 mg/dL from day 3 after the start of administration, showing a significant blood glucose-lowering effect. Compound 11b also showed a significant blood glucose-lowering effect, although the lag time was longer than that in the case of 5 (Figure 5a). Moreover, 11b significantly reduced serum insulin concentration (Figure 5d) and HbA1c (hemoglobin A1c, which is correlated with blood glucose levels over a period of time) (Figure 5e) and produced an improvement in the oral glucose tolerance test (OGTT) (Figure 5c), showing significant antitype 2 diabetes effects. Since 11b improved insulin resistance in KK-Ay mice, we quantitated adiponectin, which is reported to be associated with insulin resistance, and found that 5 increased adiponectin significantly, whereas 11b did so only moderately (Figure 5f). Since a low adiponectin level is related to insulin resistance, adiponetin elevation by RXR agonists may be correlated with their antitype 2 diabetes effects.
Figure 5.
Evaluation of antitype 2 diabetes effects of repeated oral administration of 5 or 11b at 10 mg/kg/day to male KK-Ay mice for 14 consecutive days. (a) Time course of blood glucose levels. (b) Time course of body weight change. (c) Results of oral glucose tolerance tests (OGTT) in KK-Ay mice treated with vehicle and compounds. (d) Effects of compounds on serum insulin levels. (e) Effects of compounds on serum HbA1c levels. (f) Effects of compounds on adiponectin levels. (g–i) Effects of compounds on liver weight, serum triglyceride, and total cholesterol, respectively. The white, gray, and black bars indicate vehicle, 5, and 11b treatment, respectively. The data (n = 3–7) represent the mean ± sem. Data for vehicle control and 5 were taken from ref (18), because these experiments were performed at the same time. Statistical analysis was performed by analysis of variance (ANOVA). Significant differences: * p < 0.05 vs vehicle. ** p < 0.01 vs vehicle.
We also evaluated adverse effects in male KK-Ay mice treated with RXR full agonist 5 or RXR partial agonist 11b. Examination of body weight change, liver weight, serum TG, and total cholesterol revealed that while 5 induced significant increases similar to those seen in ICR mice, 11b did not alter these parameters in comparison with the vehicle (Figure 5b,g–i). Thus, 11b appears to have a favorable profile of therapeutic and side effects. The reason why 11b showed antitype 2 diabetes effects at 10 mg/kg p.o. but did not produce significant side effects even when orally administered at 30 mg/kg/day, which provides a serum concentration sufficient to produce the Emax, is considered to be its RXR partial agonist character, though other factors such as differences in timing or mechanism of action may also be involved.
To address the mechanism of the antitype 2 diabetes activity of 11b, we examined changes in the expression levels of genes associated with glucose/lipid metabolism in the liver of KK-Ay mice by means of RT-PCR (Figure 6). It is reported that RXR agonists increase Slc2a1 (GLUT1) and Slc2a2 (GLUT2), thereby inducing an increase of liver glucose intake.23Gck expression is also increased by RXR agonists.23 Other changes include suppression of G6p and Pck expression, and increase in expression of Gck (related to glycolysis), Scd1, Fasn, and Srebp1c, which are associated with increased lipid synthesis induced by LXR agonists.6 We also examined changes in expression of Irs1 and Irs2. Neither 5 nor 11b influenced gene expression of Irs1, Irs2, Slc2a1, Slc2a2, G6pc, and Pck. However, 11b increased Gck expression significantly, indicating that one of the mechanisms of the glucose-lowering effect of 11b is induction of glycolysis via Gck. While 5 markedly increased the gene expression of Scd1 and Fasn, 11b did not. Since Scd1 and Fasn are associated with lipid synthesis, this result may explain the lack of serum TG elevation by 11b.
Figure 6.
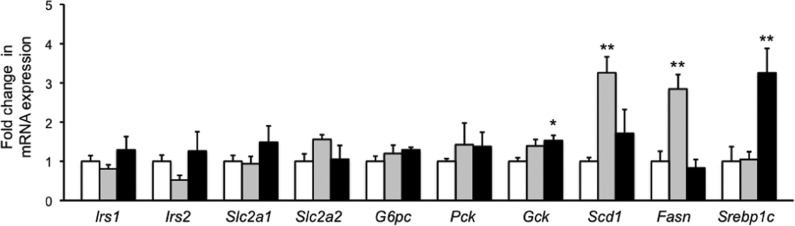
Fold changes in mRNA expression of Irs1 (a), Irs2 (b), Slc2a1 (c), Slc2a2 (d), G6pc (e), Pck (f), Gck (g), Scd1 (h), Fasn (i), and Srebp1c (j) in the liver tissue of male KK-Ay mice treated with vehicle, 5, or 11b at 10 mg/kg/day for 14 consecutive days. These measurements were performed using the same mice as in the case of Figure 5. The white, gray, and black bars indicate vehicle, 5, and 11b treatment, respectively. The data (n = 3–7) represent the mean ± sem. Statistical analysis was performed by analysis of variance (ANOVA). Significant differences: * p < 0.05 vs vehicle. ** p < 0.01 vs vehicle.
Expression of Gck, Scd1, and Fasn is regulated by Srebp1c,24−26 but 5 had no effect on the expression of Srebp1c. It has been reported that Sreb1c expression in liver is suppressed by adiponectin.27 Therefore, the reason why 5 had no effect on Srebp1c expression may be that it induced a significant increase of adiponectin, as shown in Figure 6. On the other hand, the partial agonist 11b activates RXR only moderately, and this may be sufficient to induce expression of Srebp1c, which lowers blood glucose, without causing overexpression of Scd1 and Fasn, which induce lipid synthesis. These results are consistent with our hypothesis that there is a threshold difference between the therapeutic and adverse effects of RXR activation. Although an appropriate low dosage of a RXR full agonist may show similar beneficial effects to a RXR partial agonist 11b, in the case of the overdose medical malpractice, RXR full agonists can cause several adverse effects. Therefore, RXR partial agonists will be more attractive antitype 2 diabetes drug candidates than RXR full agonists.
In summary, we hypothesized that there are different thresholds for the therapeutic effects and side effects of RXR activation. Therefore, we aimed to synthesize RXR partial agonists by reducing the molecular flexibility of the representative RXR agonist structure. As we had hoped, ring formation between the acidic and linking domains with a triazole structure afforded a RXR partial agonist 11b. This compound showed a significant antitype 2 diabetes effect in KK-Ay diabetic model mice but did not induce the side effects associated with RXR full agonists in ICR mice or SD rats. Compound 11b did not induce expression of genes associated with lipid synthesis, whereas the full agonist 5 did induce expression of these genes. These results support our hypothesis of a threshold difference for the therapeutic and side effects of RXR agonists. We believe that RXR partial agonists such as 11b represent a promising class of candidate antitype 2 diabetes agents.
Acknowledgments
We thank Dr. Miyachi (Okayama University) for kindly providing TIPP703 and carba-T0901317. We also thank Dr. Kagechika (School of Biomedical Science, Tokyo Medical and Dental University) for kindly providing PA452. The authors are also grateful to Professor Yoshio Naomoto and Dr. Takuya Fukazawa (Department of General Surgery, Kawasaki Medical School) for preparing plasmides. We also thank Dr. Aiba (Okayama University) for assistance with dissection.
Glossary
Abbreviations
- RXR
retinoid X receptor
- PPAR
peroxisome proliferator-activated receptors
- LXR
liver X receptor
- RAR
retinoic acid receptor
- TG
triglyceride
- RT-PCR
reverse transcriptase polymerase chain reaction
- Irs
insulin receptor substrate
- GLUT
glucose transporter
- G6p
glucose-6-phosphatase
- Pck
phosphoenolpyruvate carboxykinase
- Gck
glucokinase
- Scd1
stearoyl-CoA desaturase 1
- Fasn
fatty acid synthase
- Srebp1c
sterol regulatory element-binding protein 1c
Supporting Information Available
General information, synthetic procedures, combustion analysis data, HPLC charts, luciferase reporter gene assay, and in vivo experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
H.K. conceived and designed the project. N.Y., R.S., and M.H. synthesized compounds. F.O., S.Y., and Y.O. performed reporter gene assays. M.M. prepared plasmids. K.K., M.N., and C.F. performed in vivo experiments with ICR mice and SD rats. A.T. performed HPLC analysis. Y.Y. and H.Y. performed in vivo experiments with KK-Ay mice. C.F., S.U., A.M., M.N., and T.O. performed PCR analysis. The manuscript was written by H.K. and F.O.
This work was supported by Health and Labour Science research grants for Research on seeds for Publicly Essential Drugs and Medical Devices (23080401) from the Ministry of Health, Labour, and Welfare of Japan. We also thank the Ministry of Education, Science, Culture and Sports of Japan, and Takeda Science Foundation for financial support.
The authors declare no competing financial interest.
Supplementary Material
References
- Kanda S.; Nakashima R.; Takahashi K.; Tanaka J.; Ogawa J.; Ogata T.; Yachi M.; Araki K.; Ohsumi J. Potent antidiabetic effects of rivoglitazone, a novel peroxisome proliferator-activated receptor-gamma agonist, in obese diabetic rodent models. J. Pharmacol. Sci. 2009, 111, 155–166. [DOI] [PubMed] [Google Scholar]
- Mitro N.; Mak P. A.; Vargas L.; Godio C.; Hampton E.; Molteni V.; Kreusch A.; Saez E. The nuclear receptor LXR is a glucose sensor. Nature 2007, 445, 219–213. [DOI] [PubMed] [Google Scholar]
- Svensson S.; Ostberg T.; Jacobsson M.; Norström C.; Stefansson K.; Hallén D.; Johansson I. C.; Zachrisson K.; Ogg D.; Jendeberg L. Crystal structure of the heterodimeric complex of LXRalpha and RXRbeta ligand-binding domains in a fully agonistic conformation. EMBO J. 2003, 22, 4625–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lera A. R.; Bourguet W.; Altucci L.; Gronemeyer H. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat. Rev. Drug Discovery 2007, 6, 811–820. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J.; Evans R. M. The RXR heterodimers and orphan receptors. Cell 1995, 83, 841–850. [DOI] [PubMed] [Google Scholar]
- Laffitte B. A.; Chao L. C.; Li J.; Walczak R.; Hummasti S.; Joseph S. B.; Castrillo A.; Wilpitz D. C.; Mangelsdorf D. J.; Collins J. L.; Saez E.; Tontonoz P. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 5419–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara T.; Chuang J. C.; Vestermark G. L.; Garmey J. C.; Ketchum R. J.; Huang X.; Brayman K. L.; Thorner M. O.; Repa J. J.; Mirmira R. G.; Evans-Molina C. Liver X receptor agonists augment human islet function through activation of anaplerotic pathways and glycerolipid/free fatty acid cycling. J. Biol. Chem. 2010, 285, 5392–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman A. I.; Larson C.; Mangelsdorf D. J.; Ranganathan R. Structural determinants of allosteric ligand activation in RXR heterodimers. Cell 2004, 116, 417–429. [DOI] [PubMed] [Google Scholar]
- Mukherjee R.; Davies P. J.; Crombie D. L.; Bischoff E. D.; Cesario R. M.; Jow L.; Hamann L. G.; Boehm M. F.; Mondon C. E.; Nadzan A. M.; Paterniti J. R. Jr.; Heyman R. A. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature 1997, 386, 407–410. [DOI] [PubMed] [Google Scholar]
- Davies P. J.; Berry S. A.; Shipley G. L.; Eckel R. H.; Hennuyer N.; Crombie D. L.; Ogilvie K. M.; Peinado-Onsurbe J.; Fievet C.; Leibowitz M. D.; Heyman R. A.; Auwerx J. Metabolic effects of rexinoids: tissue-specific regulation of lipoprotein lipase activity. Mol. Pharmacol. 2001, 59, 170–176. [DOI] [PubMed] [Google Scholar]
- Li X.; Hansen P. A.; Xi L.; Chandraratna R. A.; Burant C. F. Distinct mechanisms of glucose lowering by specific agonists for peroxisomal proliferator activated receptor gamma and retinoic acid X receptors. J. Biol. Chem. 2005, 280, 38317–38327. [DOI] [PubMed] [Google Scholar]
- Lenhard J. M.; Lancaster M. E.; Paulik M. A.; Weiel J. E.; Binz J. G.; Sundseth S. S.; Gaskill B. A.; Lightfoot R. M.; Brown H. R. The RXR agonist LG100268 causes hepatomegaly, improves glycaemic control and decreases cardiovascular risk and cachexia in diabetic mice suffering from pancreatic beta-cell dysfunction. Diabetologia 1999, 42, 545–554. [DOI] [PubMed] [Google Scholar]
- Liu S.; Ogilvie K. M.; Klausing K.; Lawson M. A.; Jolley D.; Li D.; Bilakovics J.; Pascual B.; Hein N.; Urcan M.; Leibowitz M. D. Mechanism of selective retinoid X receptor agonist-induced hypothyroidism in the rat. Endocrinology 2002, 143, 2880–2885. [DOI] [PubMed] [Google Scholar]
- Nishimaki-Mogami T.; Tamehiro N.; Sato Y.; Okuhira K.; Sai K.; Kagechika H.; Shudo K.; Abe-Dohmae S.; Yokoyama S.; Ohno Y.; Inoue K.; Sawada J. The RXR agonists PA024 and HX630 have different abilities to activate LXR/RXR and to induce ABCA1 expression in macrophage cell lines. Biochem. Pharmacol. 2008, 76, 1006–1013. [DOI] [PubMed] [Google Scholar]
- Fujii S.; Ohsawa F.; Yamada S.; Shinozaki R.; Fukai R.; Makishima M.; Enomoto S.; Tai A.; Kakuta H. Modification at the acidic domain of RXR agonists has little effect on permissive RXR-heterodimer activation. Bioorg. Med. Chem. Lett. 2010, 20, 5139–5142. [DOI] [PubMed] [Google Scholar]
- Takamatsu K.; Takano A.; Yakushiji N.; Morohashi K.; Morishita K.; Matsuura N.; Makishima M.; Tai A.; Sasaki K.; Kakuta H. The first potent subtype-selective retinoid X receptor (RXR) agonist possessing a 3-isopropoxy-4-isopropylphenylamino moiety, NEt-3IP (RXRalpha/beta-dual agonist). ChemMedChem 2008, 3, 780–787. [DOI] [PubMed] [Google Scholar]
- Ohsawa F.; Morishita K.; Yamada S.; Makishima M.; Kakuta H. Modification at the Lipophilic Domain of RXR Agonists Differentially Influences Activation of RXR Heterodimers. ACS Med. Chem. Lett. 2010, 1, 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuta H.; Ohsawa F.; Yamada S.; Makishima M.; Tai A.; Yasui H.; Yoshikawa Y. Feasibility of Structural Modification of Retinoid X Receptor Agonists to Separate Blood Glucose-Lowering Action from Adverse Effects: Studies in KK-Ay Type 2 Diabetes Model Mice. Biol. Pharm. Bull. 2012, 35, 629–633. [DOI] [PubMed] [Google Scholar]
- Yamauchi T.; Waki H.; Kamon J.; Murakami K.; Motojima K.; Komeda K.; Miki H.; Kubota N.; Terauchi Y.; Tsuchida A.; Tsuboyama-Kasaoka N.; Yamauchi N.; Ide T.; Hori W.; Kato S.; Fukayama M.; Akanuma Y.; Ezaki O.; Itai A.; Nagai R.; Kimura S.; Tobe K.; Kagechika H.; Shudo K.; Kadowaki T. Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J. Clin. Invest. 2001, 108, 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Zhou R.; Li L.; Chen J.; Chen L.; Li C.; Ding H.; Yu L.; Hu L.; Jiang H.; Shen X. Danthron functions as a retinoic X receptor antagonist by stabilizing tetramers of the receptor. J. Biol. Chem. 2011, 286, 1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga J.; Oyama T.; Hirakawa Y.; Makishima M.; Morikawa K.; Hashimoto Y.; Miyachi H. Improvement of the transactivation activity of phenylpropanoic acid-type peroxisome proliferator-activated receptor pan agonists: effect of introduction of fluorine at the linker part. Bioorg. Med. Chem. Lett. 2008, 18, 4525–8. [DOI] [PubMed] [Google Scholar]
- Aoyama A.; Aoyama H.; Dodo K.; Makishima M.; Hashimoto Y.; Miyachi H. LXR antagonists with a 5-substituted phenanthridin-6-one skeleton: synthesis and LXR transrepression activities of conformationally restricted carba-T0901317 analogs. Heterocycles 2008, 76, 137–142. [Google Scholar]
- Heim M.; Johnson J.; Boess F.; Bendik I.; Weber P.; Hunziker W.; Fluhmann B. Phytanic acid, a natural peroxisome proliferator-activated receptor (PPAR) agonist, regulates glucose metabolism in rat primary hepatocytes. FASEB J. 2002, 16, 718–720. [DOI] [PubMed] [Google Scholar]
- Kim S. Y.; Kim H. I.; Kim T. H.; Im S. S.; Park S. K.; Lee I. K.; Kim K. S.; Ahn Y. H. SREBP-1c mediates the insulin-dependent hepatic glucokinase expression. J. Biol. Chem. 2004, 279, 30823–30829. [DOI] [PubMed] [Google Scholar]
- Miyazaki M.; Dobrzyn A.; Man W. C.; Chu K.; Sampath H.; Kim H. J.; Ntambi J. M. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J. Biol. Chem. 2004, 279, 25164–25171. [DOI] [PubMed] [Google Scholar]
- Horton J. D.; Goldstein J. L.; Brown M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002, 109, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awazawa M.; Ueki K.; Inabe K.; Yamauchi T.; Kaneko K.; Okazaki Y.; Bardeesy N.; Ohnishi S.; Nagai R.; Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem. Biophys. Res. Commun. 2009, 382, 51–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.