Abstract
Herein, the synthesis of novel hydrophobic and hydrophilic cobinamides via aminolysis of vitamin B12 derivatives that activate soluble guanyl cyclase (sGC) is presented. Unlike other sGC regulators, they target the catalytic domain of sGC and show higher activity than (CN)2Cbi.
Keywords: vitamin B12, cobinamide, aminolysis, soluble guanyl cyclase
Over the past decade, there has been much interest in NO-independent soluble guanyl cyclase (sGC) activators.1,2 sGC plays an important role in cardiovascular homeostasis, platelet function, angiogenesis, and neurotransmission. It operates via activation by nitric oxide (NO), which binds to the sGC heme moiety. This induces the conversion of guanosine triphosphate (GTP) into a second messenger cyclic guanosine monophosphate (cGMP), resulting in physiological effects.3,4 Currently, pharmacological regulation of sGC is achieved via the use of various NO releasing organic nitrates or nitrovasodilators such as nitroglycerin, isosorbide dinitrate, and isosorbide-5-mononitrate. NO-releasing activators, although effective, are known to rapidly induce tolerance and have side-effects. Therefore, the next logical step was to explore alternative methods of sGC regulation. 3-[5-Hydroxymethyl-2-furyl]-1-benzylindazole (YC-1) was the first identified NO-independent sGC regulator whose mechanism of action requires the presence of heme.5 The discovery of YC-1 was followed by other regulators with the same mechanism of action, some with similar structural features, while others had a different structure.6−8 Later, several heme replacing sGC activating compounds were shown to be effective regulators of sGC both in vitro and in vivo. Despite their differences, all of these regulators function by targeting the regulatory domain of sGC.
Recently, we have reported that dicyanocobinamide (CN)2Cbi (Figure 1) is a novel NO-independent sGC activator with a unique mechanism of action.9 Unlike other sGC regulators, (CN)2Cbi directly targets the catalytic domain of sGC, responsible for cGMP synthesis. Moreover, it was found that (CN)2Cbi exhibits a much higher activity than vitamin B12 itself and its cofactors adenosylcobalamine and methylcobalamine.
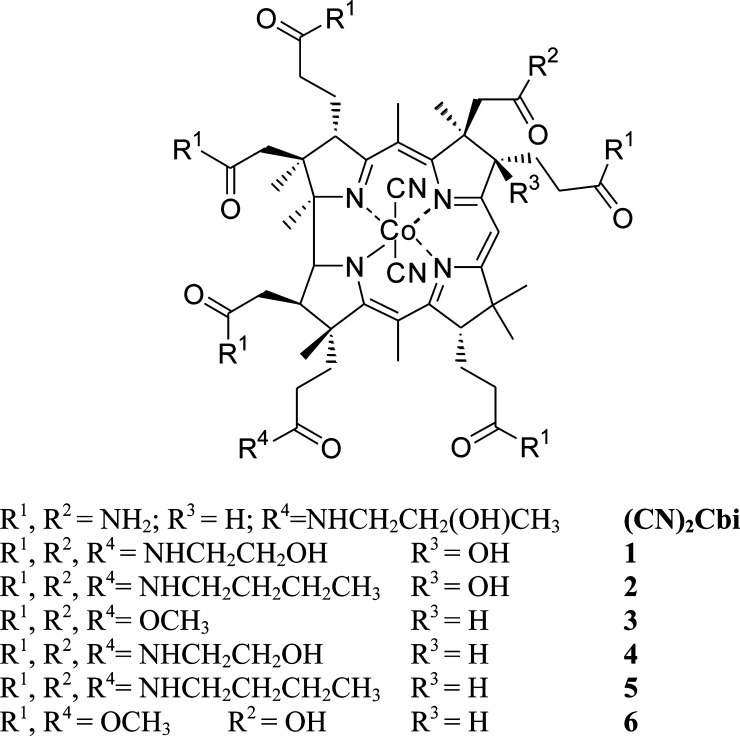
Figure 1.
Hydrophobic and hydrophilic cobinamides.
The unique activation mechanism and properties of (CN)2Cbi highlight the importance of developing new cobinamide derivatives to enhance sGC-targeting therapeutics. (CN)2Cbi possesses seven amide groups at the periphery of the macrocycle and the central cobalt bearing two axial cyano ligands. In our previous report, we suggested that changes in sGC-activating properties of (CN)2Cbi may be altered with introduction of different peripheral substitutents around the corrin macrocycle.9 Unfortunately, there are no reports detailing the chemical synthesis of such compounds. Herein, we disclose the synthesis of new hydrophilic and hydrophobic cobinamides (Figure 1) by the aminolysis of vitamin B12 derivatives and their biological properties as potential NO-independent sGC activators.
In our previous report, the selective synthesis of mono- and diamides from c-lactone 7 was detailed.10 It was found that prolongation of reactions with ethanolamine, especially in toluene and carbon tetrachloride (CCl4), gave polar byproduct consisting of tri- and tetra-amides. Högberg's and Montalbetti's groups reported that cyanide and azide facilitated the aminolysis of simple esters.11,12 Therefore, we envisaged that the synthesis of hydrophilic cobinamides could be accomplished using c-lactone 7 (Scheme 1).
Scheme 1.
The reaction of c-lactone 7 with ethanolamine was conducted in the presence of tetrabutylammonium cyanide (Bu4N+CN–) in CCl4 and afforded cobinamide 1 in 61% yield (Table 1, entry 1). Interestingly, also using NaN3 as a catalyst, regardless of the solvent, gave cobinamide 1 in good yields (Table 1, entries 3 and 4).
Table 1. Optimization of Hydrophilic Cobinamide 1 from 7a.
| entry | solvent | catalyst | time (h) | yield (%) of 1 |
|---|---|---|---|---|
| 1 | CCl4 | Bu4N+CN– | 24 | 61 |
| 2 | toluene | Bu4N+CN– | 24 | – |
| 3 | CCl4 | NaN3 | 24 | 46 |
| 4 | toluene | NaN3 | 24 | 54 |
Reactions were carried out using 15 mg (1.4 μmol) of 7 in solvent (1.0 mL) with ethanolamine (0.1 mL) at 50 °C.
Subsequently, the developed conditions were used for the synthesis of hydrophobic cobinamides, in which ethanolamine was replaced with n-butylamine. Because of the low reactivity of n-butylamine observed in c-lactone ring-opening reactions, the concentration of the amine was increased 10-fold.10 Unfortunately, the reaction did not yield desired cobinamide 2. However, short optimization studies showed that replacing the solvent with CCl4 and employing either NaCN or NaN3 dramatically changed the outcome of the reaction, generating cobinamide 2 in good yields (Table 2, entries 1 and 2). Interestingly, reactions in toluene formed a mixture of compounds, in which no evidence of desired cobinamide 2 was found.
Table 2. Optimization of Hydrophobic Cobinamide 2 from 7a.
| entry | solvent | catalyst | time (h) | yield (%) of 2 |
|---|---|---|---|---|
| 1 | CCl4 | KCN | 72 | 56 |
| 2 | CCl4 | NaN3 | 72 | 62 |
| 3 | toluene | KCN | 72 | – |
| 4 | toluene | NaN3 | 72 | – |
Reactions were carried out using 15 mg (1.4 μmol) of 7 in solvent (1.0 mL) with n-butylamine (1.0 mL) at 50 °C.
Both hydrophilic and hydrophobic cobinamides possessed the C8-hydroxyl group, originating from c-lactone ring opening. Its presence could possibly cause lactone ring closure to occur under acidic conditions.13 Therefore, using heptamethylester 3 as a starting material, more robust cobinamides might be synthesized. Moreover, this would not only allow comparison between hydrophilic and hydrophobic cobinamides but also examination of the influence of the hydroxyl group on sGC activation.
When the synthesis of hydrophilic cobinamide 4 was first attempted using conditions described above, a complex mixture of products was observed, indicating a sluggish reaction. However, once again, the choice of solvent turned out to be crucial; employing toluene instead of CCl4 with an increase in the amine concentration led to cobinamide 4 in 67% yield.
Unfortunately, the synthesis of hydrophobic cobinamide 5, using ester 3, was much more problematic. The conditions used for cobinamide 2, as well as for other cobinamides, did not work for the synthesis of cobinamide 5 (see the Supporting Information for a full optimization study). Under close scrutiny, it was found that the major products in these reactions were hexa-amides, indicating that there are differences in the reactivity of the peripheral methyl esters, even though they seem almost identical. By comparing the reactions of amine with c-lactone 7 and heptamethylester 3, it was envisaged that the c-position is somehow less reactive. Therefore, c-acid 6(14) was utilized as a starting material.
c-Acid 6 was first coupled with n-butylamine, giving C7-amide 8 in 89% yield.13 Then, it was reacted with n-butylamine in the presence of NaN3 in CCl4, giving a hydrophobic cobinamide. According to the MS analysis, cobinamide 2 was isolated instead of compound 5 (Scheme 2), but the exact position of the hydroxyl group is unclear. This unusual hydroxylation was only observed in hydrophobic cobinamide synthesis and is now under investigation. To omit hydroxylation processes, a new approach was undertaken (Scheme 3).
Scheme 2.
Scheme 3.
It was reported that the presence of the C8-hydroxyl group could cause c-lactone formation under acidic conditions.13 Therefore, c-lactone 9 was formed from cobinamide 2 followed by its reduction to c-acid 10. Subsequently, it was coupled with n-butylamine, leading to desired hydrophobic cobinamide 5 in 62% yield.
To evaluate sGC-activating properties of these compounds, we tested how they affect the cGMP-forming activity of the purified enzyme (Figure 2). Consistent with our previous report, dicyanocobinamide displayed a dose-dependent stimulation of sGC with an estimated 8.1-fold maximal activation. We also observed that at higher dose sGC activation reaches a plateau, indicating a 64 μM half maximal effective concentration (EC50).
Figure 2.
Activation of sGC by cobinamide derivatives. A 0.5 μg amount of sGC purified as described previously15 was incubated for 10 min with indicated concentrations of cobinamides, and the cGMP-forming activity was measured as reported previously.9 The symbols are the means ± SDs from three independent measurements performed in triplicate (n = 9), while the solid lines are the curve fits obtained using the GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
Although all newly synthesized corrinoids retained their ability to stimulate sGC, they differed in their sGC activiation properties and displayed different EC50 values. Changing the peripheral groups to ethanolamides resulted in cobinamide derivative with stronger activation potency. Hydrophilic cobinamide 1, bearing the C8-hydroxyl group, displayed a lower EC50 (25 μM) and a stronger (9.8-fold) maximal activation of sGC. Cobinamide 4, lacking the C8-hydroxyl group, was even a more potent sGC activator at higher concentrations (11.3-fold activation) but had a higher EC50 (92 μM). Furthermore, hydrophobic cobinamide 5, lacking the C8-hydrolxyl group, displayed a more modest potency (6.5-fold activation) but had an improved EC50 (32 μM). It should be noted that sGC stimulation diminished at higher concentrations of 5 (Figure 2, solid triangles). Such a bell-shaped dose–response curve strongly suggests that cobinamide 5 is capable of binding to an additional inhibitory site with an apparent IC50 ∼ 356 μM. Finally, cobinamide 2, bearing the C8-hydroxyl group, also displayed a diminished potency (5.4-fold activation), although its EC50 (∼69 μM) was not substantially different from (CN)2Cbi.
In summary, we have described a novel synthesis of a small library of unknown dicyanocobinamide derivatives via efficient aminolysis of terminal methyl ester groups on vitamin B12 derivatives 3 and 7. These new derivatives confirmed our previous hypothesis that modifications in the periphery of the corrin macrocycle of cobinamide would modify the sGC activating potency of the compounds. Biochemical studies demonstrated that the introduction of hydrophilic moieties improves maximal sGC activation. Moreover, the bell-shaped dose–response curve for one derivative reveals that sGC has an additional corrinoid binding site, which is recognized at higher concentrations and may have an inhibitory function. Our detailed studies into new cobinamide sGC activators is ongoing, the results of which will be reported in due course.
Supporting Information Available
Experimental procedures and characterization of all new cobainamides and optimization table for the attempted synthesis of cobinamide 5 from cobalamin 3. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
All authors contributed equally.
This work was supported by the European Regional Development Found with the TEAM program, Grant No. TEAM/2009-3/4 (D.G.) and U.S. Public Health Service Grant HL088128 (E.M.).
The authors declare no competing financial interest.
Supplementary Material
References
- Stasch J. P.; Hobbs A. J. NO-Independent, Haem-Dependent Soluble Guanylate Cyclase Stimulators. Handb. Exp. Pharmacol. 2009, 191, 277–308. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H. H. W.; Schmidt P. M.; Stasch J. P. NO- and Haem-Independent Soluble Guanylate Cyclase Activators. Handb. Exp. Pharmacol. 2009, 191, 309–339. [DOI] [PubMed] [Google Scholar]
- Ignarro L. Nitric Oxide: A Unique Endogenous Signaling Molecule in Vascular Biology. J. Biosci. Rep. 1999, 19, 51–71. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A.; Barry B. K.; McNamara D. B.; Grutter D. Y.; Kadowitz P. J.; Ignarro L. Relaxation of Bovine Coronary Artery and Activation of Coronary Arterial Guanylate Cyclase by Nitric Oxide, Nitroprusside and a Carcinogenic Nitrosoamine. J. Cyclic Nucleotide Res. 1979, 5, 211–224. [PubMed] [Google Scholar]
- Wu C. C.; Ko F. N.; Kuo S. C.; Lee F. Y.; Teng C. M. YC-I Inhibited Human Platelet Aggregation Through NO-Independent Activation of Soluble Guanylate Cyclase. Br. J. Pharmacol. 1995, 116, 1973–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko F. N.; Wu C. C.; Kuo S. C.; Lee F. Y.; Teng C. M. YC-1, a Novel Activator of Platelet Guanylate Cyclase. Blood 1994, 84, 4226–4233. [PubMed] [Google Scholar]
- Becker E. M.; Alonso-Alija C.; Apeler H.; Gerzer R.; Minuth T.; Pleiß U.; Schmidt P.; Schramm M.; Schröder H.; Schroeder W.; Steinke W.; Straub A.; Stasch J. P. NO-Independent Regulatory Site of Direct sGC Stimulators like YC-1 and BAY 41–2272. BMC Pharmacol. 2001, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerrigter G.; Burnett J. C. Nitric Oxide-Independent Stimulation of Soluble Guanylate Cyclase with BAY 41–2272 in Cardiovascular Disease. Cardiovasc. Drug Rev. 2007, 25, 30–45. [DOI] [PubMed] [Google Scholar]
- Sharina I.; Sobolevsky M.; Doursout M. F.; Gryko D.; Martin E. Cobinamides are Novel Coactivators of Nitric Oxide Receptor that Target Soluble Guanylyl Cyclase Catalytic Domain. J. Pharmacol. Exp. Ther. 2012, 340, 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ó Proinsias K.; Sessler J. L.; Kurcoń S.; Gryko D. New Hydrophobic Vitamin B12 Derivatives via Ring-Opening Reactions of c-Lactone. Org. Lett. 2010, 12, 4674–4677. [DOI] [PubMed] [Google Scholar]
- Högberg T.; Ström P.; Ebner M.; Rämsby S. Cyanide as an Efficient and Mild Catalyst in the Aminolysis of Esters. J. Org. Chem. 1987, 52, 3033–3036. [Google Scholar]
- Montalbetti C. A. G. N.; Falque V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar]
- ó Proinsias K.; Giedyk M.; Loska R.; Chromiński M.; Gryko D. Selective Modifications of Hydrophobic Vitamin B12 Derivatives at c-and d-Positions. J. Org. Chem. 2011, 76, 6806–6812. [DOI] [PubMed] [Google Scholar]
- Pfammatter M. J.; Darbre T.; Keese R. Synthesis of Vitamin-B12 Derivatives with Peripheral Tris(oxyethylene) Chains. Helv. Chim. Acta 1998, 81, 1105–1116. [Google Scholar]
- Martin E.; Berka V.; Tsai A. L.; Murad F. Soluble Guanylyl Cyclase: The Nitric Oxide Receptor. Methods Enzymol. 2005, 396, 478–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.