Abstract
Thirty-two diverse compounds were evaluated for their ability to inhibit both Pgp-mediated efflux in mouse T-lymphoma L5178 MDR1 and NorA-mediated efflux in S. aureus SA-1199B. Only four compounds were strong inhibitors of both efflux pumps. Three compounds were found to inhibit Pgp exclusively and strongly, while seven compounds inhibited only NorA. These results demonstrate that Pgp and NorA inhibitors do not necessarily overlap, opening the way to safer therapeutic use of effective NorA inhibitors.
Keywords: Multiple drug resistance, NorA, P-glycoprotein, promiscuous activity, efflux pump inhibitor, S. aureus
Human P-glycoprotein (Pgp), also known as ABCB1 or MDR1, is one of the most studied human transporters, since it influences the metabolism and the distribution of a high number of marketed drugs in gut, liver, brain, heart, and many other tissues.1 The S. aureus efflux pump NorA is a bacterial membrane transport protein which is of great clinical importance, since it is known to play a major role in the development of resistance to the fluoroquinolone drugs.2 Both these membrane transporters reduce the concentration of a number of structurally diverse and apparently unrelated xenobiotics, including drugs, from inside their host cells without alteration or degradation.3,4 However, they differ in their mechanism, since they belong to different protein families: Pgp is an ATP Binding Cassette (ABC) type pump and utilizes the energy of ATP hydrolysis directly, while NorA is a Major Facilitator Superfamily (MFS) type pump and utilizes the H+ gradient for active efflux.5,6
While Pgp inhibition is generally considered to be an unwanted effect, in oncology it is a long sought-after goal, since multidrug resistance (MDR) in cancer cells is often associated with Pgp overexpression.7,8 However, due to the key role played in the elimination and distribution of its substrates, Pgp inhibition is generally an unwanted property for therapeutics not employed in the oncologic field, since it might alter the pharmacokinetics parameters of coadministered drugs (for example transporter–enzyme interplay).9 NorA is responsible for the phenomenon of MDR in some pathogenic strains S. aureus and is not considered to be an antitarget. Its inhibition is potentially beneficial, since when certain antimicrobials, including for example most fluoroquinolones, are being used as antibacterials against pump-related resistant strains, the inhibition of NorA by efflux pump inhibitors (EPIs) may restore the original efficacy of the compounds, unless some other resistance mechanism is also present.10,11
Recent studies have revealed four compounds which inhibit both efflux pumps: biricodar and timcodar,12 elacridar13 and tariquidar.14 Few other compounds are known to inhibit both pumps, such as reserpine (1) and verapamil.15 This study takes into consideration both pumps together in order to investigate whether the activities of Pgp and NorA are correlated or not. Results presented here show that most of the recently discovered novel NorA inhibitors do not significantly inhibit the human Pgp pump at a concentration of 10–4 M. Furthermore, few compounds have been shown to inhibit Pgp activity while being noninhibitors of the NorA efflux pump. In conclusion, results show that in a significant number of cases these promiscuous targets do not necessarily share common inhibitors. This supports the investigation and development of effective NorA inhibitors which are nontoxic to humans.
Our group has been involved in both NorA16,17 and Pgp18 in silico modeling. The entire set of compounds in the NorA data set have been projected into the Pgp in silico model,18 and a number of compounds for which NorA inhibitory activity is already available have been selected and tested for their activity against Pgp. Similarly, the entire Pgp data set was virtually screened using the NorA in silico model, and a number of compounds have been selected and tested for their NorA inhibitory activity. This preliminary analysis guaranteed an optimal selection of compounds for the experimental study of the selectivity between the pumps. Five compounds which were untested in both experiments were also acquired in order to balance the data set.
A total of 32 compounds are presented here (Table 1): 21 compounds for which NorA inhibition experimental data were available which were tested for Pgp inhibition, six compounds for which Pgp inhibition experimental data were available which were tested for NorA inhibition, and five compounds which were tested in both experiments. The latter set of compounds is composed entirely of marketed or previously marketed drugs: amlodipine (2), astemizole (3), dipyridamole (4), loperamide (5), and quinidine (6).
Table 1. Inhibition of the NorA-Mediated Efflux of EtBr in SA-1199B Cells and of the Pgp-Mediated Cell Efflux of R123 in Mouse T Lymphoma L5178 MDR1 Cells.
| Cpd IDa | common name | % inhibition of EtBr efflux | % inhibition of R123 efflux |
|---|---|---|---|
| 1b | reserpine | 84.8c | N.A. |
| 2 | amlodipine | 60.6 | 52.8d |
| 3 | astemizole | 80.6 | 12.8d |
| 4 | dipyridamole | 42.3 | 4.7e |
| 5 | loperamide | 77.7 | 4.5d |
| 6 | quinidine | 31.5 | 21.1e |
| 7 | aripiprazole | 92.3 | 99e,f |
| 8 | ebastine | 88.3 | 99e,f |
| 9 | sertindole | 81.8 | 74e,f |
| 10 | ziprasidone | 86.6 | 19e,f |
| 11 | aprindine | 51.6 | 16e,f |
| 12 | repaglinide | 14.0 | 35e,f |
| 13g | cyclosporine A | N.A. | 92e,f |
| 14h | alprenolol | N.A. | 5.0e,f |
| 15 | 22.8i | 44.1e | |
| 16 | 23.6i | 64.7e | |
| 17 | 33.9j | 1.9e | |
| 18 | 1.7j | 2.6d | |
| 19 | 4.4j | 3.2e | |
| 20 | 91.2k | 40.6e | |
| 21 | 19.7f | 16.8e | |
| 22 | 88.2c | 4.3e | |
| 23 | 88.4c | 1.6e | |
| 24 | 0c | 2.3e | |
| 25 | 30.6c | 21.7e | |
| 26 | 86.6c | 7.8d | |
| 27 | 0c | 2.2e | |
| 28 | 2.8c | 2.1e | |
| 29 | 71.7c | 0.6d | |
| 30 | 41.8c | 2.6e | |
| 31 | 41.3c | 20.4e | |
| 32 | 81.0c | 0.7d | |
| 33 | 15.5c | 2.6e | |
| 34 | 74.7c | 1.7d | |
| 35 | 0c | 11.3e |
Structures for compounds 1–35, IUPAC names for compounds 15–35, and SMILES are given as Supporting Information.
Reserpine was used as a positive control for EtBr efflux inhibition.
From ref (16).
Tested at 10–5 M concentration.
Tested at 10–4 M concentration.
From ref (18).
Cyclosporine A was used as a positive control for R123 efflux inhibition.
Alprenolol was used as a negative control for R123 efflux inhibition.
From ref (21).
From ref (22).
From ref (17).
Eleven compounds were evaluated for their ability to inhibit the efflux of ethidium bromide (EtBr). Tests were performed at a concentration of 50 μM against SA-1199B using 1 as a positive control. The SA-1199B strain contains a point mutation in grlA (topoisomerase IV A subunit gene) resulting in an amino acid substitution in GrlA (A116E), and it also overexpresses the NorA efflux pump (norA++) by way of a promoter up-mutation.19,20
Compounds 3, 5, aripiprazole (7), ebastine (8), sertindole (9), and ziprasidone (10) demonstrate an inhibition of EtBr efflux in the SA-1199B strain of >70%, which is comparable to that of the reference compound, 1 (≥80%) (Table 1). These six compounds were tested for their intrinsic antibacterial activity. Only 9 displayed weak intrinsic antibacterial activity (25 μg/mL, Table 2). Aprindine (11) and repaglinide (12) did not inhibit EtBr efflux.
Table 2. Minimum Inhibitory Concentration (MIC) of the Compounds That Have an EtBr Efflux Inhibition >70% against the S. aureus Strain SA-1199B.
| compd | common name | MIC (μg/mL) |
|---|---|---|
| 3 | astemizole | 100 |
| 5 | loperamide | >100 |
| 7 | aripiprazole | >100 |
| 8 | ebastine | >100 |
| 9 | sertindole | 25 |
| 10 | ziprasidone | >100 |
Twenty-seven compounds were subjected to Pgp inhibition experiments, carried out by measuring the ability of these compounds to inhibit Pgp-mediated cell efflux of rhodamine 123 (R123) in mouse T lymphoma L5178 MDR1 cells. Cyclosporine A (13) was used as a positive control, and alprenolol (14) was used as a negative control.
As can be seen in Table 1, most NorA inhibitors were not effective Pgp inhibitors. In particular, compounds 5, 22, 23, 26, 29, 32, and 34 are inhibitors of NorA, but not Pgp, while compounds 4, 17, 18, 19, 24, 27, 28, 30, and 33 are clearly inhibitors of neither NorA nor Pgp efflux. Compounds 12, 15, and 16, and to a lesser extent compounds 6, 21, 25, 31, and 35, are inhibitors of Pgp but not NorA. Only compounds 7, 8, 9, and 20, and to a lesser extent compounds 2, 3, 10, and 11, are inhibitors of both NorA and Pgp efflux.
Of the 12 marketed drugs tested, 6 (quinidine) is known in the literature to be one of the least potent drugs reported to affect the pharmacokinetics of other coadministered drugs by inhibiting Pgp activity.23 This is also confirmed in a list of clinically relevant drug–drug interactions due to the alteration of transporters’ activity which has recently been made available by the FDA.24 Hence, we used the percent of inhibition of Pgp mediated R123 efflux caused by 100 μM of compound 6 as a threshold for defining Pgp inhibitors and noninhibitors. This value was 21.1%. The threshold for NorA inhibition was set at 70%, while molecules with a percentage inhibition <50% were considered to be noninhibitors, as in previous publications.16,17,21
At least three compounds which were measured to be NorA inhibitors do not inhibit Pgp. Six other compounds tested showed similar results but cannot be directly compared to compound 6, since they were tested at lower concentration due to solubility problems. These results seem to be mostly comparable to those obtained by others.25,26 In fact, compounds 2 and 6 are known effective Pgp inhibitors. Compounds 3 and 5, that at 100 μM are better Pgp inhibitors than compound 6, were less potent in this study due to the lower concentration at which they were tested (10 vs 100 μM).27 Compound 4, that was previously reported to inhibit over 90% of Pgp mediated calcein-AM efflux,25 did not affect R123 efflux from L5173 MDR1 cells. However, a significant degree of variability in the IC50 values depending on the different probe substrates used (generally within 5-fold, in few cases over 10-fold) has already been observed by Rautio et al.23 In this case, the use of different cell lines adds a further variable.
Several fluoroquinolones used to treat bacterial infections are known in vivo Pgp substrates. By inactivating Pgp in mice, several authors showed a marked influence of Pgp mediated transport in sparfloxacin and grepafloxacin brain penetration and in grepafloxacin heart disposition and renal excretion.28,29 In particular, Sasabe and co-workers suggested a possible involvement of Pgp inactivation in grepafloxacin cardiac side effects.28
Pgp is also known to share a large number of inhibitors with CYP3A4, and this lack of selectivity is a major problem in the research of effective human chemosensitizers.30 The results presented here show that this sort of promiscuity does not necessarily exist between Pgp and NorA and, hence, does not bar the development of clinically useful NorA inhibitors.
A simple qualitative analysis of the compounds was carried out by means of multivariate statistics (Principal Component Analysis, PCA) on VolSurf+31,32 molecular descriptors in order to obtain clear indications for the design of safer NorA inhibitor compounds. Only the compounds with a marked inhibition of either or both pumps and the compounds which were clearly noninhibitors of both pumps were used in the analysis. This study revealed the following:
-
(1)
A significant apolarity (hydrophobicity) is necessary to guarantee affinity with both pumps, since the compounds inhibiting both pumps are markedly more hydrophobic.
-
(2)
The “size” of the molecule is determinant in the distinction between noninhibitors (smaller-size) and strong inhibitors (larger-size) of the two proteins. Interestingly, medium-size compounds are able to inhibit only NorA.
-
(3)
A previous analysis18 concluded that at least one polar feature (HB accepting group) is necessary for binding with Pgp. Here we confirm this hypothesis for both the proteins. The only compound with the “right” size and apolarity that occurs in this study among the inhibitors of both pumps is compound 27, which lacks polar groups. Contrarily, too many polar groups make the molecule unable to interact with the pumps, most likely because of a low permeability (for example, compound 4).
-
(4)
A significant “polar capacity”,31,32 namely the ratio of polar volumes with respect to overall molecular volume, can play a critical role in the selectivity in favor of NorA. In particular, acid groups are present in compounds 29, 32, and 34. These observations are highlighted in Figure 1.
Figure 1.
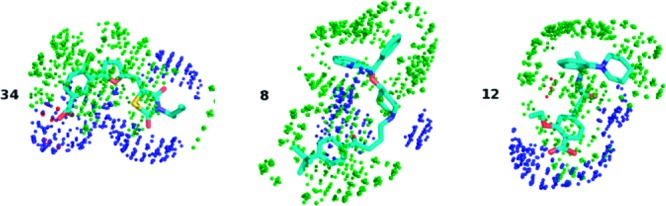
Compound 34 is an inhibitor of NorA, but not Pgp. Compound 12 is an inhibitor of Pgp, but not NorA. Compound 8 is an inhibitor of both NorA and Pgp. The green dots represent the GRID DRY field at −0.5 kcal mol–1, and the blue dots represent the N1 field at −3.5 kcal mol–1. The O field is not visible at −3.5 kcal mol–1. The polar capacity values (CW5, energy = −3.0 kcal mol–1) are 0.24 for compound 34, 0.07 for compound 8, and 0.12 for compound 12. The capacity values at the same energy level for the entire data set and, for comparison, for the compounds biricodar, elacridar, tariquidar, and timcodar are given in the Supporting Information.
Compound 34, which is an inhibitor of NorA but not Pgp, has a more pronounced GRID N1 field (blue dots) than the other two molecules. This highlights the importance of a hydrophilic hydrogen-bond acceptor atom in the inhibitor molecules. In all three cases, the DRY field (green dots) is relatively strong, while the O field (red dots) is practically not present.
In conclusion, the design of selective inhibitors of the NorA bacterial efflux pump should focus on medium-size molecules with a marked polar surface area and, preferably but not necessarily, a benzoic acid group.
Glossary
Abbreviations
- Pgp
P-glycoprotein, also known as ABCB1 and MDR1
- ABC
ATP-binding cassette
- MFS
major facilitator superfamily
- MDR
multidrug resistance
- EPI
efflux pump inhibitor
- EtBr
ethidium bromide
- R123
Rhodamine 123
- MIC
minimum inhibitory concentration
- FDA
Food and Drug Administration
- CYP3A4
cytochrome P450 3A4
- PCA
principal components analysis
Supporting Information Available
Compound molecular structures, SMILES codes, and polar capacity values; common or IUPAC names; score plot and loading plot of the PCA model; detailed graphical comparison of NorA and Pgp inhibitors; detailed experimental methods; and experimental data demonstrating the purity of the active compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Shugarts S.; Benet L. Z. The Role of Transporters in the Pharmacokinetics of Orally Administered Drugs. Pharm. Res. 2009, 26, 2039–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H.; Bogaki M.; Nakamura S.; Ubukata K.; Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J. Bacteriol. 1990, 172, 6942–6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J. Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 2006, 4, 629–636. [DOI] [PubMed] [Google Scholar]
- Poole K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 162–176. [DOI] [PubMed] [Google Scholar]
- McKeegan K. S.; Borges-Walmsley M. I.; Walmsley A. R. Structural understanding of efflux-mediated drug resistance: potential routes to efflux inhibition. Curr. Opin. Pharmacol. 2004, 4, 479–486. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Ma S. Efflux Pump Inhibitors: A Strategy to Combat P-Glycoprotein and the NorA Multidrug Resistance Pump. ChemMedChem 2010, 5, 811–822. [DOI] [PubMed] [Google Scholar]
- Broccatelli F.; Carosati E.; Cruciani G.; Oprea T. I. Transporter-Mediated Efflux Influences CNS Side Effects: ABCB1, from Antitarget to Target. Mol. Inf. 2010, 29, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser H.; Fromm M. F.; König J.. Transporters and Drugs—An Overview. In Antitargets Prediction and Prevention of Drug Side Effects; Vaz R. J., Klabunde T., Eds.; WILEY-VCH: Weinheim, 2008; pp 341–366. [Google Scholar]
- Cummins C. L.; Jacobsen W.; Benet L. Z. Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. J. Pharmacol. Exp. Ther. 2002, 300, 1036–1045. [DOI] [PubMed] [Google Scholar]
- Lomovskaya O.; Lee A.; Hoshino K.; Ishida H.; Mistry A.; Warren M. S.; Boyer E.; Chamberland S.; Lee V. J. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1999, 43, 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham P. N. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug efflux inhibitor reserpine. Antimicrob. Agents Chemother. 1999, 43, 988–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin S.; Mani N.; Grossman T. H. Inhibition of Antibiotic Efflux in Bacteria by the Novel Multidrug Resistance Inhibitors Biricodar (VX-710) and Timcodar (VX-853). Antimicrob. Agents Chemother. 2004, 48, 4171–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons S.; Oluwatuyi M.; Kaatz G. W. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. J. Antimicrob. Chemother. 2003, 51, 13–17. [DOI] [PubMed] [Google Scholar]
- Leitner I.; Nemeth J.; Feurstein T.; Abrahim A.; Matzneller P.; Lagler H.; Erker T.; Langer O.; Zeitlinger M. The third-generation P-glycoprotein inhibitor tariquidar may overcome bacterial multidrug resistance by increasing intracellular drug concentration. J. Antimicrob. Chemother. 2011, 66, 834–839. [DOI] [PubMed] [Google Scholar]
- Aeschlimann J. R.; Dresser L. D.; Kaatz G. W.; Rybak M. J. Effects of NorA Inhibitors on In Vitro Antibacterial Activities and Postantibiotic Effects of Levofloxacin, Ciprofloxacin, and Norfloxacin in Genetically Related Strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 1999, 43, 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincat J. P.; Carosati E.; Sabatini S.; Manfroni G.; Fravolini A.; Raygada J. L.; Patel D.; Kaatz G. W.; Cruciani G. Discovery of Novel Inhibitors of the NorA Multidrug Transporter of Staphylococcus aureus. J. Med. Chem. 2011, 54, 354–365. [DOI] [PubMed] [Google Scholar]
- Pieroni M.; Dimovska M.; Brincat J. P.; Sabatini S.; Carosati E.; Massari S.; Kaatz G. W.; Fravolini A. From 6-Aminoquinolone Antibacterials to 6-Amino-7-thiopyranopyridinylquinolone Ethyl Esters as Inhibitors of Staphylococcus aureus Multidrug Efflux Pumps. J. Med. Chem. 2010, 53, 4466–4480. [DOI] [PubMed] [Google Scholar]
- Broccatelli F.; Carosati E.; Neri A.; Frosini M.; Goracci L.; Oprea T. I.; Cruciani G. A Novel Approach for Predicting P-glycoprotein (ABCB1) Inhibition Using Molecular Interaction Fields. J. Med. Chem. 2011, 54, 1740–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W.; Seo S. M. Mechanisms of Fluoroquinolone Resistance in Genetically Related Strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 1997, 41, 2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. T. D.; Kaatz G. W.; Gustafson J. E. The multidrug efflux pump NorA is not required for salicylate-induced reduction in drug accumulation by Staphylococcus aureus. Int. J. Antimicrob. Agents 2002, 20, 206–213. [DOI] [PubMed] [Google Scholar]
- Sabatini S.; Kaatz G. W.; Rossolini G. M.; Brandini D.; Fravolini A. From Phenothiazine to 3-Phenyl-1,4-benzothiazine Derivatives as Inhibitors of the Staphylococcus aureus NorA Multidrug Efflux Pump. J. Med. Chem. 2008, 51, 4321–4330. [DOI] [PubMed] [Google Scholar]
- Sabatini S.; Gosetto F.; Manfroni G.; Tabarrini O.; Kaatz G. W.; Patel D.; Cecchetti V. Evolution from a natural flavones nucleus to obtain 2-(4-Propoxyphenyl)quinoline derivatives as potent inhibitors of the S. aureus NorA efflux pump. J. Med. Chem. 2011, 54, 5722–5736. [DOI] [PubMed] [Google Scholar]
- Rautio J.; Humphreys J. E.; Webster L. O.; Balakrishnan A.; Keogh J. P.; Kunta J. R.; Serabjit-Singh C. J.; Polli J. W. In vitro p-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: a recommendation for probe substrates. Drug Metab. Dispos. 2006, 34, 786–792. [DOI] [PubMed] [Google Scholar]
- http://bts.ucsf.edu/fdatransportal/.
- Polli J. W.; Wring S. A.; Humphreys J. E.; Huang L.; Morgan J. B.; Webster L. O.; Serabjit-Singh C. S. Rational use of in vitro P-glycoprotein assays in drug discovery. J. Pharmacol. Exp. Ther. 2001, 299, 620–628. [PubMed] [Google Scholar]
- Katoh M.; Nakajima M.; Yamazaki H.; Yokoi T. Inhibitory Potencies of 1,4-dihydropyridine Calcium Antagonists to P-glycoprotein-Mediated Transport: Comparison with the Effects on CYP3A4. Pharm. Res. 2000, 17, 1189–1197. [DOI] [PubMed] [Google Scholar]
- Mahar Doan K. M.; Humphreys J. E.; Webster L. O.; Wring S. A.; Shampine L. J.; Serabjit-Singh C. J.; Adkison K. K.; Polli J. W. Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J. Pharmacol. Exp. Ther. 2002, 303, 1029–1037. [DOI] [PubMed] [Google Scholar]
- Sasabe H.; Kato Y.; Suzuki T.; Itose M.; Miyamoto G.; Sugiyama Y. Differential Involvement of Multidrug Resistance-Associated Protein 1 and P-Glycoprotein in Tissue Distribution and Excretion of Grepafloxacin in Mice. J. Pharmacol. Exp. Ther. 2004, 310, 648–655. [DOI] [PubMed] [Google Scholar]
- de Lange E. C.; Marchand S.; van den Berg D.; van der Sandt I. C.; de Boer A. G.; Delon A.; Bouquet S.; Couet W. In vitro and in vivo investigations on fluoroquinolones; effects of the P-glycoprotein efflux transporter on brain distribution of sparfloxacin. Eur. J. Pharm. Sci. 2000, 12, 85–93. [DOI] [PubMed] [Google Scholar]
- Wandel C.; Kim R. B.; Kajiji S.; Guengerich P.; Wilkinson G. R.; Wood A. J. J. P-Glycoprotein and Cytochrome P-450 3A Inhibition: Dissociation of Inhibitory Potencies. Cancer Res. 1999, 59, 3944–3948. [PubMed] [Google Scholar]
- Volsurf+ is distributed by Molecular Discovery: http://www.moldiscovery.com/soft_vsplus.php.
- Cruciani G.; Pastor M.; Guba W. VolSurf: a new tool for the pharmacokinetic optimization of lead compounds. Eur. J. Pharm. Sci. 2000, 11, 29–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


