Abstract
Calcitonin gene-related peptide (CGRP) receptor antagonists have been clinically shown to be effective in the treatment of migraine, but identification of potent and orally bioavailable compounds has been challenging. Herein, we describe the conceptualization, synthesis, and preclinical characterization of a potent, orally active CGRP receptor antagonist 5 (BMS-846372). Compound 5 has good oral bioavailability in rat, dog, and cynomolgus monkeys and overall attractive preclinical properties including strong (>50% inhibition) exposure-dependent in vivo efficacy in a marmoset migraine model.
Keywords: migraine, CGRP, CGRP receptor, antagonist
Migraine is a chronic debilitating disease, affecting roughly 12% of the U.S. population, with women being at least 2-fold more susceptible than men.1 One early hypothesis, put forward by Wolff, was that migraine pathophysiology was associated with the dilation of cranial blood vessels.2 More contemporary efforts have focused on neural processes and the potential roles of peripheral3 and central sensitization4 in the neurobiology of headache. The triptan class of 5-HT1B/1D agonists is the current standard of care for treating migraines, and they are believed to primarily act by vasoconstriction of cranial vessels with concomitant inhibition of neurotransmitter release from trigeminal afferents.5 However, triptans are associated with a number of unpleasant side effects including chest and neck tightening and are contraindicated in patients with cardiovascular disease and hypertension.6
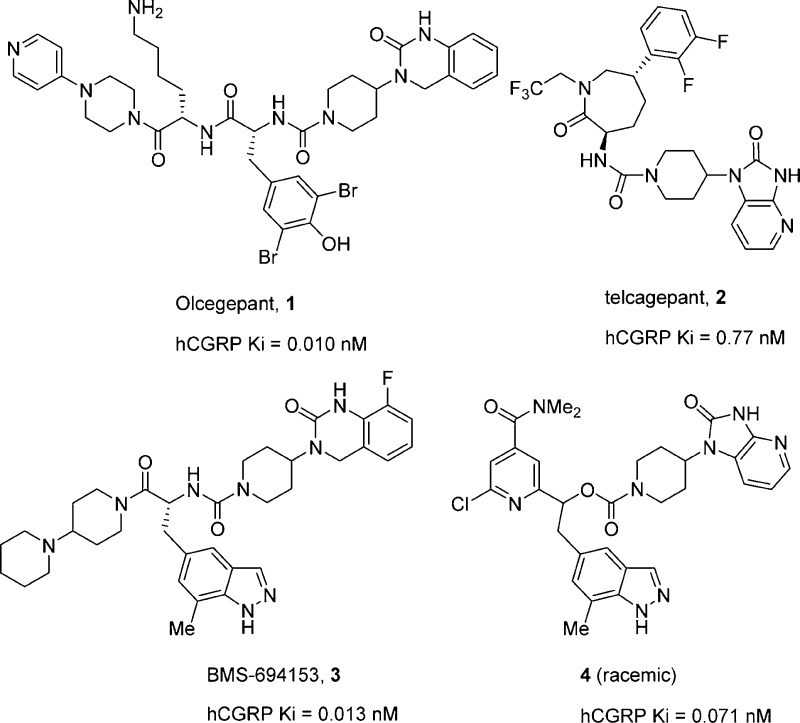
The calcitonin gene-related peptide (CGRP), a 37 amino-acid peptide, is widely distributed in the nervous system.7 CGRP is an extremely potent vasodilator that has been implicated in the pathogenesis of migraine.8−8c Studies have shown that plasma levels of CGRP are elevated during migraine attacks.9,9b The CGRP receptor is composed of a family B G-protein-coupled receptor (GPCR), named the calcitonin receptor-like receptor (CLR), along with a receptor activity modifying protein 1 (RAMP1).10 CGRP receptor antagonists are attractive therapeutic targets for the treatment of migraine, and several have demonstrated clinical efficacy. Intravenous administration of the highly potent CGRP receptor antagonist BIBN4096BS (1, Chart 1) was accompanied by alleviation of pain in migraineurs without the cardiovascular side effects associated with the use of triptans.11 While this compound effectively demonstrated the first clinical proof of concept, its route of administration and chemical instability precluded further development.12 More recently, an oral CGRP receptor antagonist, telcagepant (MK-0974)13 (2, Chart 1), was advanced to phase III trials and demonstrated clinical efficacy but was discontinued possibly due to toxicity issues.14,14b
Chart 1. Selected CGRP Receptor Antagonists.
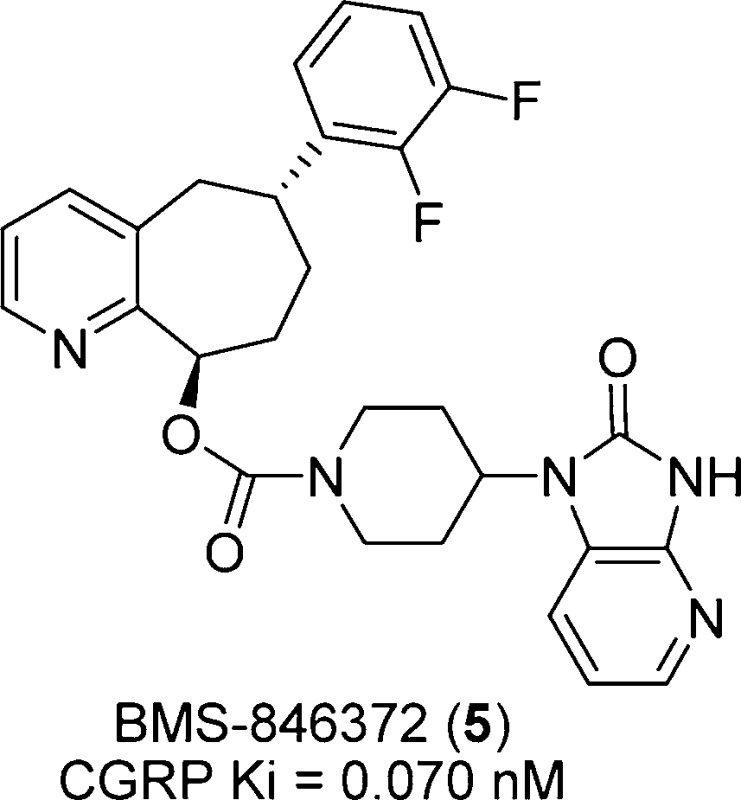
A recent publication from our laboratories disclosed a potent, indazole-based CGRP receptor antagonist (BMS-694153) (3, Chart 1) that showed rapid and efficient intranasal exposure.15 However, BMS-694153 did not have significant oral bioavailability in either a cynomolgus monkey or a rat primarily due to its poor intrinsic cellular permeability. While intranasal delivery is potentially attractive for migraine treatment, another one of our goals was to discover novel, orally active CGRP antagonists. In a recent publication, we disclosed that pyridine served as an efficient mimetic of the secondary amide in BMS-694153, providing antagonists with excellent binding potency and improved bilayer permeability.16 Yet, despite this improvement, significant oral exposure was still difficult to achieve in this series. It proved difficult to incorporate optimal physiochemical and pharmacokinetic properties in a single molecule. However, one pyridine derivative (4, Chart 1) did demonstrate moderate efficacy (31% inhibition) in the marmoset facial blood flow model15 at 10 mg/kg when administered orally. To improve oral exposure, we sought to reduce the number of rotatable bonds and improve metabolic stability in our compounds by constraining the pyridyl-containing core and further reducing polarity by replacing the indazole moiety by a 2,3-difluorophenyl ring. Herein, we describe the synthesis of BMS-846372 (5), a potent CGRP receptor antagonist with good oral bioavailability (Figure 1).
Figure 1.
Structure of BMS-846372.
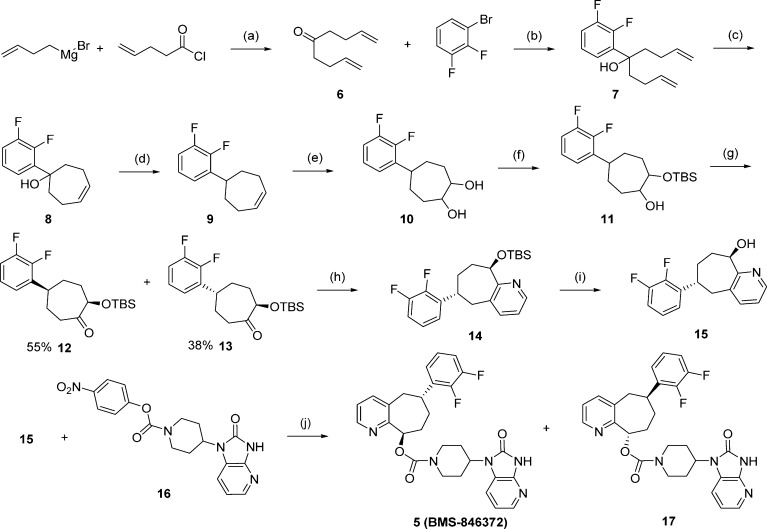
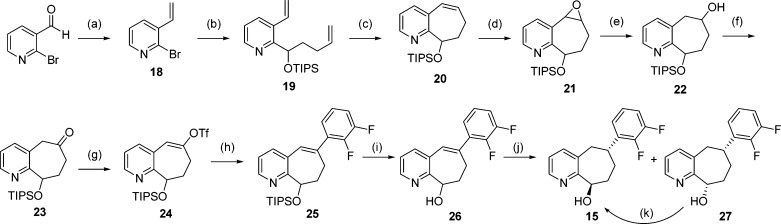
In compound 5, we incorporated an all-carbon 7-membered ring fused to the pyridine to restrict its conformational flexibility. A survey of the literature provided some simple cycloheptapyridine structures, but there were no precedents for our desired substitution pattern, particularly with the incorporation of the two chiral centers. Even without a specific stereogenic synthesis, the core bicyclic ring itself and the trans substitution pattern posed some synthetic challenges. As a potentially general route for exploration of pyridine substitution, our initial attempts focused on the formation of a suitable 7-membered ring on which the pyridine ring could be constructed. As such, the first successful synthetic route to racemic 5 is outlined in Scheme 1 with the formation of a key target intermediate ketone 13. Thus, the dienyl ketone 6 was formed by reaction of commercially available but-3-enylmagnesium bromide and pent-4-enoyl chloride.17 The addition of 2,3-difluorophenyl lithium generated from 1-bromo-2,3-difluorobenzene18,18b afforded alcohol 7 in a 55% yield. The formation of the 7-membered ring 8 was achieved by ring-closure metathesis (RCM) with benzylidene-bis(tricyclohexylphosphine)dichlororuthenium (Grubbs-I) catalyst in 89% yield under high dilution (0.012 M in CH2Cl2).19 Deoxygenation to 9 took place in 88% yield using triethylsilane and trifluoroacetic acid (TFA) in CH2Cl2.20 Dihydroxylation was effected under standard osmium-catalyzed 4-methyl morpholine N-oxide (NMO) conditions21 to give diol 10, followed by tri-tert-butyl-silyl (TBS) protection to afford the monoprotected, inseparable diastereomeric mixture of 11 in 84% yield (two steps). Following Dess-Martin oxidation of the alcohol intermediate 15, the two diastereomers of the ketone intermediate 12 and 13 were separated. The structure of the trans isomer 13 was identified by X-ray crystallography.22 Using literature conditions,23 the formation of the pyridine derivative 14 from the ketone 13 with propargylamine under gold(III) catalysis occurred in 20% yield. Treatment of 14 with tetrabutylammonium fluoride (TBAF) afforded the racemic penultimate intermediate 15 in good yield. The alcohol 15 was coupled with 16(16) to provide racemic 5 in good yield. The final desired product, 5 (BMS-846372), was separated by chiral preparative high-pressure liquid chromatography (HPLC), from its enantiomer 17.24 This 10-step synthesis was used to synthesize the original lots of BMS-846372.
Scheme 1. First Synthesis of 5 (BMS-846372).
Reagents and conditions: (a) THF, −78 °C to rt, 3 h (68%). (b) n-BuLi, THF, −78 °C to rt, 1 h (55%). (c) Grubbs-I, CH2Cl2 (0.012 M), 40 °C, 2 h (89%). (d) Triethylsilane, TFA, CH2Cl2, rt, 3 h (88%). (e) OsO4, NMO, acetone/water, 1 h. (f) TBS-Cl, DMF, rt, 5 h (84% for two steps). (g) Dess-Martin periodinane, CH2Cl2, rt, 17 h (55% of 12 + 38% of 13). (h) Propargylamine, NaAuCl4·2H2O, EtOH, 80 °C, 5 h (20%). (i) TBAF, THF, rt, 19 h (84%). (j) NaH, THF; rt, 18 h (46%), then chiral separation.
The primary issues with our original overall scheme were an early high dilution RCM reaction, the low yields of pyridine from the ketone 13, and the difficulty in controlling both diastereomeric and enantiomeric selectivities. Thus, when gram quantities of 5 were needed for more extensive preclinical evaluations, the route shown in Scheme 1 proved difficult to scale up. Because formation of the pyridine ring (14) in Scheme 1 was a very low yielding step, a more focused approach using commercially available pyridines became more attractive. Accordingly, a second route was developed for the synthesis of the penultimate 15, starting with commercially available 2-bromo-3-pyridinecarboxaldehyde (Scheme 2). A simple Wittig reaction led to the formation of 2-bromo-3-vinylpyridine 18 in good yield.25 Lithium-halogen exchange of 18 followed by addition of penten-4-al and TIPS-Cl afforded the desired diene 19 in 74% yield. While direct RCM with 19 gave no reaction, treatment of the HCl salt26,26b of 19 under standard RCM conditions with the (1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium (Grubbs-II) catalyst gave the desired cycloheptene 20 in 86% yield. Racemic epoxidation of 20 with a racemic Jacobson's catalyst27,27b afforded the epoxide 21 in 70% yield. After hydrogenolysis of the epoxide 21, the resulting alcohol 22 was converted to the ketone 23 by Swern oxidation in good yield. Triflate 24 was formed under standard conditions in 86% yield, and Suzuki coupling with commercially available 2,3-difluorophenylboronic acid also proceeded smoothly to afford 25. Deprotection with TBAF afforded the unsaturated alcohol 26 in 81% yield. Hydrogenation of 26 gave a mixture of the desired alcohol 15 and its diastereomer 27. After separation using flash column chromatography (FCC), 27 was converted to 15 by a Mitsunobu reaction. Multigram quantities of 5 were able to be prepared by this route, again through chiral HPLC separation, which allowed for extensive preclinical evaluation of 5. Compound 5 was crystallized, and its relative stereochemistry was proved by X-ray crystallography.24
Scheme 2. Second Synthesis of BMS-846372 (5).
Reagents and conditions: (a) Methyltriphenylphosponium bromide, n-BuLi, THF, 72 h (89%). (b) n-BuLi, THF, 4-pentenylaldehyde, then TIPS-Cl, −78 °C to rt (74%). (c) HCl; Grubbs-II, CH2Cl2, 40 °C, 4 h (86%). (d) (±)-[1,2-Cyclohexanediamino-N,N′-bis(3,5-di-t-butylsalicylidene)]manganese(III) chloride, NaOCl, Na2HPO4, CH2Cl2 (70%). (e) Pd/C/hydrogen (25 psi), EtOH, rt, 4 h (99%). (f) Oxalyl chloride, DMF (cat.), −50 °C, CH2Cl2, Et3N (49%). (g) LDA, PhNTf2, THF, −78 °C to rt, 18 h (86%). (h) 2,3-Difluorophenylboronic acid, Na2CO3, Pd(PPh3)4, toluene, 110 °C, 1.5 h (65%). (i) TBAF, THF, rt, 1 h (81%). (j) Pd/C/hydrogen (1 atm) (16% 15 and 74% 27). (k) 4-Nitrobenzoic acid, PPh3, DIAD, THF, 0 °C to rt, 5 h; LiOH, rt, 3 h (77%).
Binding affinities for the human CGRP receptor were determined by inhibition of 125I-CGRP binding in SK-N-MC cell membranes as previously disclosed.15 Compound 5 displaced 125I-CGRP with a Ki = 0.070 ± 0.021 nM (n = 13), while its enantiomer 17 had significantly reduced affinity with a Ki = 940 nM (Table 1). Functional receptor antagonism for 5 was determined by measuring inhibition of CGRP-stimulated cAMP production in SK-N-MC cells.15 The compound was shown to be an antagonist with an IC50 = 0.22 ± 0.05 nM (n = 2). BMS-846372 completely inhibited CGRP-mediated elevation of cAMP.
Table 1. In Vitro Data for 5 and Its Enantiomer 17.
| compd | 5 | 17 |
|---|---|---|
| hCGRP Ki (nM) | 0.070 (±0.021) | 940 |
| cAMP IC50 (nM) | 0.22 | |
| PAMPA (pH 5.5/pH 7.4) (nm/s) | 1500/880 |
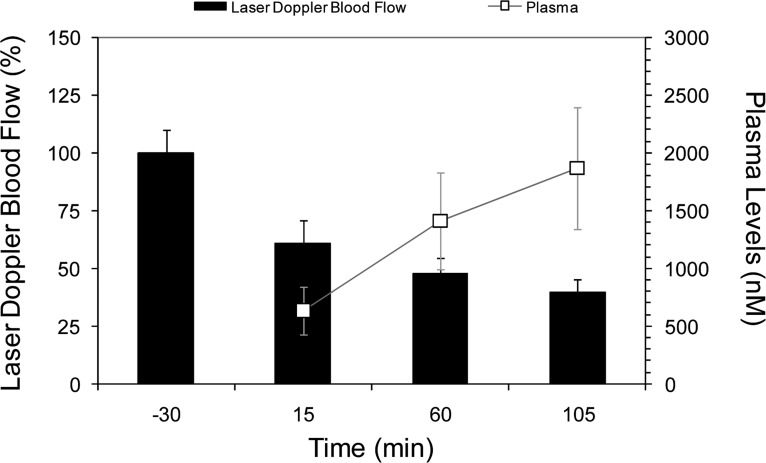
A novel noninvasive marmoset recovery model for in vivo efficacy assessment of CGRP-receptor antagonists was developed in our laboratories, which utilized facial blood flow as a surrogate for intracranial artery diameter, and the dilation of arteries, which characterize a migraine.15 Briefly, marmosets were anesthetized, and facial blood flow was increased by intravenous (iv) administration of hαCGRP (10 μg/kg) at 45 min intervals (−30, 15, 60, and 105 min). The effect of different doses of antagonist 5, delivered SC at 0 min, on the hαCGRP-induced increases in facial blood flow was measured by laser Doppler flowmetry. In this model, compound 5 showed exposure-dependent inhibition of CGRP-induced increases in marmoset facial blood flow upon subcutaneous (sc) dosing (Figure 2).28 As compared to predose vehicle control (−30 min), strong (>50%) inhibition of CGRP-induced effects on facial blood flow was observed with 7 mg/kg of 5 at 60 and 105 min postdose (52–60%) (Figure 2). In comparing exposure versus efficacy with 7 mg/kg of 5 at the 60 and 105 min postdose test times, plasma levels of 5 were above 1000 and 1500 nM, respectively, and both times were associated with strong in vivo efficacy (>50% inhibition).
Figure 2.
Marmoset facial blood flow.
With a 10 mg/kg dose po and 1 mg/kg iv, compound 5 exhibited significant oral bioavailability in the rat (Fpo = 29%), dog (Fpo = 34%), and cynomolgus monkey (Fpo = 38%), certainly in part because of its high bilayer permeability (PAMPA: 880–1500 nm/s, Table 1). Compound 5 is reasonably stable in human liver microsome with 74% remaining after 10 min (0.5 μM) and with a t1/2 = 24 min. In addition, compound 5 at 10 μM showed no significant potential for off-target liabilities in a panel of receptor, ion channel, and enzyme activity assays.
In conclusion, BMS-846372 is a potent CGRP antagonist, with good oral bioavailability, strong exposure-dependent in vivo efficacy, and acceptable off-target liabilities. It is active in vivo and represents an opportunity for human dosing. Additional preclinical studies of BMS-846372 will be reported in due course.
Acknowledgments
We thank Dr. Baoqin Ma for the X-ray analysis of the intermediate 13 and 5 and the members of Discovery Analytical Sciences for their help in the chiral separation and full characterization of 5.
Glossary
Abbreviations
- CGRP
calcitonin gene-related peptide
- GPCR
G-protein-coupled receptor
- CLR
calcitonin receptor-like receptor
- RAMP1
receptor activity modifying protein 1
- THF
tetrahydrofuran
- RCM
ring-closure metathesis
- TFA
trifluoroacetic acid
- NMO
4-methyl morpholine N-oxide
- TBS
tri-tert-butyl-silyl
- TBAF
tetrabutylammonium fluoride
- DIAD
diisopropyl azodicarboxylate
- HPLC
high-pressure liquid chromatography
- TIPS
tri-isopropyl-silyl
- LCMS
liquid chromatography mass spectrometry
- Grubbs-I
benzylidene-bis(tricyclohexylphosphine)dichlororuthenium, bis(tricyclohexylphosphine)benzylidine ruthenium(IV) dichloride
- Grubbs-II
(1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium
- FCC
flash column chromatography
- NMR
nuclear magnetic resonance
- sc
subcutaneous
- po
taken orally
- iv
intravenous
Supporting Information Available
Experimental details and analytical data for the preparation of compounds 6–15 and 17–27 and full characterization of BMS-846372. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Lipton R. B.; Stewart W. F.; Diamond S.; Diamond M. L.; Reed M. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache 2001, 41, 646–657. [DOI] [PubMed] [Google Scholar]
- Graham J. P.; Wolff H. G. Mechanism of migraine headache and the action of ergotamine tartrate. Arch. Neurol. Psychiatry 1938, 39, 737–763. [Google Scholar]
- Olesen J.; Burstein R.; Ashina M.; Tfelt-Hansen P. Origin of pain in migraine: Evidence for peripheral sensitisation. Lancet Neurol. 2009, 8, 679–690. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J; Charbit A. R.; Andreou A. P; Akerman S; Holland P. R. Neurobiology of migraine. Neuroscience 2009, 161, 327–341. [DOI] [PubMed] [Google Scholar]
- Wackenfors A.; Jarvius M.; Ingemansson R.; Edvinsson L.; Malmsjoe M. Triptans induce vasoconstriction of human arteries and veins from the thoracic wall. J. Cardiovasc. Pharmacol. 2005, 45, 476–484. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Lipton R. B.; Ferrari M. D. Migraine—Current understanding and treatment. N. Engl. J. Med. 2002, 346, 257–270. [DOI] [PubMed] [Google Scholar]
- van Rossum D.; Hanisch U. K.; Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci. Biobehav. Rev. 1997, 21, 649–678. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J. Calcitonin gene-related peptide antagonists as treatments of migraine and other primary headaches. Drugs 2005, 65, 2557–2567. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. Blockade of CGRP receptors in the intracranial vasculature: A new target in the treatment of headache. Cephalalgia 2004, 24, 611–622. [DOI] [PubMed] [Google Scholar]
- Williamson D. J.; Hargreaves R. J. Neurogenic inflammation in the context of migraine. Microsc. Res. Tech. 2001, 53, 167–178. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Edvinsson L; Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Edvinsson L. The trigeminovascular system and migraine. Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 1993, 33, 48–56. [DOI] [PubMed] [Google Scholar]
- Recober A.; Russo A. F. Calcitonin gene-related peptide: An update on the biology. Curr. Opin. Neurol. 2009, 22, 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J.; Diener H. C.; Husstedt I. W.; Goadsby P. J.; Hall D.; Meier U.; Pollentier S.; Lesko L. M. Calcitonin gene related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004, 350, 1104–1110. [DOI] [PubMed] [Google Scholar]
- IDDB. Thomson Scientific reports that the development of BIBN4096BS has been discontinued.
- Paone D. V.; Shaw A. W.; Nguyen D. N.; Burgey C. S.; Deng J. Z.; Kane S. A.; Koblan K. S.; Salvatore C. A.; Mosser S. D.; Johnston V. K.; Wong B. K.; Miller-Stein C. M.; Hershey J. C.; Graham S. L.; Vacca J. P.; Williams T. M. Potent, orally bioavailable calcitonin gene-related peptide receptor antagonists for the treatment of migraine: Discovery of N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1- (2,2,2-trifluoroethyl)azepan-3-yl]-4- (2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin- 1-yl)piperidine-1-carboxamide (MK-0974). J. Med. Chem. 2007, 50, 5564–5567. [DOI] [PubMed] [Google Scholar]
- Hewitt D. J.; Martin V.; Lipton R. B.; Brandes J.; Ceesay P.; Gottwald R.; Schaefer E.; Lines C.; Ho T. W. Randomized controlled study of telcagepant plus Ibuprofen or Acetaminophen in migraine. Headache 2011, 51, 533–543. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P. Excellent tolerability but relatively low initial clinical efficacy of telcagepant in migraine headache. Headache 2011, 51, 118–123Merck publicly announced the discontinuation of further development of telcagepant, but no specific reasons were given. [DOI] [PubMed] [Google Scholar]
- Degnan A. P.; Chaturvedula P. V.; Conway C. M.; Cook D.; Davis C. D.; Denton R.; Han X.; Macci R.; Mathias N. R.; Moench P.; Pin S. S.; Ren S. X.; Schartman R.; Signor L.; Thalody G.; Widmann K. A.; Xu C.; Macor J. E.; Dubowchik G. M. Discovery of (R)-4-(8-fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-yl)-N-(3-(7-methyl-1H-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-yl)piperidin-1-yl)propan-2-yl)piperidine-1-carboxamide (BMS-694153): A potent antagonist of the human calcitonin gene- related peptide receptor for migraine with rapid and efficient intranasal exposure. J. Med. Chem. 2008, 51, 4858–4861. [DOI] [PubMed] [Google Scholar]
- Luo G.; Chen L.; Civiello R.; Pin S. S.; Xu C.; Kostich W.; Kelley M.; Conway C. M.; Macor J. E.; Dubowchik G. M.. Calcitonin gene-related peptide (CGRP) receptor antagonists: Pyridine as a replacement for a core amide group. Bioorg. Med. Chem. Lett. 2012, in press. DOI: doi.org/10.1016/j.bmcl.2012.02.065. [DOI] [PubMed] [Google Scholar]
- Thommen M.; Veretenov A. L.; Guidetti-Grept R.; Keese R. The tandem Pauson-Khand reaction. Helv. Chim. Acta 1996, 79, 461–476. [Google Scholar]
- Henry A. A.; Olsen A. G.; Matsuda S.; Yu C.; Geierstanger B. H.; Romesberg F. E. Efforts to expand the genetic alphabet: Identification of a replicable unnatural DNA self-pair. J. Am. Chem. Soc. 2004, 126, 6923–6931. [DOI] [PubMed] [Google Scholar]
- 2,3-Difluorophenyl lithium was also directly generated from 2,3-difluorobenzene:; Gray G. W.; Hird; Lacey D.; Toyne K. J. The synthesis and transition temperatures of some 4,4″-dialkyl- and 4,4″-alkoxyalkyl-1,1′:4′,1″-terphenyls with 2,3- or 2′,3′-difluoro substituents and of their biphenyl analogues. J. Chem. Soc. Perkin Trans. 2: Phys. Org. Chem. 1989, 2041–2054. [Google Scholar]
- Louie J.; Bielawski C. W.; Grubbs R. H. Tandem catalysis: The sequential mediation of olefin metathesis, hydrogenation, and hydrogen transfer with single-component Ru complexes. J. Am. Chem. Soc. 2001, 123, 11312–11313. [DOI] [PubMed] [Google Scholar]
- Semmelhack M. F.; Yamashita A. Arene-metal complexes in organic synthesis. Synthesis of acorenone and acorenone B. J. Am. Chem. Soc. 1980, 102, 5924–5926. [Google Scholar]
- Spiegel D. A.; Njardarson J. T.; Wood. J. L. CP-263,114 Synthetic studies. Construction of an isotwistane ring system via rhodium carbenoid C-H insertion. Tetrahedron 2002, 58, 6545–6554. [Google Scholar]
- See the Supporting Information for details.
- Abbiati G.; Arcadi A.; Bianchi G.; Giuseppe S. D.; Marinelli F.; Rossi E. Sequential amination/annulation/aromatization reaction of carbonyl compounds and propargylamine: A new one-pot approach to functionalized pyridines. J. Org. Chem. 2003, 68, 6959–6966. [DOI] [PubMed] [Google Scholar]
- See the Supporting Information for details. The absolute configuration was eventually proved by chiral synthesis of BMS-846372:Leahy D.; et al. Org. Lett. Manuscript in preparation. [Google Scholar]
- Spivey A. C.; Shukla L.; Hayler J. F. Conjugate addition of 2- and 4-pyridylcuprates: An expeditious asymmetric synthesis of natural (−)-evoninic acid. Org. Lett. 2007, 9, 891–894. [DOI] [PubMed] [Google Scholar]
- Felpin F. -X; Girard S.; Vo-Thanh G.; Robins R. J.; Villiéras J.; Lebreton J.. Efficient enantiomeric synthesis of pyrrolidine and piperidine alkaloids from tobacco. J. Org. Chem. 2001, 66, 6305–6312. [DOI] [PubMed] [Google Scholar]
- Welter C.; Moreno R. M.; Streiff S.; Helmchen G. Enantioselective synthesis of (+)(R)- and (−)(S)-nicotine based on Ir-catalysed allylic amination. Org. Biomol. Chem. 2005, 3, 3266–6268. [DOI] [PubMed] [Google Scholar]
- For chiral epoxidation:; Morel A. F.; Larghi E. L. First asymmetric total synthesis of (−)-(R)- and (+)-(S)-geibalansine. Tetrahedron: Asymmetry 2004, 15, 9–10. [Google Scholar]
- Jacobson E. N.; Zhang W.; Muci A. R.; Ecker J. R.; Deng L. Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane. J. Am. Chem. Soc. 1991, 113, 7063–7064The catalyst used here was an equal mixture of commercially available enantiomers. [Google Scholar]
- Keavy D.; et al. To be published.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.