Abstract
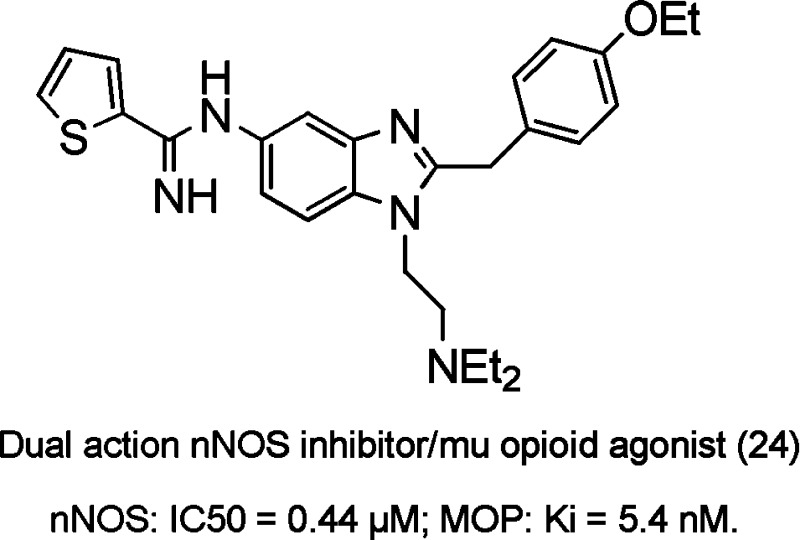
A novel series of benzimidazole designed multiple ligands (DMLs) with activity at the neuronal nitric oxide synthase (nNOS) enzyme and the μ-opioid receptor was developed. Targeting of the structurally dissimilar heme-containing enzyme and the μ-opioid GPCR was predicated on the modulatory role of nitric oxide on μ-opioid receptor function. Structure–activity relationship studies yielded lead compound 24 with excellent nNOS inhibitory activity (IC50 = 0.44 μM), selectivity over both endothelial nitric oxide synthase (10-fold) and inducible nitric oxide synthase (125-fold), and potent μ-opioid binding affinity, Ki = 5.4 nM. The functional activity as measured in the cyclic adenosine monosphospate secondary messenger assay resulted in full agonist activity (EC50 = 0.34 μM). This work represents a novel approach in the development of new analgesics for the treatment of pain.
Keywords: nitric oxide, opioid, dual action, benzimidazole, NOS inhibitor, NOpiate, NOpioid
Opioid analgesics are one of the most important classes of drugs and represent the cornerstone of pain management. Although opiates have a long history of successful management of pain, there is a continued interest in the development of new opioid analgesics to overcome their numerous liabilities that include respiratory depression, nausea, sedation, constipation, dependency, abuse, and the development of tolerance.1,2 In the case of chronic and neuropathic pain, opiate use fails to adequately address the features of hyperalgesia and allodynia, yielding suboptimal pain relief in many patients.3−5 Paradoxically, chronic administration of opiates may be self-limiting as a result of the development of opioid-induced hyperalgesia (OIH),6 an enhancement of the pain response attributed to adaptative changes in the central nervous system such as upregulation of the cyclic adenosine monosphospate (cAMP) pathway and increased production of nitric oxide (NO).
It has been suggested that compounds acting upon multiple mechanisms of action could better address the shortcomings of current pain therapies by enhancing efficacy and reducing side effects.3,7,8 The importance of multiple mechanisms acting in concert either additively or synergistically to treat pain has been exemplified by the polypharmacology of opioid combinations such as oxycodone-acetominaphen, morphine-oxycodone, morphine-δ antagonists, and morphine-gabapentin.9−11 However, the development of combination drugs is complicated by a need to optimally match pharmacokinetic properties and by difficulties in predicting drug interactions, dissolution rates, and stability in the formulated product.12
In our approach to address the pressing need for the development of improved analgesics targeting novel pain pathways,3,13 we adopted the dual action or designed multiple ligand (DML)14 paradigm with the specific aim of incorporating μ-opioid agonism and nitric oxide synthase (NOS) inhibition into a single new chemical entity (NCE).
NO is synthesized by three highly related isoforms (neuronal or nNOS, endothelial or eNOS, and inducible or iNOS) and regulates neurotransmission, blood pressure, and inflammatory responses, respectively.15 NO is known to play an important role mediating nociceptive processing in sensitized pain states,16 and NOS inhibitors are effective in animal models of neuropathic and inflammatory pain.16−19 In addition, nonselective NOS inhibitors l-nitro arginine methyl ester (L-NAME) and 7-nitroindazole (7-NI) potentiate opioid analgesia in pain models and reduce morphine tolerance. However, this effect is lost in nNOS knockout mice, suggesting that the neuronal form is important in modulating opioid analgesia and tolerance.20,21
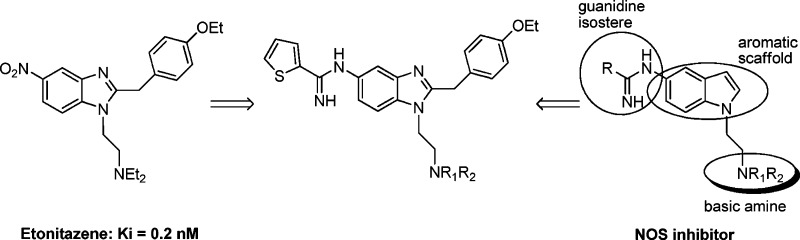
With this in mind, while recognizing the difficulty inherent in integrating the pharmacophoric elements from two highly dissimilar targets14,22 (nNOS, a heme-containing enzyme, and the μ-opioid GPCR), we initiated a program to develop a dual-acting selective nNOS inhibitor (i.e., lacking eNOS and iNOS inhibition) with μ-opioid agonist activity. Common structural features between etonitazene,23 a potent μ-agonist (Ki = 0.2 nM), and previously published nNOS inhibitors were envisioned (Figure 1).24,25
Figure 1.
Proposed dual action structure.
Both the central aryl scaffold and the basic amine side chain were apparent in the etonitazene framework. Determination as to whether incorporation of a guanidine isostere [which makes an important bidentate interaction with the conserved glutamic acid residue at the arginine (substrate) binding site of the NOS enzyme] would be tolerated without significant loss of μ-opioid binding ability was paramount. Although little has been published on the binding interactions of etonitazene,26 two literature reports suggested that upon replacement of the nitro group, antinociceptive activity could be retained albeit weaker than etonitazine itself.27,28 Herein, we report the synthesis of a series of dual action substituted benzimidazoles with selective inhibitory activity toward the human nNOS isoform and activity at the μ-opioid receptor.
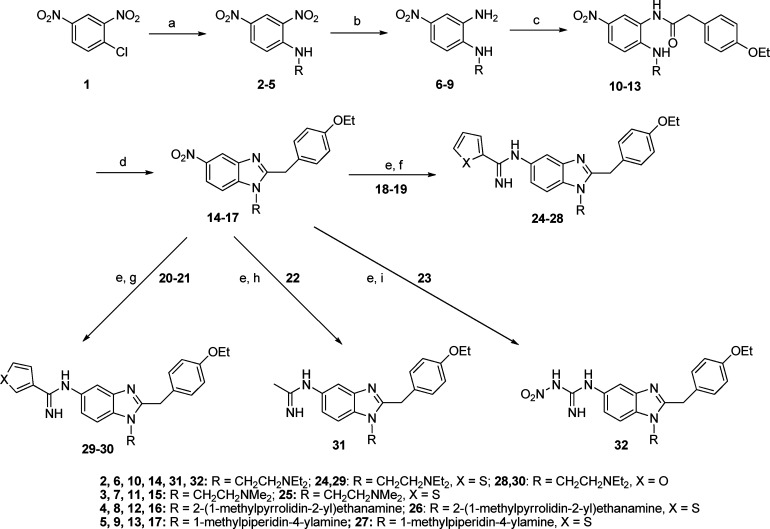
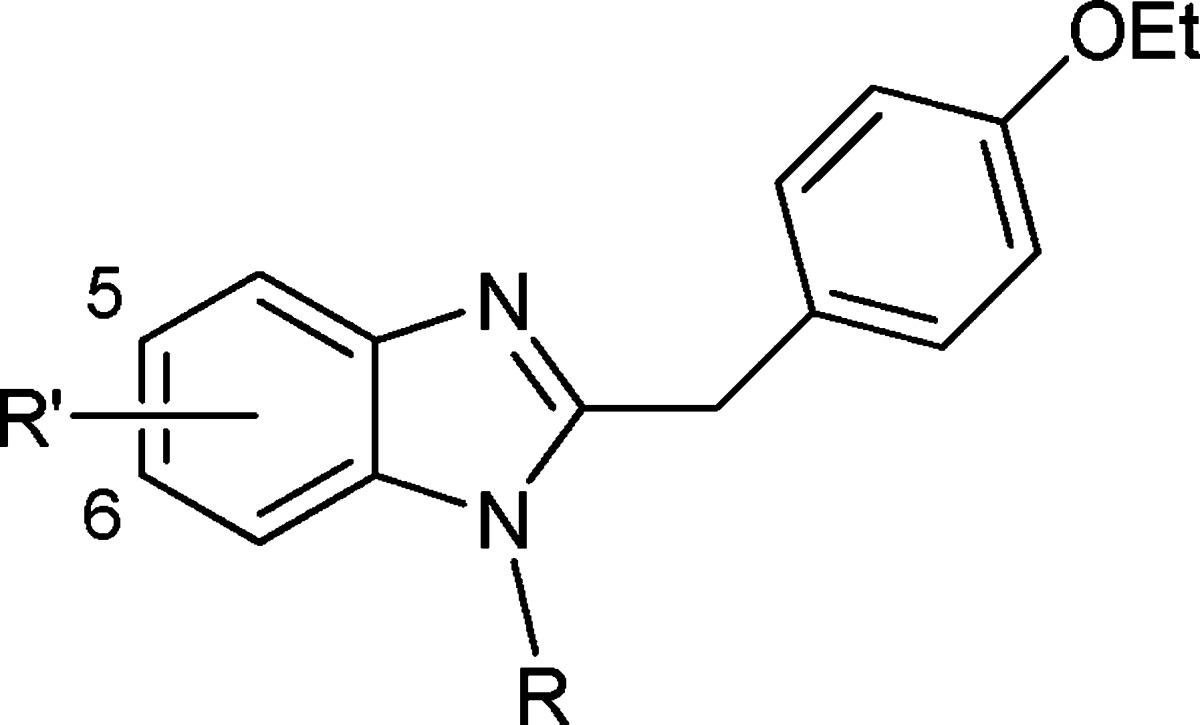
Preparation of the 1,2,5-trisubstituted benzimidazole analogues was achieved as outlined in Scheme 1 utilizing a modified version of the original synthesis of etonitazene.23
Scheme 1. Synthesis of Trisubstituted Benzimidazole Final Compounds 24–32.
Reagents and conditions: (a) Various diamines (H2NR), EtOH, reflux. (b) Aqueous (NH4)2S, EtOH, H2O, 70 °C. (c) 2-(4-Ethoxyphenyl)acetic acid, EEDQ, CH2Cl2 or THF, 35–60 °C. (d) PCl5, CHCl3, reflux. (e) Pd–C/H2, EtOH, room temperature. (f) Methyl thiophene-2-carbimidothioate·HI (18) or benzyl furan-2-carbimidothioate·HBr (19), EtOH, room temperature. (g) Benzyl thiophene-3-carbimidothioate·HBr (20) or benzyl furan-3-carbimidothioate·HBr (21), EtOH, room temperature. (h) Naphthalen-2-ylmethyl ethanimidothioate·HBr (22), EtOH, room temperature. (i) 1-Methyl-3-nitro-1-nitrosoguanidine (23), EtOH, reflux.
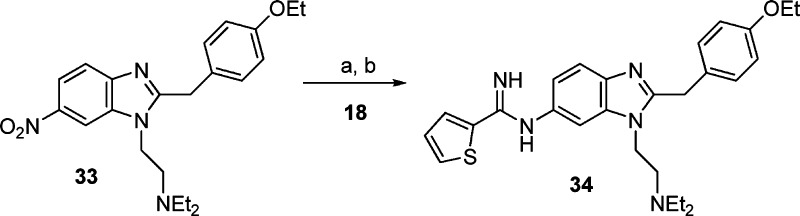
The tertiary amino side chains were introduced via nucleophilic aromatic substitution of the chloro group of 1-chloro-2,4-dinitrobenzene 1 with various diamines. Recrystallization from EtOH yielded the 2,4-dinitro-substituted anilines 2–5. Preferential reduction (commonly >5:1) of the nitro group ortho to the secondary amine was achieved by a Zinin reduction.29 Subsequent separation from the para diamino regioisomer exploiting dry column chromatography techniques yielded the 1,2-diaminobenzenes 6–9. Condensation with 2-(4-ethoxyphenyl)acetic acid in the presence of 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ) followed by PCl5-induced cyclization30 yielded the key benzimidazole intermediates 14–17. Reduction of the nitro group to the corresponding amino group under atmospheric hydrogenation conditions and subsequent reaction in situ with one of methyl thiophene-2-carbimidothioate·hydroiodide (HI) (18), benzyl furan-2-carbimidothioate·hydrobromide (HBr) (19), benzyl thiophene-3-carbimidothioate·HBr (20), benzyl furan-3-carbimidothioate·HBr (21), naphthalen-2-ylmethyl ethanimidothioate·HBr (22), or 1-methyl-3-nitro-1-nitrosoguanidine (23) yielded final compounds 24–32.31 Utilizing the reduction/amidine formation sequence (vide supra), the six-substituted regioisomer of 24 was synthesized from known compound 33,25 as shown in Scheme 2. All compounds were converted into their corresponding dihydrochloride salts.
Scheme 2. 6-Regioisomer of Compound 24.
Reagents and conditions: (a) Pd–C/H2, EtOH, room temperature. (b) Methyl thiophene-2-carbimidothioate·HI (18), EtOH, room temperature.
The inhibitory activities of the target compounds against human NOS isoforms,32 their binding affinity to the human μ opioid receptor,33 and a functional measurement of agonist-like activity (the ability to inhibit forskolin mediated cAMP production)33 were assessed (Table 1).
Table 1. Inhibition of Human NOS Enzymes and MOP Binding and Functional Dataa.
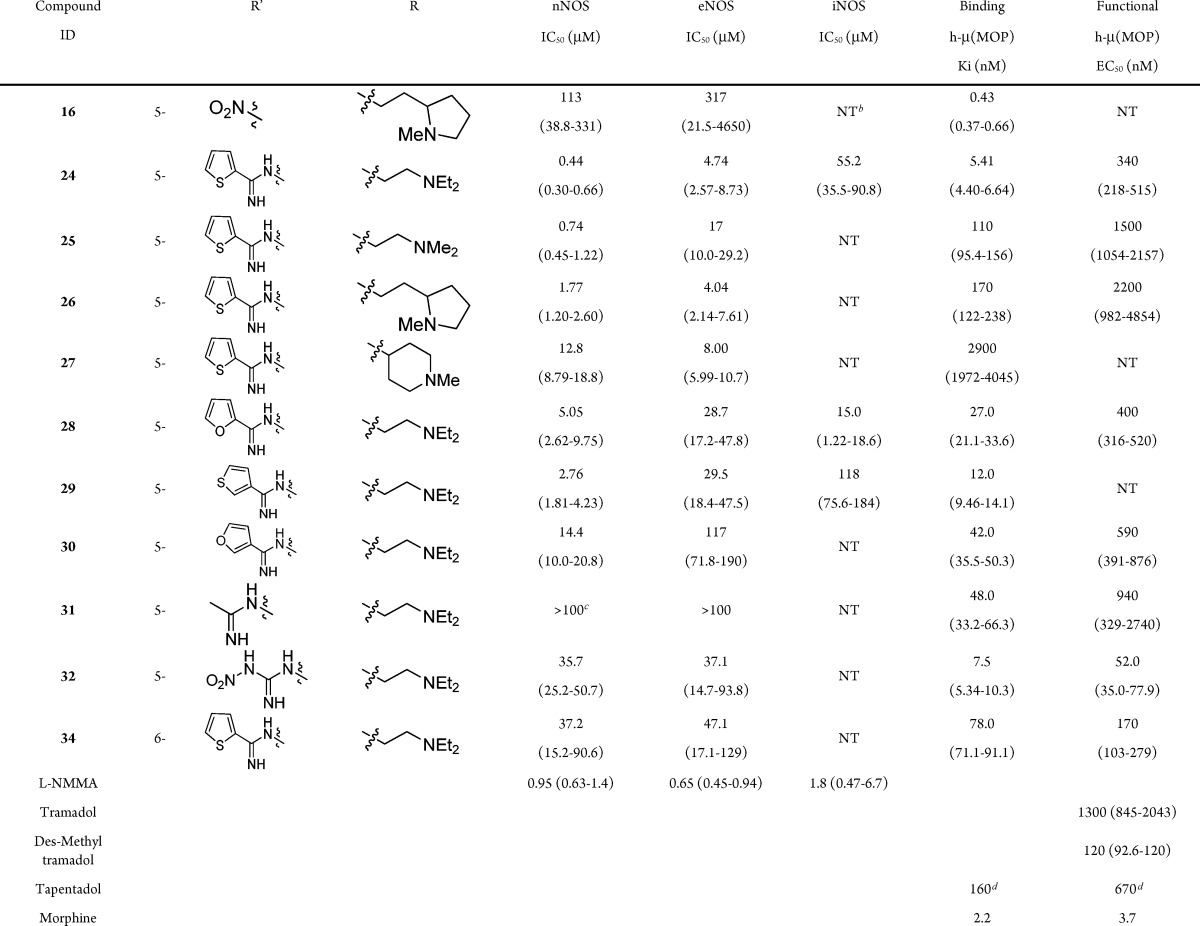
Values reported in parentheses are 95% confidence intervals.
NT, not tested.
>100, not active at the maximum test concentration of 100 μM.
Data from ref (38).
Compound 24 was identified as the most potent nNOS inhibitor [IC50 = 0.44 μM, more potent than the clinically active nonselective NOS inhibitor (L-NMMA)], while demonstrating selectivity over eNOS (10-fold preference for nNOS); iNOS (125-fold) and importantly showed potent binding affinity (Ki = 5.4 nM, comparable to morphine) at the μ-opioid receptor in a competitive radioligand binding assay.
Compounds 24, 25, 28, 29, and 30 were selective (5–23-fold) for the nNOS over the eNOS isoform. To obtain compounds devoid of the cardiovascular liabilities associated with eNOS inhibition,34 selective nNOS inhibition is required. In this series of compounds, the acyclic basic amine side chains showed improved nNOS/eNOS selectivity in comparison to the cyclic amino side chain 27. Thiophene amidines 24 and 29 were more potent for the nNOS and eNOS isoforms when compared to the corresponding furanyl amidines 28 and 30, respectively. Suprisingly, compounds 31 and 32 show weak inhibitory activity at NOS despite the presence of the acetamidine (31) and nitroguanidine (32) moieties, two functional motifs that have been utilized successfully in previous NOS inhibitors.35 However, 32 displayed excellent activity in the μ-opioid functional assay (52 nM), suggesting an important interaction of the nitro group of etonitazene and potentially 32 that facilitates potent functional activity. In contrast to the 5-substituted analogue 24 and other 1,6-substituted bicyclic scaffolds,36 the six-substituted regioisomer 34 shows much weaker nNOS inhibition (85-fold).
Select compounds showed nanomolar level potency in the opioid binding assay but with reduced functional activity. However, these compounds displayed full agonist properties at the μ-opioid receptor. Because of the potential synergies of the dual mechanisms, the functional activity may not need to be as potent as morphine. For example, both Tramadol (and its more active desmethyl metabolite; see Table 1) and Tapentadol (30-fold weaker than morphine in a [35S]GTPγS functional assay) are clinically utilized centrally acting analgesics despite showing modest functional activity at the μ-opioid receptor, likely due to the synergy of nonopioid mechanisms (primarily monoamine reuptake inhibition).37,38
In conclusion, we have designed and synthesized a series of novel dual action nNOS inhibitors with μ-opioid agonist activity and selectivity for nNOS over eNOS. This is the first report of a DML combining μ-opioid activity and selective nNOS inhibitory activity. It is notable that this represents one of the few cases of the successful design for two structurally distinct macromolecular targets (GPCR and oxygenase enzyme) as the majority of reported DMLs target similar subclasses.14,22 The lead compound 24 inhibited nNOS more potently than L-NMMA and displayed a level of potency similar to morphine in a μ-opioid binding assay. Thus, having achieved proof of concept of dual targeting of these dissimilar pain targets, future efforts will be focused on evaluating the potential synergistic effects of combined nNOS/μ-opioid mechanisms in animal models of acute and chronic pain.
Acknowledgments
We are grateful to NoAb BioDiscoveries Inc. (Mississauga, ON, Canada); Asinex Ltd (Moscow, Russia) for performing the human NOS inhibition assays; and Cerep SA (France) for the MOP binding and functional assays.
Glossary
Abbreviations
- cAMP
cyclic adenosine monosphospate
- DML
designed multiple ligand
- EEDQ
2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline
- eNOS
endothelial nitric oxide synthase
- HBr
hydrobromide
- HI
hydroiodide
- iNOS
inducible nitric oxide synthase
- L-NAME
l-nitro arginine methyl ester
- NCE
new chemical entity
- 7-NI
7-nitroindazole
- NO
nitric oxide
- NOS
nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- OIH
opioid-induced hyperalgesia
Supporting Information Available
Synthetic procedures, analytical characterization and purity assessment of final products, and biological assay protocols. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
† Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, ON, M5G 1L7, Canada.
We thank the Natural Sciences and Engineering Research Council (NSERC) of Canada for providing an undergraduate student research award (USRA) to B.G.
The authors declare no competing financial interest.
Supplementary Material
References
- Candiotti K. A.; Gitlin M. C. Review of the effect of opioid related side effects on the undertreatment of moderate to severe chronic non-cancer pain: Tapentadol, a step toward a solution?. Curr. Med. Res. Opin. 2010, 26, 1677–1684. [DOI] [PubMed] [Google Scholar]
- Compton W. M.; Volkow N. D. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug Alcohol Depend. 2006, 81, 103–107. [DOI] [PubMed] [Google Scholar]
- Woodcock J.; Witter J.; Dionne R. A. Stimulating the development of mechanism-based, individualized pain therapies. Nat. Rev. Drug Discovery 2007, 6, 703–710. [DOI] [PubMed] [Google Scholar]
- Dray A.; Perkins M. N.. New pain treatments in late development. In Pharmacology of Pain; Beaulieu P., Lussier D., Porreca F., Dickenson A. H., Eds.; IASP Press: Seattle, 2010; p 383. [Google Scholar]
- Ulugol A.; Aslantas A.; Karadag H. C.; Bulbul E. D.; Tuncer A.; Dokmeci I. The effect of combined systemic administration of morphine and L-NAME, a NOS inhibitor, on behavioural signs of neuropathic pain in rats. Neurosci. Res. Commun. 2002, 30, 143–153and references cited therein. [Google Scholar]
- Chu L. F.; Clark D. J.; Angst M. J. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: A preliminary prospective study. J. Pain 2006, 7, 43–48. [DOI] [PubMed] [Google Scholar]
- Afilalo M.; Stegman J.-U.; Upmalis D. Tapentadol immediate release: A new treatment option for acute pain management. J. Pain Res. 2010, 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon K. E.; Herman D. S.; Agnes R. S.; Largent-Milnes T. M.; Kumarasinghe I. R.; Ma S. W.; Guo W.; Lee Y. S.; Ossipov M. H.; Hruby V. J.; Lai J.; Porreca F.; Vanderah T. W. Novel peptide ligands with dual acting pharmacophores designed for the pathophysiology of neuropathic pain. Brain Res. 2011, 1395, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards P; Riff D.; Keen R.; Stern W. Analgesic and adverse effects of a fixed-ration morphine-oxycodone combination (MoxDuo) in the treatment of postoperative pain. J. Opioid Manage. 2011, 7, 217–28. [DOI] [PubMed] [Google Scholar]
- Daniels D. J.; Lenard N. R.; Etienne C. L.; Law P. Y.; Roerig S. C.; Portoghese P. S. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 19208–19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron I.; Bailey J. M.; Tu D.; Holden R. R.; Weaver D. F.; Houlden R. L. Morphine, Gabapentin, or their combination for neuropathic pain. N. Engl. J. Med. 2005, 352, 1324–1334. [DOI] [PubMed] [Google Scholar]
- Frantz S. The trouble with making combination drugs. Nat. Rev. Drug Discovery 2006, 5, 881–882. [DOI] [PubMed] [Google Scholar]
- Wortman M.Novel Pain Therapeutics: When Will Life Get Better on the Island of Pain? Start Up, April 2011, Elsevier Business Intelligence. [Google Scholar]
- Morphy R.; Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 1–21. [DOI] [PubMed] [Google Scholar]
- Moncada S.; Palmer R. M. J.; Higgs E. A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [PubMed] [Google Scholar]
- Schmidtko A.; Tegeder I.; Geissleinger G. No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci. 2009, 32, 339–346. [DOI] [PubMed] [Google Scholar]
- Tao F.; Tao Y.-X.; Mao P.; Zhao C.; Li D.; Liaw W.-J.; Raja S. N.; Johns R. A. Intact carrageenan-induced thermal hyperalgesia in mice lacking inducible nitric oxide synthase. Neuroscience 2003, 120, 847–854. [DOI] [PubMed] [Google Scholar]
- Hao J.-X.; Xu X.-J. Treatment of a chronic allodynia-like response in spinally injured rats: effects of systemically administered nitric oxide synthase inhibitors. Pain 1996, 66, 313–319. [DOI] [PubMed] [Google Scholar]
- Tanabe M.; Nagatani Y.; Saitoh K.; Takasu K.; Ono H. Pharmacological assessments of nitric oxide synthase isoforms and downstream diversity of NO signaling in the maintenance of thermal and mechanical hypersensitivity after peripheral nerve injury in mice. Neuropharmacology 2009, 56, 702–708. [DOI] [PubMed] [Google Scholar]
- Toda N.; Kishioka S.; Hatano Y.; Toda H. Modulation of opioid actions by nitric oxide signaling. Anesthesiology 2009, 110, 166–81. [DOI] [PubMed] [Google Scholar]
- Heinzen E. L.; Pollack G. M. The development of morphine antinociceptive tolerance in nitric oxide synthase-deficient mice. Biochem. Pharmacol. 2004, 67, 735–741. [DOI] [PubMed] [Google Scholar]
- Morphy R.; Rankovic Z. The physicochemical challenges of designing multiple ligands. J. Med. Chem. 2006, 49, 4961–4970. [DOI] [PubMed] [Google Scholar]
- Hunger A.; Keberle J.; Rossi A.; Hoffmann K. Benzimidazol-derivate und verwandte heterocyclen III. Synthese von 1-aminoalkyl-2-nenzyl-nitro-benzimidazolen. Helv. Chim. Acta 1960, 43, 1032–1046. [Google Scholar]
- Renton P.; Speed J.; Maddaford S.; Annedi S. C.; Ramnauth J.; Rakhit S. 1,5-Disubstituted indole derivatives as selective human neuronal nitric oxide synthase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 5301–5304. [DOI] [PubMed] [Google Scholar]
- Maddaford S.; Annedi S. C.; Ramnauth J.; Rakhit S. Advancements in the developments of nitric oxide synthase inhibitors. Annu. Rep. Med. Chem. 2009, 44, 27–50and references cited therein. [Google Scholar]
- Feinberg A. P.; Creese I.; Snyder S. H. The opiate receptor: A model explaining structure-activity relationships of opiate agonists and antagonists. Proc. Natl. Acad. Sci. U.S.A. 1976, 73, 4215–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T. R.; Bajwa B. S.; Jacobsen A. E.; Rice K. C.; Streaty R. A.; Klee W. A. Probes for narcotic receptor mediated phenonmena 7. Synthesis and pharmacological properties of irreversible ligands specific for μ or δ opiate receptors. J. Med. Chem. 1984, 27, 1570–1574. [DOI] [PubMed] [Google Scholar]
- Lecolier S.Nouveaux dialkylaminoalkyl-1 p-alkoxyphenyl alkyl-2 benzimidazoles 5-substitues. Fr. Patent FR1481049, 1967.
- Porter H. K. The Zinin reduction of nitroarenes. Org. React. 1973, 20, 455–481. [Google Scholar]
- Carroll F. I.; Coleman M. C. Etonitazene. An improved synthesis. J. Med. Chem. 1975, 18, 318–320. [DOI] [PubMed] [Google Scholar]
- Refer to the Supporting Information for the synthesis of reagents.
- Recombinant human nNOS, eNOS, and iNOS were produced in Baculovirus-infected Sf9 cells. The inhibitory activities of the compounds were measured by following the conversion of [3H]-l-arginine into [3H]-l-citrulline in the presence of the requisite cofactors. The enzymatic reaction was carried out in the presence or absence of varying concentrations of the compound. Following that, [3H]-l-citrulline was separated from the [3H]-l-arginine using DOWEX ion-exchange resin. The inhibition of enzyme activity by the compound is measured by dividing the enzymatic conversion in the presence of compound divided by the enzymatic conversion in the absence of compound. The IC50 value is the concentration of compound that gives rise to 50% inhibition. Complete assay protocols are available in the accompanying Supporting Information.
- Wang J. B.; Johnson P. S.; Persico A. M.; Hawkins A. L.; Giffin C. A.; Uhl G. R. Human μ-opiate receptor. cDNA and genomic clones, pharmacological characterization and chromosomal assignment. FEBS Lett. 1994, 338, 217–222. [DOI] [PubMed] [Google Scholar]
- Cayatte A. J.; Palacino J. J.; Horten K.; Cohen R. A. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler., Thromb., Vasc. Biol. 1994, 14, 753–759. [DOI] [PubMed] [Google Scholar]
- Erdal E. P.; Litzinger E. A.; Seo J.; Zhu Y.; Ji H.; Silverman R. B. Selective neuronal nitric oxide synthase inhibitors. Curr. Top. Med. Chem. 2005, 5, 603–624. [DOI] [PubMed] [Google Scholar]
- Maddaford S. P.; Renton P.; Speed J.; Annedi S. C.; Ramnauth J.; Rakhit S.; Andrews J.; Mladenova G.; Majuta L.; Porreca F. 1,6-Disubstituted indole derivatives as selective human neuronal nitric oxide synthase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 5234–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa R. B. A novel approach to the pharmacology of analgesics. Am. J. Med. 1996, 101Suppl. 1A40S–46S. [DOI] [PubMed] [Google Scholar]
- Tzschentke T. M.; Christoph T.; Kögel B.; Schiene K.; Hennies H. H.; Englberger W.; Haurand M.; Jahnel U.; Cremers T. I. F. H.; Friderichs E.; De Vry J. (−)-(1R,2R)-3-(3-Dimethylamino-1-ethyl-2-methyl-propyl)-phenol Hydrochloride (Tapentadol HCl): A Novel μ-Opioid Receptor Agonist/Norepinephrine Reuptake Inhibitor with Broad-Spectrum Analgesic Properties. J. Pharmacol. Exp. Ther. 2007, 323, 265–276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.