Abstract
Respiratory infections caused by human rhinovirus are responsible for severe exacerbations of underlying clinical conditions such as asthma in addition to their economic cost in terms of lost working days due to illness. While several antiviral compounds for treating rhinoviral infections have been discovered, none have succeeded, to date, in reaching approval for clinical use. We have developed a potent, orally available rhinovirus inhibitor 6 that has progressed through early clinical trials. The compound shows favorable pharmacokinetic and activity profiles and has a confirmed mechanism of action through crystallographic studies of a rhinovirus−compound complex. The compound has now progressed to phase IIb clinical studies of its effect on natural rhinovirus infection in humans.
Keywords: antiviral, rhinovirus, capsid, inhibitor
The family picornaviridae includes a diverse range of pathogens causing disease in both humans and other animals.1 Of these, the rhinovirus, a species of the enterovirus genera, is perhaps the most ubiquitous of human respiratory pathogens causing the majority of cases of the common cold2 as well as being responsible for sometimes severe exacerbations of underlying illnesses such as asthma, cystic fibrosis, and chronic obstructive pulmonary disease (COPD).3 The difficulties inherent in the development of a specific therapeutic for the treatment of human rhinovirus (HRV) infection have become almost proverbial, with the lack of a “cure for the common cold” being used to highlight perceived flaws in scientific and medical progress. Despite this popular misconception, a range of specific rhinovirus inhibitors have been identified in recent years, and some have progressed into clinical development,1,4 although none have as yet been approved for use.
The most productive viral targets for the identification of HRV inhibitors have been the protein capsid and the 3C protease. Processing of the viral polyprotein is strictly dependent on the 3C protease following an initial cleavage by a second virus-encoded 2A protease.5 Because of its essential role in the viral lifecycle, the 3C protease has been the target of numerous research programs with several inhibitory compounds identified.1,4,6 An irreversible 3C protease inhibitor incorporating an unsaturated ethyl ester Michael acceptor, rupintrivir (AG7088), demonstrated potent antiviral activity against all enteroviruses including multiple HRV serotypes and HRV clinical isolates6 as well as showing low toxicity and acceptable safety and tolerability in intranasal dosing.7 Rupintrivir performed well in experimental HRV challenge studies, reducing the severity of both viral load and symptomatic illness,8 but failed to show a clinically significant impact in a natural infection study.9 Following this lack of efficacy, further clinical development of rupintrivir for HRV ceased.
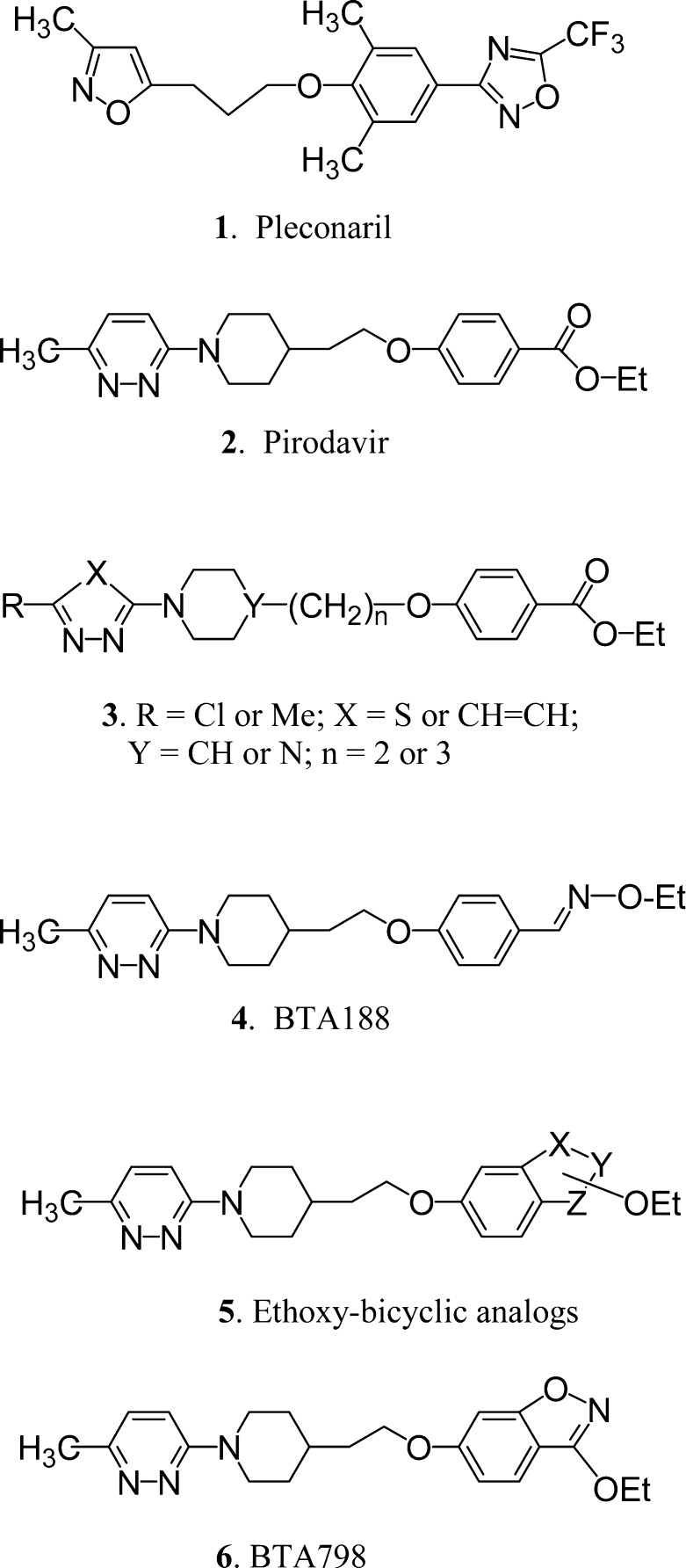
Pleconaril 1 (Chart 1), the only HRV inhibitor so far to be submitted for regulatory approval to the U.S. Food and Drug Administration, shows broad spectrum antiviral activity across a range of enterovirus species, inhibiting 90% of clinical isolates at a concentration of 0.18 μM.10 A phase III clinical trial showed that pleconaril 1 caused a significant reduction in both the severity and the duration of disease symptoms.11,12 Safety and drug interaction concerns, however, led the assessment panel to reject the application for pleconaril 1.13 Subsequently, the compound is being developed as an intranasal treatment with a phase II study completed in 2007, although no results have been released to date (http://www.clinicaltrials.gov/ct/gui/show/NCT00394914).
Chart 1. Structures of Representative Antirhinoviral Capsid-Binding Compounds Discussed in the Text.
On the basis of reported in vitro results, the pyridazine derivative pirodavir 2 is among the most active of the known capsid binders and at a concentration of 0.064 μg mL−1 inhibits 80% of 100 HRV strains.14 While effective in experimental challenge studies when administered very frequently intranasally,15,16 the ester functionality of pirodavir 2 has been shown to undergo facile hydrolysis in vivo to the carboxylic acid, which is inactive,17 and this ease of hydrolysis renders the molecule unsuitable for oral administration.
Several years ago, we began a research project to identify novel orally active HRV capsid binders, and part of our investigations was to try to identify metabolically stable isosteric alternatives to the labile ester functionality on pirodavir 2. In total, we prepared and tested several hundred new compounds, confirming in general terms the structure−activity relationships (SAR) that have been reported for pirodavir 2.17 In summary, the most active HRV inhibitors are those of general structure 3 comprising a methyl or chloro-substituted-pyridazine linked via an N-piperidinyl-alkyl group to a 4-oxybenzoate ethyl ester. With regard to the ethyl ester moiety, we found that the anti-HRV SAR is quite demanding, the only good alternative being an oxime ether group so that compound 4 (BTA188) was found to have equivalent activity to pirodavir 2 against a panel of HRV18 and superior activity against a wider range of picornaviruses.19
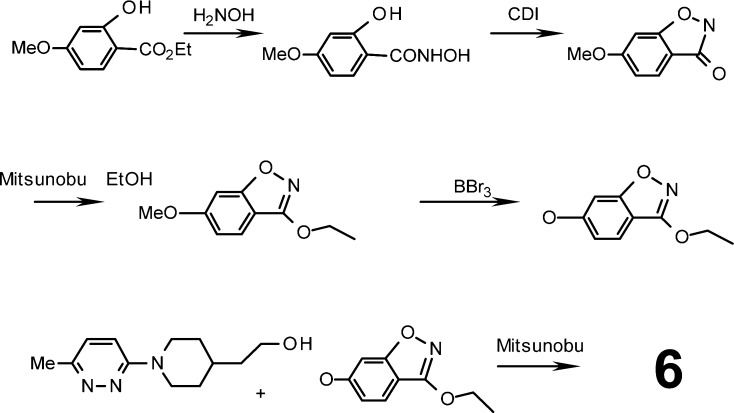
While BTA188 4 was sufficiently potent in vitro and demonstrated good pharmacokinetic and toxicity profiles in initial studies, subsequent indications of undesirable metabolism both in vitro and in vivo suggested that the liabilities of the ester functionality had not been completely resolved, prompting further improvement of the series. Given that the HRV activity of pirodavir 2 requires the precise chemical characteristics of a terminal ethyl ester functionality, it occurred to us that ethoxy-substituted 6,5-fused-benzoheterocycles of general structure 5 could be suitable isosteres of the ethyl benzoate group and would be much less prone to hydrolysis. Thus, we devised suitable synthetic methods (Scheme 1) and prepared many compounds of type 5 including benzisoxazole and benzisothiazole analogues 6−12 of pirodavir 2 (Table 1).20 Compound 6, for example, was prepared by Mitsunobu reaction of the appropriate hydroxyethylpiperidine with 3-ethoxy-6-hydroxy-1,2-benzisoxazole.
Scheme 1. Summary of the Synthetic Route for BTA798 6.
Mitsunobu reactions were prepared in anhydrous THF with diisopropylazodicarboxylate at 0 °C and then allowed to react at room temperature overnight. CDI, carbonyldiimidazole.
Table 1. Structure and Anti-HRV Activity of Compounds 6−12.
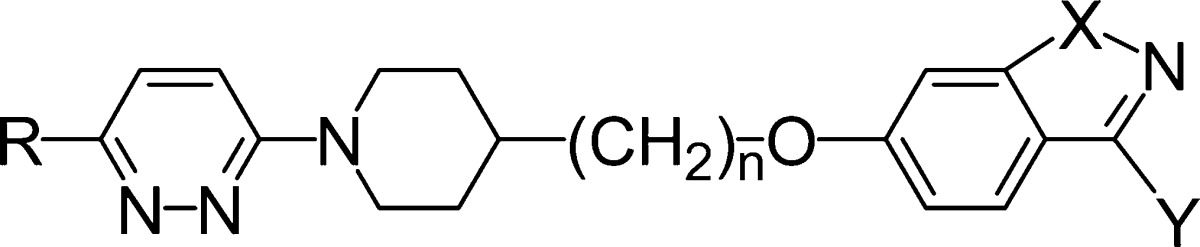
| EC50a |
||||||
|---|---|---|---|---|---|---|
| compd | R | n | X | Y | HRV2 | HRV14 |
| 6 | Me | 2 | O | OEt | 0.001 | 0.005 |
| 7 | Me | 2 | O | Et | 0.024 | 0.088 |
| 8 | Cl | 2 | O | OEt | 0.003 | 0.019 |
| 9 | Me | 2 | O | O-nPr | 0.003 | 0.029 |
| 10 | Me | 2 | O | nPr | 0.084 | 0.013 |
| 11 | Me | 2 | S | OEt | 0.003 | 0.029 |
| 12 | Cl | 3 | O | OEt | 0.003 | 0.009 |
| 2 | Me | 2 | 0.003 | 0.004 | ||
Activity in μg mL−1.
All new compounds were initially tested for activity against two HRV strains (HRV-2 and HRV-14) using cell culture cytopathic effect (CPE)-based assays as summarized in Table 1. The results indicated that the ethoxy-benzisoxazole compound 6 (BTA798), the closest structural analogue of pirodavir 2, is the most active compound and has similar activity to 2 against HRV2 and HRV14. Various other ethoxy-substituted 6,5- and 6,6-benzoheterocyclic analogues of pirodavir, including 6-linked 2-ethoxybenzoxazole21 and 7-linked 4-ethoxy-quinazoline20 analogues, were also prepared, but testing against HRV showed lower activity. Overall, our antiviral testing results indicated that the ethoxy-benzisoxazole of 6 is the preferred bicyclic ring system.
A panel of 32 HRV serotypes comprising viruses from species groups A and B, antiviral susceptibility groups A and B, and both major and minor receptor groups was assessed for sensitivity to BTA798 6 with comparative data generated for pleconaril 1, pirodavir 2, rupintrivir,6 and BTA188 4 (Table 2). The results indicate that BTA798 6 has potent antiviral activity in vitro for HRV serotypes from both species groups A and B, both susceptibility groups, and both receptor groups, with a median EC50 value of 4.3 ng mL−1 (11.2 nM) for the 32 viruses tested. Similarly, BTA798 6 showed good activity against a panel of 39 clinical HRV isolates with a median EC50 of 7.3 ng mL−1 (19.1 nM) and like BTA188 4 is highly potent against a range of enterovirus species including echovirus, Coxsackie virus A9, poliovirus, and enterovirus 71.22
Table 2. Comparative Activity of BTA798 and Other HRV Inhibitors Across a Panel of 32 HRV Serotypes.
| species |
antiviral susceptibility group |
receptor group |
|||||
|---|---|---|---|---|---|---|---|
| virus classification methoda (no. of virus) | A (26) | B (6) | A (8) | B (24) | major (27) | minor (5) | |
| BTA798 | median EC50b | 3.8 | 5.0 | 5.7 | 2.7 | 5.2 | 1.9 |
| EC50 range | <0.9−>1000 | 0.8−21.0 | 0.8−>1000 | <0.9−147.3 | <0.9−>1000 | 0.5−15.9 | |
| fold difference | 1.3 | 2.1 | 2.7 | ||||
| pleconaril | median EC50 | 37.4 | 224.1 | 387.6 | 33.8 | 49.2 | 23.2 |
| EC50 range | 7.6−1539.7 | 31.7−>1000 | 31.7−>1000 | 7.6−277.1 | 9.5−>1000 | 7.6−45.3 | |
| fold difference | 6.0 | 11.5 | 2.1 | ||||
| pirodavir | median EC50 | 4.0 | 11.1 | 20.6 | 2.6 | 5.6 | 2.3 |
| EC50 range | 0.2−>5000 | 2.5−35.2 | 2.5−>5000 | 0.2−157.4 | 0.2−>5000 | 0.9−34.2 | |
| fold difference | 2.8 | 8.1 | 2.4 | ||||
| BTA188 | median EC50 | 5.0 | 7.9 | 13.0 | 4.6 | 5.45 | 3.4 |
| EC50 range | 0.5−>5000 | <0.05−17 | <0.05−>5000 | 0.5−>1000 | <0.05−>5000 | 0.8−6.2 | |
| fold difference | 1.6 | 2.8 | 1.6 | ||||
| rupintrivir | median EC50 | 2.0 | 1.2 | 1.2 | 2.0 | 1.5 | 3.0 |
| EC50 range | 0.5−7.2 | 0.7−2.1 | 0.5−3.0 | 0.8−7.2 | 0.5−7.2 | 1.7−6.4 | |
| fold difference | 1.7 | 1.7 | 2.0 | ||||
The specificity of BTA798 6 was examined by testing the in vitro efficacy of the compound against representative nonpicornaviral species including bovine viral diarrhea virus (Nadl strain), respiratory syncytial virus (A-2 strain), human influenza virus (A/Sydney/5/97 strain and B/Harbin/7/94 strain), and human herpes simplex virus type 2 at concentrations up to 5000 nM (data not shown). No effect of BTA798 6 on CPE for these viruses was observed, indicating that BTA798 6 lacks antiviral activity for representatives from five different virus families that include those with both positive and negative strand RNA genomes as well as those with a double-stranded DNA genome.
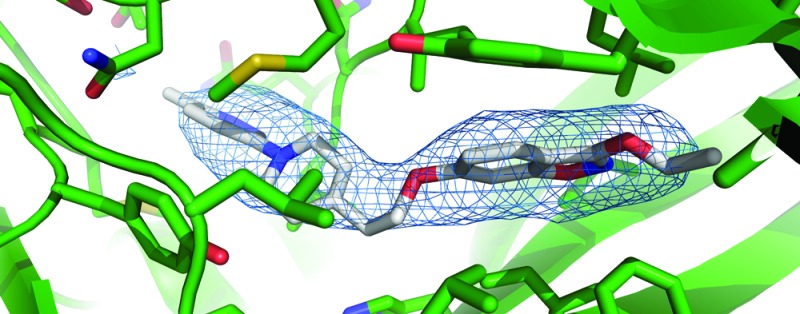
As an analogue of pirodavir 2, it was presumed that BTA798 6 would act by a similar mechanism as a capsid binder interfering with the normal function of the viral protein coat. Analysis of HRV variants selected for resistance to pleconaril 1 indicated that cross-resistance was seen to BTA798 6 as well as pirodavir 2 (see the Supporting Information). All variants, however, remained sensitive to the 3C protease inhibitor rupintrivir. As further confirmation of the mechanism of action of BTA798 6, the structure of HRV2 in complex with BTA798 6 was determined by X-ray crystallography (Figure 1a). The electron density map derived from the crystallographic data delineated the position of the amino acids in the protein capsid at 3.0 Å resolution and the cavity within the VP1 protein of the capsid protomer where other capsid inhibitors have previously been identified23 was found to contain a region of electron density consistent with the presence of BTA798 6. Inclusion of a molecule of BTA798 6 in the structure and further refinement confirmed the position and orientation in the VP1 pocket (Figure 1b).
Figure 1.
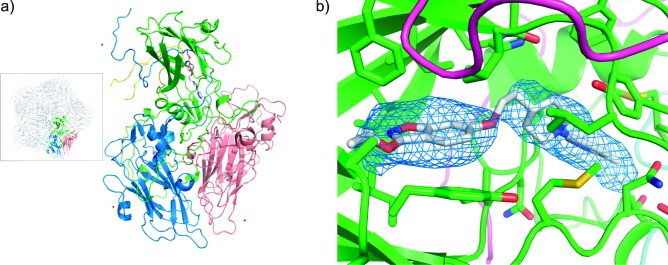
Crystal structure of the HRV2−BTA798 6 complex. (a) Diagrammatic representation of the protomer with VP1 (green), VP2 (pink), VP3 (blue), and VP4 (yellow) shown as cartoons, waters are shown as red spheres, and BTA798 6 are shown as rods colored by atom type. The inset shows one of the capsid pentamers with a single protomer highlighted. (b) View of BTA798 6 in the VP1 binding “canyon”. The protein is shown as a cartoon with binding residues shown as rods colored by atom type. BTA798 6 is shown as rods colored by atom type with carbons gray. The omit map Fo − Fc electron density corresponding to BTA798 6 is shown at a σ level of 1.8 as a blue mesh.
The preclinical pharmacokinetic profile of BTA798 6 in rat and dog (Table 3) was found to be better than that of BTA188 4(18) with improved exposure at a lower dose. The metabolic stability of BTA798 6 is also improved over that of BTA188 4 and now appears to have completely resolved the liability seen with pirodavir 2. After oral or iv dosing of either rat or dog, unmetabolized BTA798 6 is the major component identified in both plasma and excreta. These observations contrast with BTA188 4 where a number of metabolites were identified, including the inactive carboxylic acid (data not shown).
Table 3. Pharmacokinetic Parameters of BTA798 Following Oral Administrationa in Rat and Dog.
| rat |
dog |
|||
|---|---|---|---|---|
| parameter | male (n = 28) | female (n = 27) | male (n = 3) | female (n = 3) |
| Cmax (ng mL−1) | 22500 | 17700 | 11000 | 6100 |
| tmax (h) | 16.0 | 11.2 | 14.7 | 7.7 |
| AUC (ng h mL−1) | 483000 | 489000 | 202000 | 121000 |
| t1/2 (h) | 51.6 | 46.4 | 18.3 | 15.9 |
| bioavailability (%) | 94 | 95 | b | b |
Single oral dose of 50 mg kg−1 of BTA798 in 1% carboxymethylcellulose. See the Supporting Information for details.
Calculated bioavailability was in excess of 100% indicative of enterohepatic recirculation.
BTA798 6 showed excellent pharmacokinetic and toxicological profiles during phase I studies, with a Cmax of 1890 ng mL−1, Tmax of 1.03 h, t1/2 of 11.8 h, and an AUC0−∞ of 13758 ng h mL−1 following a single 400 mg dose. No dose-limiting toxicities or trends in adverse events were seen with single doses up to 1600 mg or multiple doses of 400 mg twice daily for seven days. BTA798 6 is progressing through clinical trials, having shown efficacy in an experimental rhinovirus infection model phase IIa trial (manuscript in preparation). A phase IIb trial of BTA798 6 in patients with natural rhinovirus infection is underway.
Acknowledgments
This research was undertaken on the MX1 beamline at the Australian Synchrotron, Victoria, Australia. M.W.P. is an Australian Research Council Federation Fellow and a National Health and Medical Research Council Honorary Fellow. Infrastructure support from the National Health and Medical Research Council Independent Research Institutes Infrastructure Support Scheme and the Victorian State Government Operational Infrastructure Support Program is gratefully acknowledged.
Glossary
Abbreviations
- COPD
chronic obstructive pulmonary disease
- HRV
human rhinovirus
- SAR
structure−activity relationship
Supporting Information Available
Detailed experimental procedures for the synthesis of compounds, antiviral assays, and crystallography. This material is available free of charge via the Internet at http://pubs.acs.org.
Project funding through the AusIndustry Start Grant scheme is acknowledged.
The authors declare the following competing financial interest(s): All authors were either consultants for (SCF, MWP) or employees of (others) Biota while working on this project. SH, BL, AL, MWP, JR, SPT and CJM declare financial interest in Biota Holdings Ltd.
Author Present Address
∥ Baker IDI Heart and Diabetes Institute, 75 Commercial Road Melbourne, Victoria 3000, Australia.
Author Present Address
⊥ The Department of Medicinal Chemistry, Boehringer Ingelheim RCV GmbH & Co KG, Dr. Boehringer-Gasse 5-11, Vienna, Austria.
Supplementary Material
References
- De Palma A. M.; Vliegen I.; De Clercq E.; Neyts J. Selective inhibitors of picornavirus replication. Med. Res. Rev. 2008, 286823–884. [DOI] [PubMed] [Google Scholar]
- Anzueto A.; Niederman M. S. Diagnosis and treatment of rhinovirus respiratory infections. Chest 2003, 12351664–1672. [DOI] [PubMed] [Google Scholar]
- Gern J. E. The ABCs of rhinoviruses, wheezing, and asthma. J. Virol. 2010, 84157418–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patick A. K. Rhinovirus chemotherapy. Antiviral Res. 2006, 712−3391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seipelt J.; Guarne A.; Bergmann E.; James M.; Sommergruber W.; Fita I.; Skern T. The structures of picornaviral proteinases. Virus Res. 1999, 622159–168. [DOI] [PubMed] [Google Scholar]
- Patick A. K.; Binford S. L.; Brothers M. A.; Jackson R. L.; Ford C. E.; Diem M. D.; Maldonado F.; Dragovich P. S.; Zhou R.; Prins T. J.; Fuhrman S. A.; Meador J. W.; Zalman L. S.; Matthews D. A.; Worland S. T. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 1999, 43102444–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsyu P. H.; Pithavala Y. K.; Gersten M.; Penning C. A.; Kerr B. M. Pharmacokinetics and safety of an antirhinoviral agent, ruprintrivir, in healthy volunteers. Antimicrob. Agents Chemother. 2002, 462392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. G.; Turner R. B.; Gwaltney J. M.; Chi-Burris K.; Gersten M.; Hsyu P.; Patick A. K.; Smith G. J. 3rd; Zalman L. S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003, 47123907–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patick A. K.; Brothers M. A.; Maldonado F.; Binford S.; Maldonado O.; Fuhrman S.; Petersen A.; Smith G. J. 3rd; Zalman L. S.; Burns-Naas L. A.; Tran J. Q. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 2005, 4962267–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C.; Tull T. M.; Seipel M. E.; Groarke J. M. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 1999, 4392109–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. G.; Herrington D. T.; Coats T. L.; Kim K.; Cooper E. C.; Villano S. A.; Liu S.; Hudson S.; Pevear D. C.; Collett M.; McKinlay M. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: Results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 2003, 36121523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C.; Hayden F. G.; Demenczuk T. M.; Barone L. R.; McKinlay M. A.; Collett M. S. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob. Agents Chemother. 2005, 49114492–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior K. FDA panel rejects common cold treatment. Lancet Infect. Dis. 2002, 25264. [DOI] [PubMed] [Google Scholar]
- Andries K.; Dewindt B.; Snoeks J.; Willebrords R.; van Eemeren K.; Stokbroekx R.; Janssen P. A. In vitro activity of pirodavir (R 77975), a substituted phenoxy-pyridazinamine with broad-spectrum antipicornaviral activity. Antimicrob. Agents Chemother. 1992, 361100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. G.; Andries K.; Janssen P. A. Safety and efficacy of intranasal pirodavir (R77975) in experimental rhinovirus infection. Antimicrob. Agents Chemother. 1992, 364727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. G.; Hipskind G. J.; Woerner D. H.; Eisen G. F.; Janssens M.; Janssen P. A.; Andries K. Intranasal pirodavir (R77,975) treatment of rhinovirus colds. Antimicrob. Agents Chemother. 1995, 392290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries K.Discovery of Pirodavir, a Broad-Spectrum Inhibitor of Rhinoviruses. In The Search for Antiviral Drugs; Adams J., Merluzzi V. J., Eds.; Brikhauser: Boston, 1993; pp 179−209. [Google Scholar]
- Watson K. G.; Brown R. N.; Cameron R.; Chalmers D. K.; Hamilton S.; Jin B.; Krippner G. Y.; Luttick A.; McConnell D. B.; Reece P. A.; Ryan J.; Stanislawski P. C.; Tucker S. P.; Wu W. Y.; Barnard D. L.; Sidwell R. W. An orally bioavailable oxime ether capsid binder with potent activity against human rhinovirus. J. Med. Chem. 2003, 46153181–3184. [DOI] [PubMed] [Google Scholar]
- Barnard D. L.; Hubbard V. D.; Smee D. F.; Sidwell R. W.; Watson K. G.; Tucker S. P.; Reece P. A. In vitro activity of expanded-spectrum pyridazinyl oxime ethers related to pirodavir: Novel capsid-binding inhibitors with potent antipicornavirus activity. Antimicrob. Agents Chemother. 2004, 4851766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. G.; Krippner G. Y.; Stanislawski P. C.; McConnell D. B. U.S. Patent 7,166,604, 2007.
- Brown R. N.; Cameron R.; Chalmers D. K.; Hamilton S.; Luttick A.; Krippner G. Y.; McConnell D. B.; Nearn R.; Stanislawski P. C.; Tucker S. P.; Watson K. G. 2-Ethoxybenzoxazole as a bioisosteric replacement of an ethyl benzoate group in a human rhinovirus (HRV) capsid binder. Bioorg. Med. Chem. Lett. 2005, 1582051–2055. [DOI] [PubMed] [Google Scholar]
- Thibaut H. J.; Leyssen P.; Puerstinger G.; Muigg A.; Neyts J.; De Palma A. M. Towards the design of combination therapy for the treatment of enterovirus infections. Antiviral Res. 2011, 903213–217. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. The structure of antiviral agents that inhibit uncoating when complexed with viral capsids. Antiviral Res. 1989, 1113–13. [DOI] [PubMed] [Google Scholar]
- Andries K.; Dewindt B.; Snoeks J.; Wouters L.; Moereels H.; Lewi P. J.; Janssen P. A. Two groups of rhinoviruses revealed by a panel of antiviral compounds present sequence divergence and differential pathogenicity. J. Virol. 1990, 6431117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham G.; Colonno R. J. Many rhinovirus serotypes share the same cellular receptor. J. Virol. 1984, 512340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.