Abstract
Lysosomes are involved in protein turnover and removing misfolded species, and their enzymes have the potential to offset the defect in proteolytic clearance that contributes to the age-related dementia Alzheimer's disease (AD). The weak cathepsin B and L inhibitor Z-Phe-Ala-diazomethylketone (PADK) enhances lysosomal cathepsin levels at low concentrations, thereby eliciting protective clearance of PHF-τ and Aβ42 in the hippocampus and other brain regions. Here, a class of positive modulators is established with compounds decoupled from the cathepsin inhibitory properties. We utilized PADK as a departure point to develop nonpeptidic structures with the hydroxyethyl isostere. The first-in-class modulators SD1002 and SD1003 exhibit improved levels of cathepsin up-regulation but almost complete removal of cathepsin inhibitory properties as compared to PADK. Isomers of the lead compound SD1002 were synthesized, and the modulatory activity was determined to be stereoselective. In addition, the lead compound was tested in transgenic mice with results indicating protection against AD-type protein accumulation pathology.
Keywords: Alzheimer's disease, cathepsin B, lysosomal modulator, PADK, nonpeptidic inhibitors
Reducing protein accumulation events is essential for slowing the progression of Alzheimer's disease (AD), the most frequent form of dementia. Accumulations of Aβ peptides, PHF-τ, and related paired helical filaments are implicated as contributing to the disease.1 There are no current treatments that reduce the levels of protein accumulation, although several different strategies are currently under development. There are two classes of drugs that are approved for the treatment of AD. They are (i) inhibitors of acetylcholinesterase that only affect the symptoms of AD and do little to slow or reverse the course of disease progression and (ii) an antagonist of the N-methyl-d-aspartate (NMDA) receptor that is no better than placebo in treating mild AD patients, and there is only meager evidence for a benefit in a moderate form of the condition.2 New treatments that directly affect the underlying pathology of the disease are desperately needed.
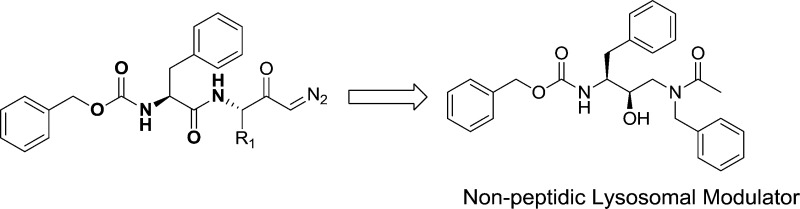
The events associated with protein accumulation pathology point to protein clearance as a treatment avenue to offset AD type synaptic pathology, which is the best neurobiological correlate of cognitive decline.3,4 In response to protein accumulation, the lysosomal system exhibits regulatory events as found in AD5−8 as well as in areas of the aged brain.9,10 Lysosomes play an important role in normal protein turnover and the clearance of misfolded or aggregated protein species. The cathepsin family of lysosomal proteases appears to be particularly responsive to accumulating proteins in neurons. Protein accumulation stress up-regulates the message, protein, and activity levels of cathepsins,11−13 including the stress produced by Aβ1–42. Such responses may keep protein accumulation partially in check and account for the gradual nature of AD type pathology that can extend over many years in patients. Enhancement of the lysosomal system has been suggested as a plausible strategy to reduce aberrant protein accumulation in age-related neurodegenerative disorders.11,13,14 Shown in Figure 1, the positive lysosomal modulators Z-Phe-Ala-diazomethylketone (PADK; 1A) and Z-Phe-Phe-diazomethylketone (PPDK; 1B) are small molecules found in many studies to enhance lysosomal enzyme levels and elicit protective clearance of PHF-τ and Aβ42 in the brain.11,12,14−16
Figure 1.
Structure of PADK and PPDK.
Recently, Butler et al.17 demonstrated that the pharmacologically controlled modulation of the lysosomal system promotes clearance of Aβ species in two mouse models of AD. The findings also indicate that reducing intracellular protein accumulation stress protects against AD type synaptic decline. These observations suggested that PADK could serve as an important lead in the development of a new class of agents with the potential to treat protein accumulation pathology. Although PADK has good therapeutic potential in treating neurodegenerative disorders, there are obvious concerns about its drug-likeness, namely, a protein reactive diazomethylketone and a metabolically active peptide bond. Herein, we report the enantioselective synthesis of peptidomimetics of PADK and PPDK with improved druglike properties, enhanced up-regulation of cathepsin B, and neuroprotective properties in a transgenic mouse model of AD.
Peptides generally suffer from poor in vivo stability and bioavailability. A peptidomimetic strategy based on a hydroxylethyl isostere was designed that would allow retention of the pendant functionality found in PADK and PPDK. We also chose to explore if the electrophilic diazoamide could be replaced with a nonreactive capping group such that the modulators would be expected to bind in a reversible manner to their targets.
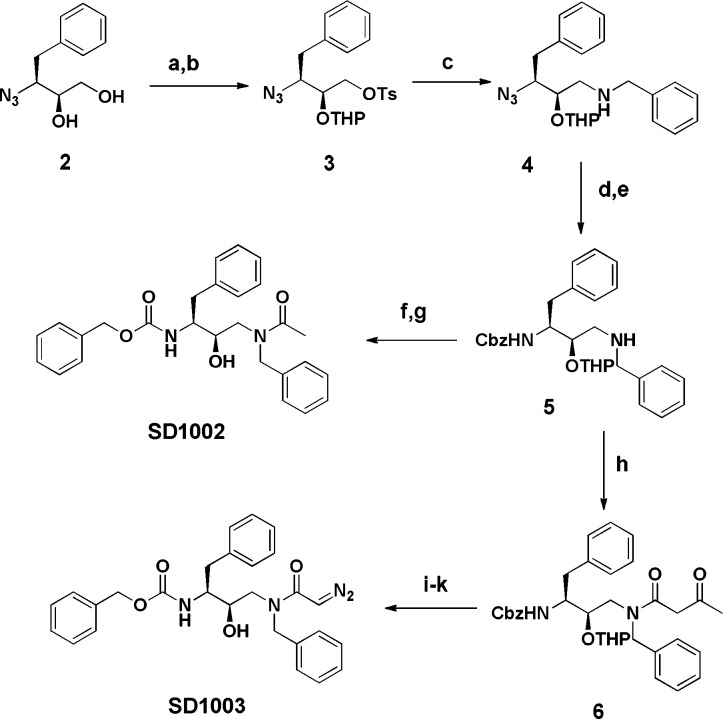
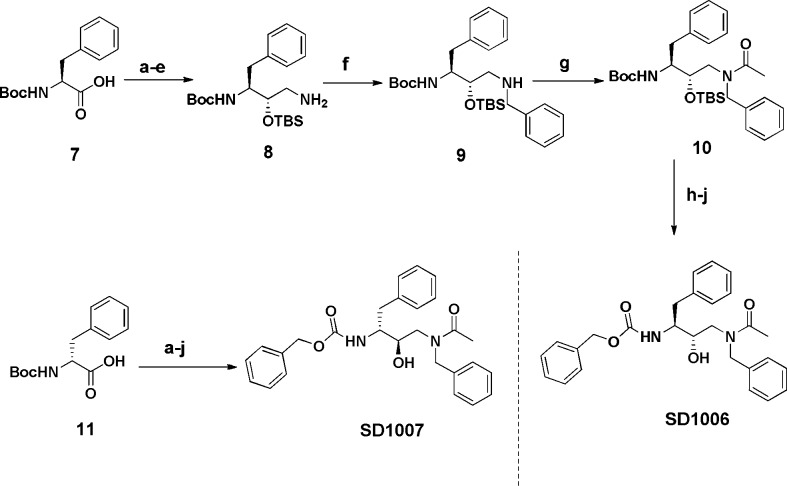
The synthesis of nonpeptidic lysosomal modulators (Scheme 1) started with the enantiometrically pure azidodiol 2, which was prepared according to literature procedures.18 The enantiomeric purity of the azidodiol was confirmed by optical rotation (+31.2), which compared favorably with reported values for this compound.19,20 Tosylation of the primary alcohol followed by protection of the secondary alcohol as the corresponding THP ether gave 3 in good overall yield. Displacement of the tosyl group proceeded smoothly with benzylamine to give the intermediate 4. Catalytic hydrogenation of the azide followed by incorporation of a carbobenzyloxy group on to the primary amine yielded the common intermediate 5. Acylation of 5 followed by deprotection of the THP group under mild acidic conditions yielded analogue SD1002. Alternatively, with the intermediate 5, acetoacetylation was achieved with diketene, and subsequent diazo transfer proceeded smoothly with tosylazide. Hydrolysis under mild basic conditions effected deacetylation to yield the desired compound SD1003, which retains the electrophilic end group.
Scheme 1. Synthesis of lead series of peptidomimetics.
Reagents and conditions. (a)TsCl, pyridine; (b) dihydropyran, PPTS, CH2Cl2; (c) benzylamine, THF, reflux; (d) H2, Pd/C, EtOH; (e) PhCH2OC(O)Cl, NaHCO3; (f) PPTS, EtOH, 60 °C; (g) Ac2O, Et3N,DMAP, THF; (h) diketene, EtOH; (i) TsN3, DBU, AcCN; (j) LiOH/H2O; (k) PPTS, EtOH, 40 °C.
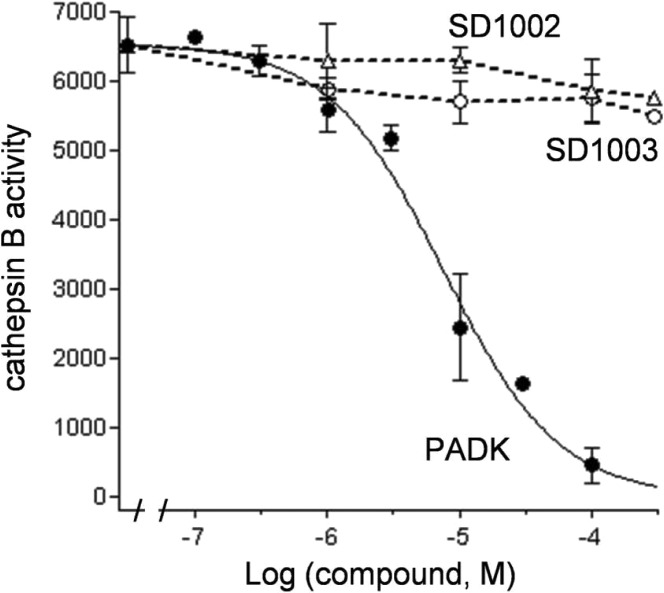
The nonpeptidic compounds SD1002 and SD1003 were evaluated for cathepsin B inhibition since the parent PADK was originally classified as an irreversible cathepsin B inhibitor (Figure 2 and Table 1). PADK weakly inhibited cathepsin B activity (IC50 = 9.4 ± 2.4 μM); however, in hippocampal slices, PADK at 3–10 μM up-regulated multiple cathepsin isoforms without affecting the levels of actin and other proteins (Figure 3a). Note that PADK was unable to enhance cathepsin levels in the presence of brefeldin A (Figure 3b), which impairs endosome to lysosome traffic,21 further indicating as in previous reports22 that the lysosomal enhancement involves modulated trafficking/maturation of cathepsins. Interestingly, cathepsin up-regulation was not observed upon treatment with the cathepsin B inhibitor CA074me that is nearly 100 times more potent than PADK (Table 1). Thus, more potent cathepsin inhibition does not correspond with improved lysosomal modulation. This led us to regard the weak inhibitor PADK more properly as a lysosomal modulator and to expect that similar phenotypic responses could be seen with analogous compounds. In fact, the nonpeptidic analogues possess very weak (SD1003) or negligible (SD1002) cathepsin B inhibition as compared to PADK (Figure 2) but maintain the ability to positively modulate cathepsin levels (Figure 3b).
Figure 2.
Cathepsin B inhibition by peptidomimetics. PADK and nonpeptidyl compounds SD1002 and SD1003 were assessed for their effects on cathepsin B activity. The cathepsin B assay used the fluorogenic substrate Z-Arg-Arg AMC, and mean fluorescence units ± SEMs were normalized to percent control.
Table 1. Extent of Cathepsin B Inhibition and Potencya.
| compd | extent of inhibition (%) | IC50 (μM) |
|---|---|---|
| PADK | 100 (P < 0.0001) | 7–10 |
| SD1002 | 8–11 (NS) | >100 |
| SD1003 | 13–17 (P < 0.01) | 2–5 |
| CA074me | 100 (P < 0.0001) | 0.08–0.11 |
The cathepsin B assay used the fluorogenic substrate Z-Arg-Arg AMC to titrate the effects of different compounds. Nonlinear regression analyses used one-site competition equations to determine percent inhibition and range of IC50 values. Unpaired t tests compared untreated control levels of activity to data from the highest compound concentrations used.
Figure 3.
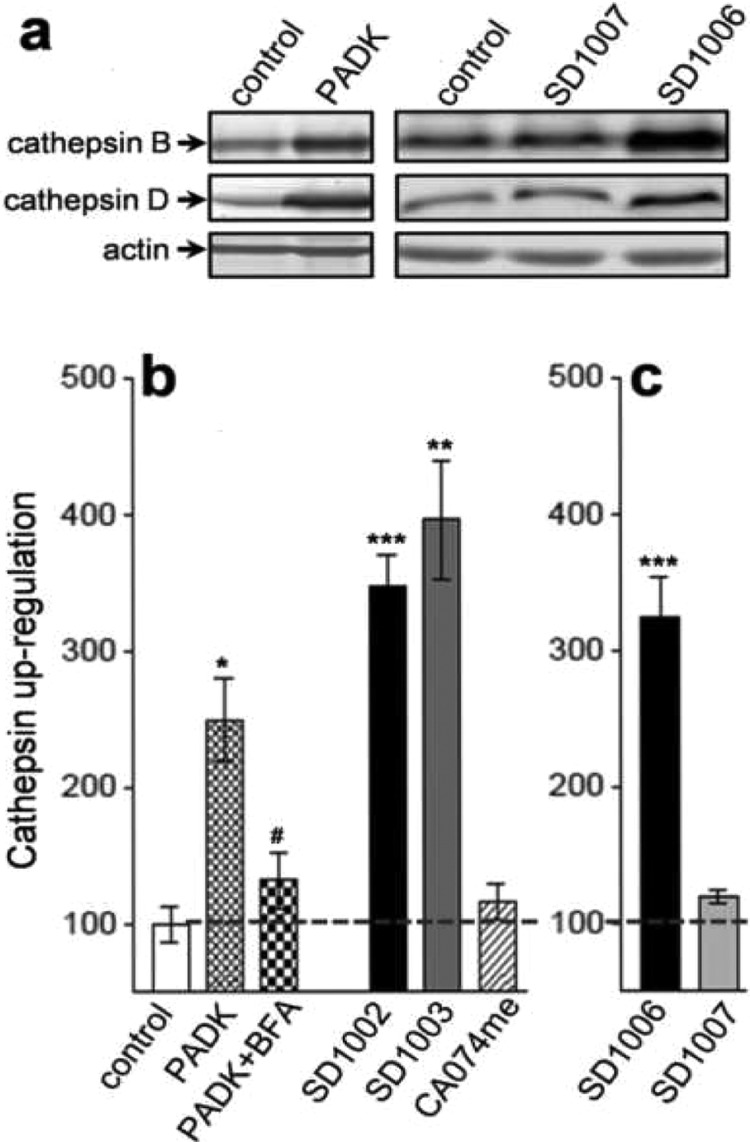
Positive lysosomal modulation in hippocampal slice cultures. Organotypic cultures prepared from rat hippocampi were treated daily with vehicle, PADK, SD1002, or SD1003 (10 μM; n = 4–5 groups of eight slices each), CA074me (0.3–1 μM; n = 6 groups), or PADK in the presence of 1 μg/mL brefeldin A (BFA). (a) After 4 days, slice groups were gently harvested and homogenized, and equal protein aliquots were analyzed by immunoblot for active cathepsin isoforms and actin. (b) The cathepsin immunoreactivity was determined by image analysis, and mean IODs ± SEMs are shown. Representative immunoblots are also shown for a separate experiment in which slice cultures were treated with the enantiomers SD1006 and SD1007 daily at 20 μM (c), twice the parent SD1002's modulatory concentration, to illustrate the stereoselective lysosomal enhancement. Unpaired t tests compared to control slices: *P < 0.01, **P < 0.001, and ***P < 0.0001; test compared to PADK: #P = 0.001.
The synthetic derivative SD1003 produced significant lysosomal modulation in hippocampal slice cultures, as much as twice the level produced by PADK (P = 0.01). Although SD1003 could be a good point to do lead modification, the presence of the reactive diazo group would produce significant concern for a drug lead. The reactive group likely explains SD1003's modest inhibitory activity with a similar IC50 as PADK (see Table 1), while SD1002 in the absence of the diazo group does not exhibit appreciable cathepsin B inhibition, especially not in the 1–10 μM range. Even without the reactive diazo moiety, SD1002 enhanced cathepsin levels to an extent that was also significantly more than that produced by PADK, and no statistical difference was found between the modulatory effects of SD1002 and SD1003 (Figure 3b). Importantly, the effects of the nonprotein reactive SD1002 suggest that the irreversible inhibition is not a prerequisite for lysosomal modulation.
On the basis of the profile of compound SD1002, it is the best suited to serve as a lead compound in future development. To establish additional structure–activity relationships for this new lead modulator, the role of configuration was probed through the synthesis of two diastereoisomers of SD1002 (Scheme 2). The commercially available amino acid derivative Boc-l-Phe 7 was converted into the known protected aminoalcohol through the intermediacy of the cyanohydrins, and reductive amination with benzaldehyde gave 8.23,24 Acylation of the amine gave acetamide 10 and the removal of the Boc group under acidic conditions yielded the free amine. Reacylation with a carbobenzyloxy group was followed by a final desilylation to yield analogue SD1006. Following the same synthetic route, the enantiomer of SD1006 (SD1007) was synthesized from Boc-d-Phe 11.
Scheme 2. Synthesis of Diastereomers of Lead Compound.
Enantioselective synthesis of diastereomers. (a) MeI, NaHCO3, DMF. (b) DIBAL, MeOH. (c) NaHSO3, KCN. (d) TBSCl, imidazole, DMF. (e) H2, 5% Rh/C, EtOH/NH3. (f) Benzaldehyde, NaCNBH3, MeOH. (g) AcCl, Et3N, CH2Cl2. (h) HCl, dioxane. (i) Cbz-Cl, Et3N, CH2Cl2. (j) HF/pyridine, acetonitrile.
The two enantiomers were tested for their ability to up-regulate cathepsins B and D in cultured hippocampal slices. In the right immunoblots of Figure 3a, only the SD1006 treatment caused the positive modulation of active cathepsin isoforms. Similar to previous reports on PADK,11,17 SD1006 has a bigger modulatory effect on cathepsin B than cathepsin D. It is clear from the quantitative results that cathepsin up-regulation is stereospecific, as the SD1007 diastereoisomer shows negligible modulation, whereas the enantiomer SD1006 produces an effect comparable to that of SD1002 (Figure 3c). Interestingly, the active enantiomer SD1006 showed no significant inhibition of cathepsin B activity, indicating that the lysosomal modulation is independent of enzyme inhibition.
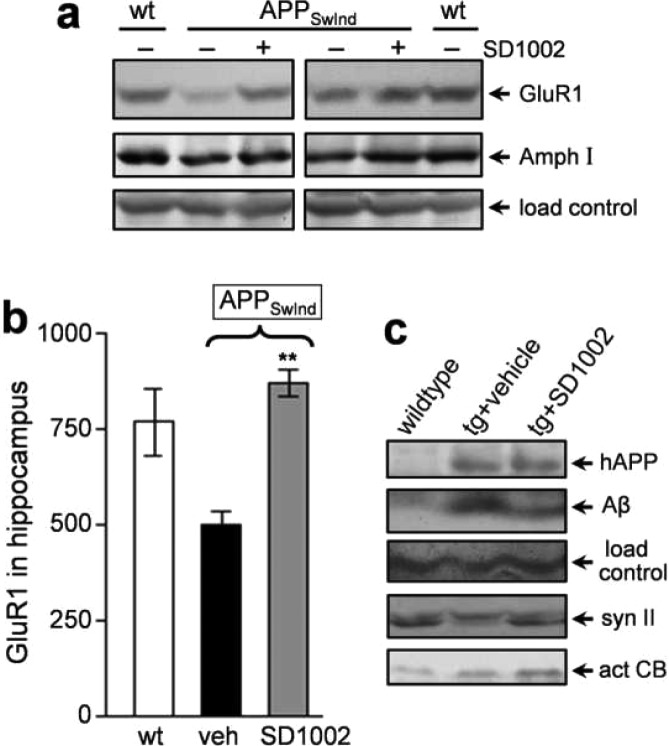
SD1002 was used in a proof-of-concept study for protective enhancement of lysosomal capacity by a nonreactive peptidomimetic in an animal model. We had previously employed this model to study the in vivo lysosomal modulation by PADK. The APPSwInd mice were previously found to exhibit synaptic decline and associated behavioral deficits,17 and here, their hippocampal samples had a 35% reduction in GluR1 as compared to age-matched wild-type mice (P < 0.01; n = 10). The lysosomal modulator SD1002 was administered daily into the APPSwInd mice that express mutant human amyloid precursor protein (hAPP), modified from the B6.Cg-Tg(PDGFB-APPSwInd)20Lms/2J strain (genotype confirmed by PCR on tail DNAs). Loss of the postsynaptic GluR1 protein and the more modest reduction in the presynaptic marker amphiphysin I were both attenuated by SD1002 (Figure 4a). Improved GluR1 levels indicate recovery of synaptic integrity in APPSwInd mice that received 9 days of SD1002 treatment at the initial testing dosage of 25 mg/kg/day (Figure 4b), reaching levels comparable to those found in the nontransgenic control mice. Future animal studies will determine the minimum effective dose. Representative hippocampal samples from the three treatment groups also exhibit a correspondence between SD1002-mediated enhancement of the active form of cathepsin B and the clearance of an 8–10-kDa oligomeric Aβ species, as well as an additional sign of synaptic protection (Figure 4c). SD1002, on the other hand, had no apparent effect on hAPP levels in the transgenic mice.
Figure 4.
SD1002 preserves synaptic markers in APPSwInd mice. The 20-month transgenic mice were injected ip daily with SD1002 (+) or vehicle (−) for 9 days, and wild-type mice (wt) were subjected to vehicle injections. (a) Equal protein aliquots of hippocampal homogenates were analyzed by standard SDS-PAGE and immunoblotting for GluR1 and amphiphysin I (Amph I) as well as a load control. (b) Mean GluR1 immunoreactivity levels ± SEMs are shown for the three treatment groups. Unpaired t test as compared to vehicle-treated transgenic group: **P < 0.001. (c) Representative hippocampal samples were also separated on tris-tricine gels to assess oligomeric Aβ species and the parent hAPP recognized by 6E10 antibody and a load control in the same blot lanes. Standard gel protocol assessed for synapsin II (syn II) and the active form of cathepsin B (act CB) in corresponding samples.
We have demonstrated that it is possible to prepare nonreactive, nonpeptidic analogues of PADK that can function as potent lysosomal modulators. Derivatives SD1002 and SD1006 produce more cathepsin up-regulation than PADK, and SD1002 protects against synaptic compromise in a transgenic model of AD. These findings could pave a way for the discovery of a new series of small molecules as lysosomal modulatory agents for treating neurodegenerative disorders such as AD.
Supporting Information Available
Experimental procedures and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported in part by University of Connecticut's Center for Science and Technology Commercialization, by the Oliver Smithies Grant from the North Carolina Biotechnology Center (Research Triangle Park, NC), and the Alzheimer's Drug Discovery Foundation and the Association for Frontotemporal Dementias. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare the following competing financial interest(s): Prof. Ben Bahr and Prof. Dennis Wright are co-founders of Synaptic Dynamics Inc..
Supplementary Material
References
- Walsh D. M.; Selkoe D. J. Aβ oligomers—A decade of discovery. J. Neurochem. 2007, 101, 1172–1184. [DOI] [PubMed] [Google Scholar]
- Schneider L. S.; Dagerman K. S.; Higgins Julian P. T.; McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch. Neurol. 2011, 68, 991–998. [DOI] [PubMed] [Google Scholar]
- Bendiske J.; Caba E.; Brown Q. B.; Bahr B. A. Intracellular deposition, microtubule destabilization, and transport failure: An “early” pathogenic cascade leading to synaptic decline. J. Neuropathol. Exp. Neurol. 2002, 61, 640–650. [DOI] [PubMed] [Google Scholar]
- Bahr B. A.; Bendiske J. The neuropathogenic contributions of lysosomal dysfunction. J. Neurochem. 2002, 83, 481–489. [DOI] [PubMed] [Google Scholar]
- Cataldo A. M.; Barnett J. L.; Berman S. A.; Li J.; Quarless S.; Bursztejn S.; Lippa C.; Nixon R. A. Gene expression and cellular content of cathepsin D in Alzheimer’s disease brain: Evidence for early up-regulation of the endosomal-lysosomal system. Neuron 1995, 14, 671–680. [DOI] [PubMed] [Google Scholar]
- Callahan L. M.; Vaules W. A.; Coleman P. D. Quantitative decrease in synaptophysin message expression and increase in cathepsin D message expression in Alzheimer disease neurons containing neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 1999, 58, 275–287. [DOI] [PubMed] [Google Scholar]
- Ginsberg S. D.; Hemby S. E.; Lee V. M. -.; Eberwine J. H.; Trojanowski J. Q. Expression profile of transcripts in Alzheimer's disease tangle-bearing CA1 neurons. Ann. Neurol. 2000, 48, 77–87. [PubMed] [Google Scholar]
- Nixon R. A.; Mathews P. M.; Cataldo A. M. The neuronal endosomal-lysosomal system in Alzheimer's disease. J. Alzheimer's Dis. 2001, 3, 97–107. [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M.; DeGuzman T.; Kenessey A.; Palkovits M.; Lajtha A. The distribution of cathepsin D activity in adult and aging human brain regions. J. Neurochem. 1992, 58, 2207–2211. [DOI] [PubMed] [Google Scholar]
- Bi X.; Yong A. P.; Zhou J.; Gall C. M.; Lynch G. Regionally selective changes in brain lysosomes occur in the transition from young adulthood to middle age in rats. Neuroscience (Oxford) 2000, 97, 395–404. [DOI] [PubMed] [Google Scholar]
- Bendiske J.; Bahr B. A. Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis—An approach for slowing Alzheimer disease?. J. Neuropathol. Exp. Neurol. 2003, 62, 451–463. [DOI] [PubMed] [Google Scholar]
- Butler D.; Brown Q. B.; Chin D. J.; Batey L.; Karim S.; Mutneja M. S.; Karanian D. A.; Bahr B. A. Cellular Responses to Protein Accumulation Involve Autophagy and Lysosomal Enzyme Activation. Rejuvenation Res. 2005, 8, 227–237. [DOI] [PubMed] [Google Scholar]
- Mueller-Steiner S.; Zhou Y.; Arai H.; Roberson E. D.; Sun B.; Chen J.; Wang X.; Yu G.; Esposito L.; Mucke L.; Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: Implications for Alzheimer's disease. Neuron 2006, 51, 703–714. [DOI] [PubMed] [Google Scholar]
- Bahr B. A. Lysosomal modulatory drugs for a broad strategy against protein accumulation disorders. Curr. Alzheimer Res. 2009, 6, 438–445. [DOI] [PubMed] [Google Scholar]
- Ryzhikov S.; Bahr B. A. Gephyrin alterations due to protein accumulation stress are reduced by the lysosomal modulator Z-Phe-Ala-diazomethylketone. J. Mol. Neurosci. 2008, 34, 131–139. [DOI] [PubMed] [Google Scholar]
- Bahr B. A.; Wisniewski M. L.; Butler D. Positive Lysosomal Modulation As a Unique Strategy to Treat Age-Related Protein Accumulation Diseases. Rejuvenation Res. 2012, 15, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D.; Hwang J.; Estick C.; Nishiyama A.; Kumar S. S.; Baveghems C.; Young-Oxendine H. B.; Wisniewski M. L.; Charalambides A.; Bahr B. A. Protective effects of positive lysosomal modulation in Alzheimer's disease transgenic mouse models. PLoS One 2011, 6, e20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K.; Thompson W. J.; Holloway M. K.; McKee S. P.; Duong T. T.; Lee H. Y.; Munson P. M.; Smith A. M.; Wai J. M.; et al. Potent HIV protease inhibitors: The development of tetrahydrofuranylglycines as novel P2-ligands and pyrazine amides as P3-ligands. J. Med. Chem. 1993, 36, 2300–2310. [DOI] [PubMed] [Google Scholar]
- Dubey A. K.; Goswami D.; Chattopadhyay A. A simple and inexpensive procedure for low valent copper mediated benzylation of aldehydes in wet medium. ARKIVOC (Gainesville, FL, U. S.) 2010, 137–145. [Google Scholar]
- Park S.; Lee S.; Kang H. Efficient synthesis of 2(S)-[1(S)-azido-2-phenylethyl]oxirane. Bull. Korean Chem. Soc. 2008, 29, 1073–1074. [Google Scholar]
- Turner M. D.; Arvan P. Protein traffic from the secretory pathway to the endosomal system in pancreatic β-cells. J. Biol. Chem. 2000, 275, 14025–14030. [DOI] [PubMed] [Google Scholar]
- Butler D.; Hwang J.; Estick C.; Nishiyama A.; Kumar S. S.; Baveghems C.; Young-Oxendine H. B.; Wisniewski M. L.; Charalambides A.; Bahr B. A. Protective effects of positive lysosomal modulation in Alzheimer’s disease transgenic mouse models. PLoS One 2011, 6, e20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris K. D.; Hoover D. J.; Damon D. B.; Davies D. R. Synthesis and crystallographic analysis of two rhizopuspepsin inhibitor complexes. Biochemistry 1992, 31, 8125–8141. [DOI] [PubMed] [Google Scholar]
- Boger J.; Payne L. S.; Perlow D. S.; Lohr N. S.; Poe M.; Blaine E. H.; Ulm E. H.; Schorn T. W.; LaMont B. I.; et al. Renin inhibitors. Syntheses of subnanomolar, competitive, transition-state analog inhibitors containing a novel analog of statine. J. Med. Chem. 1985, 28, 1779–1790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.