Abstract
In recent years, the pharmaceutical industry has faced many challenges to its business model, undergoing tremendous change and turmoil to survive. Are there any lessons to be drawn from drug discovery focused on Global Health, where there is little market incentive?
The pharmaceutical industry is in trouble. Although unlikely he had drug discovery in mind, baseball player/manager Yogi Berra may have gotten it right when he said, “We made too many wrong mistakes.” No doubt playing baseball is different from drug discovery (at least in baseball you know you have a home run even before you round first base), but it doesn't take a genius to know when something is not going well. The intellectual part comes later, when deciding what to do about it.
Most in the industry will readily admit too many wrong mistakes were made, and the data supporting this point of view (we are scientists, after all) are overwhelming. One need only pick up the business section of the newspaper to find a proliferation of articles describing companies pulling out of therapeutic areas, downsizing, reorganizing, and merging—all visible signs of the turmoil within the industry. In a growing trend, Pharma CEOs and heads of R&D publicly admit failure, loss of productivity, lack of innovation, and a need for renewal. The picture is stark and undeniable: current trends make the industry unsustainable, as poignantly illustrated by Figure 1, showing how many drugs one gets for $1B charted between 1950 and 2010. A billion dollars doesn't go very far in drug development today or, as Yogi put it, “A nickel ain't worth a dime anymore.” One could spend weeks arguing over the causes—who's to blame, how it could have been avoided—but sometimes, a picture says it all. The rest is moot.
Figure 1.
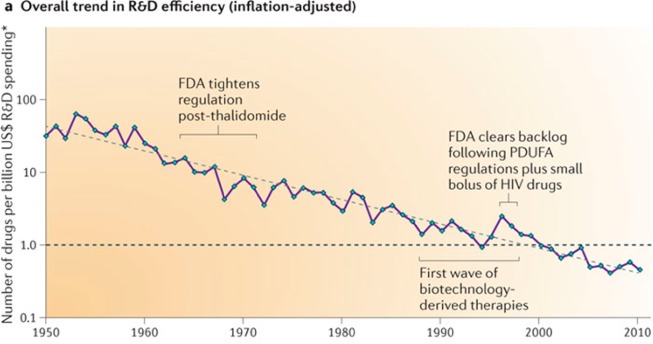
The number of drugs per billion U.S.$ R&D spending over time.1
However, the more pertinent question is not what the wrong mistakes were, but what are the correct corrections? Perhaps we can use our understanding of evolution to guide us—building off the knowledge that only the strongest survive under stringent and restricted conditions. Maybe if we are looking for examples of where R&D has succeeded, we should look where the conditions are most stringent, the funding most restricted, and (hence) the evolutionary pressure the most intense—at Global Health drug discovery.
Really, Global Health drug discovery? Granted, here too, there has been failure, and there is still much work to be done. However, take a closer look at the organizations and institutions doing this work, and you will find highly honed and motivated research and development groups (there is no fat when it comes to these diseases) developing products on a fraction of a big Pharma R&D budget. Visit the Web sites of Product Development Partnerships (PDPs) such as Medicines for Malaria Venture (MMV), Drugs for Neglected Diseases Initiative (DNDi), and the Global Alliance for TB Drug Development (TB Alliance) to view the drug development pipelines that they have established, and you will hopefully be convinced that they must be doing something right, but what are they doing, and what lessons can be drawn?
The PDPs have retained many of the best aspects of big Pharma. Like big Pharma, they employ experienced drug discoverers to guide their programs and build collaborative partnerships with a wide variety of groups, but equally important, they have assiduously avoided the aspects of big Pharma that work less well and have adapted new approaches, platforms, and collaborative models that are foreign (or anathema) to big Pharma. This is where the lessons are to be found.
Lesson #1 is obvious but often goes unheeded: go after compounds, not targets. GlaxoSmithKline embraced the genomics approach in its antibacterial program and learned this lesson the hard way, pursuing novel targets with 70 HTS target-based screens. For these heroic efforts, they were repaid with only 16 screens providing hits, and only 5 screens yielding leads.2 Likewise, a plethora of obesity targets were pursued in the pharmaceutical industry during the early 2000s—many of them chemogenomically “validated” in a rodent model, only to fall out in clinical development.3 Here, we learned a hard lesson that any toxicologist could have told us long ago—there is more than one way to stop a rat from eating, and not all of them are good. As scientists, we do not like to admit it, but reductionism does not always work well in drug discovery. Consider MMV, which partnered to screen over 5 million compounds in a phenotypic blood-stage malaria assay and identified 25,000 hits with <1 μM activity. In follow up biochemical assays, scientists were unable to link these hits to any of the top targets everyone in the field was working on. This brings up an embarrassing point; we simply are not very good at picking drug targets.
Lesson #2, a suggestion, might be considered heretical by some. Maybe some things, such as chemical libraries and HTS hits, should be considered as precompetitive. “Our compound library, the best in the industry, is unique and is the engine that drives our drug discovery program” is the retort. However, closer inspection of the published journal and patent literature belies this: your chemical library and hits are not as unique are you think they are, and there is more to be gained by sharing these than by hiding them. The PDPs have shown this to be true. “Ah, but they work in a nonprofit area, and do not need to rely on commercial return,” comes the refrain. True, but increased efficiency behooves nonprofit and for-profit endeavors alike, and the truly innovative and inventive steps (and IP) come not from the hit but from what is done with it. Medicinal chemists know this—show a group of them a hit structure, and there will be as many ideas as there are chemists. Once you accept the distinction between precompetitive and competitive, a world of new possibilities suddenly opens up. Maybe other things, like computational tools, predictive models, molecular probes and reagents, technical know-how—even technology platforms—might be shared. “This would be risky” some might say. True, but Figure 1 suggests that continuing business as usual is even riskier.
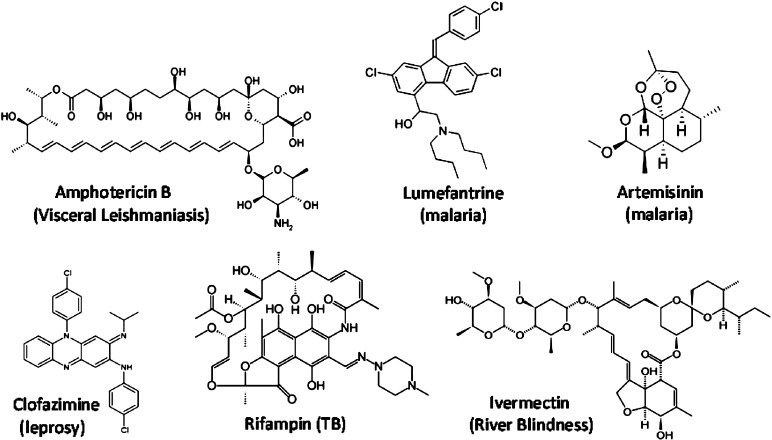
Lesson #3 is simple: be open-minded, and do not be afraid to take risks. For example, Lipinski's rules, originally developed to flag risk, have been mistranslated into “thou shalt not” rules. For some good examples of what “thou shalt not”, take a look at the chemical structure of some of the leading drugs used in the Global Health drug armamentarium (Figure 2, disease treatment in parentheses). Amphotericin B, Ivermectin, Rifampin, and Artemisinin—these do not look like “drugs”—many would claim they are downright ugly. Nonetheless, millions of lives have been saved using these compounds, ugly as they are, and the world would be much worse off without them. The lesson is simple: if you work in a risky business and risk is essential for success, then embrace it. There are many ways to do this. For example, the Foundation has established a Discovery program specifically designed to take risks, entitled Grand Challenges Explorations,4 which specifically encourages (and funds) high-risk approaches and ideas that have the potential to be game-changing. To achieve this, we are willing to accept failure—after all, how many transformational advances do you need to succeed?
Figure 2.
Structures of select Global Health drugs.
Lesson #4 is to have a long-term strategic vision and stay with it. For example, our PDP partners have identified therapeutic areas where this is great unmet medical need and are committed to their cause until the problem is solved. Patient need, not NPV based upon a series of assumptions, drives their programs and sustains their commitment. No doubt the situation is tricky in big Pharma—where the economic and leadership stability conducive to long-term vision is rapidly fading (some might argue gone) and market forces favor short-term returns over long-term success. Nonetheless, a strong argument can be made that some of the most successful companies are those that have had a sustained vision. It is not hard to find areas of unmet medical need; Alzheimer's disease, cancer, diabetes (to name a few)—why not pick a few and stay with them until the vision is fulfilled?
Drug discovery is an amazing endeavor, requiring incredible science, technology, intellect, collaboration, hard work, and perseverance. However, these qualities alone are not enough to ensure continued success—such virtues are engraved on many a mossy tombstone of now long-gone endeavors. Monks hand-scribing books, sailors hunting down whales on the open seas, photographic film developing—these all were once thriving industries. Then, the world changed— the Gutenberg printing press, oil wells, and digital cameras emerged, and these once robust industries died. Will the pharmaceutical industry suffer a similar fate? It is hard to imagine this happening, given the tremendous skill, intellect, and opportunities available. However, what is clear is that the pharmaceutical industry has come to a point where it must address some key and fundamental questions, and they must be willing and able to adjust their model and forge new paths forward. This is by no means an easy thing to achieve, but the big Pharma CEOs and Research Heads must summon their courage to voyage out into these unexplored and uncertain areas. Yogi might have some sage advice for them—“if you come to a fork in the road, take it.”
The authors declare no competing financial interest.
References
- Reproduced with permission from:Scannell J. W.; Blanckley A.; Boldon H.; Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nature Rev. Drug Discovery 2012, 11, 191–200. [DOI] [PubMed] [Google Scholar]
- Payne D. J.; Gwynn M. N.; Holmes D. J.; Pompliano D. L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nature Rev. Drug Discovery 2007, 6, 29–40. [DOI] [PubMed] [Google Scholar]
- Yao F.; MacKenzie R. G. Obesity Drug Update: The Lost Decade?. Pharmaceuticals 2010, 3, 3494–3521. [Google Scholar]
- For more information, go to www.grandchallenges.org/explorations/Pages/introduction.aspx.