Abstract
The synthesis of several 2,2-dialkyladamantyl-1-amines through the combination of a Ritter reaction with a Wagner–Meerwein rearrangement from noradamantane alcohols is reported. Several of the novel amines displayed low micromolar activities against several H1N1 influenza virus strains, including the amantadine-resistant A/PuertoRico/8/34 strain. Most of the compounds did not show cytotoxicity for MDCK cells.
Keywords: amantadine, influenza, M2 channel, Ritter reaction, Wagner−Meerwein rearrangement
Influenza A virus is a global cause of significant morbidity and mortality, which is related to its easy transmission and ability to escape from existing immunity. The possible introduction into the human population of emerging swine and highly pathogenic avian influenza viruses is considered a global health threat.1 Currently available drugs for the treatment of influenza virus infections comprise the M2 ion channel blockers amantadine and rimantadine (Chart 1)2,3 and the neuraminidase inhibitors oseltamivir and zanamivir.4 However, most of the currently circulating influenza strains are resistant to the M2 ion channel blockers, and resistance to the neuraminidase inhibitors (in particular oseltamivir) is also on rise.5−9 Accordingly, novel anti-influenza virus drugs are urgently needed.
Chart 1. Structures of Amantadine and Rimantadine.
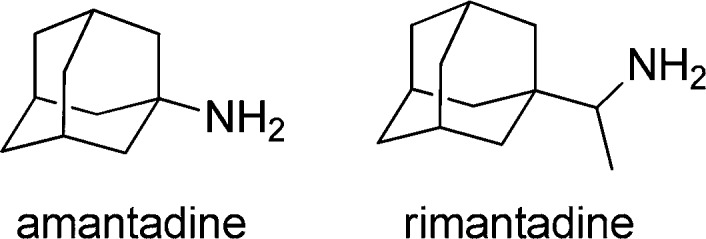
Taking into account that amantadine was initially licensed in the United States in 1966, it is not surprising that many hundreds of derivatives have been synthesized and tested, including 1-substituted adamantanes, 2-substituted adamantanes, azaspiroadamantanes, aminospiroadamantanes, 1,2-annulated adamantane derivatives, and 2-azaadamantanes.10 Interestingly, while several of them proved to be active against amantadine-sensitive strains [containing the wild-type (wt) M2 protein], only a few were active against amantadine-resistant strains.
To the best of our knowledge, 2,2-dialkylamantadines have not been synthesized so far. To explore whether the addition of two alkyl groups can be beneficial for the antiviral activity of amantadine, we decided to synthesize and evaluate the antiviral activity of a series of 2,2-dialkylamantadines starting from the known 3-noradamantanecarboxylic acid, taking advantage of a unique combination of the Ritter reaction with the Wagner–Meerwein rearrangement.
In the study reported here, we found that several 2,2-dialkylamantadines display low micromolar activity against several influenza virus strains. Interestingly, this activity was markedly subtype-dependent. While most of the compounds were active against A/H1N1 strains, whether carrying an amantadine-sensitive M2 channel or a mutated, amantadine-resistant M2 channel, neither of the compounds was active against influenza A/H3N2 strains.
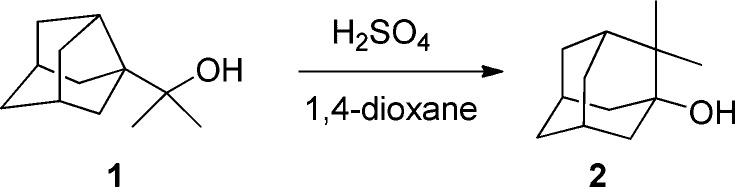
In 1993, Stoelting and Shiner reported that the solvolysis of several 1-(3-noradamantyl)ethyl sulfonates led to 2-methyl-1-adamantanol, in excellent yields. In a similar way, 2-(3-noradamantyl)propan-2-ol (1) led to 2,2,-dimethyl-1-adamantanol (2) in high yield (Scheme 1).11 Presumably, the large release of strain promotes the observed Wagner–Meerwein rearrangement.
Scheme 1. Rearrangement of Noradamantyl Alcohol 1 to Adamantanol 2.
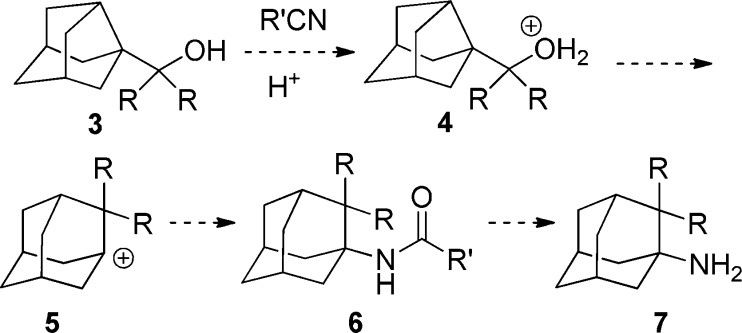
In the most familiar form of the Ritter reaction, an alcohol is treated with strong acid to generate a carbenium ion. The nitrile then reacts with it to produce a nitrilium ion whose hydrolysis affords an amide product.
Thus, we envisaged that submitting 1-(3-noradamantyl)-1,1-dialkylmethanols (3) to a Ritter reaction may led to N-(2,2-dialkyl-1-adamantyl)amides (6), through the Wagner–Meerwein rearrangement of the initially generated carbenium ion. Hydrolysis of these amides should lead to the expected 2,2-dialkylamantadine derivatives (7) (Scheme 2). Interestingly, although the combination of a Ritter reaction with a Wagner–Meerwein rearrangement has been used for mechanistic studies,12,13 its combination for synthetic purposes has been very limited.14−17
Scheme 2. Attempted Synthesis of 2,2-Dialkylamantadines 7 from Noradamanyl Alcohols 3.
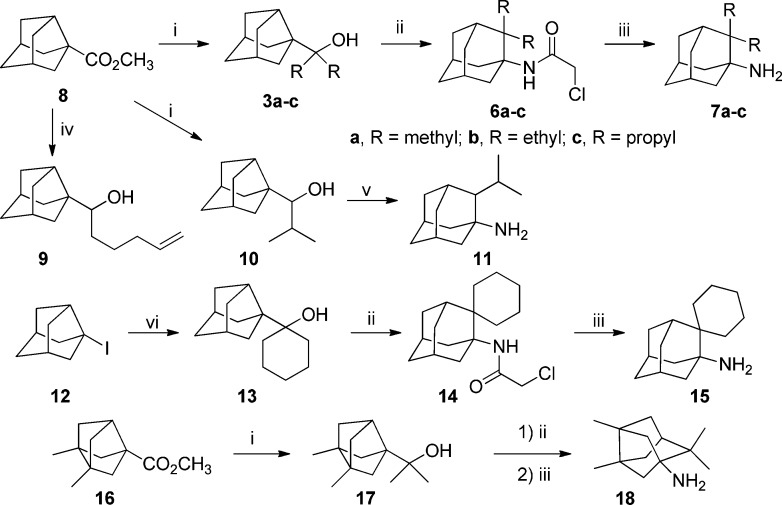
First, we studied the suitability of our approach using alcohol 3a,11 obtained in 91% yield from known ester 8.18 Jirgensons et al. had described that, while the acetamides resulting from the Ritter reaction of tertiary alcohols were very difficult to hydrolyze, the corresponding chloroacetamides underwent easy cleavage to the corresponding tert-alkylamines using thiourea.19
Thus, the reaction of tertiary alcohol 3a with chloroacetonitrile using the standard conditions developed by Jirgensons et al. led to the expected rearranged chloroacetamide 6a in 89% yield. Cleavage of the chloroacetyl group with thiourea furnished 2,2-dimethylamantadine, 7a, in 78% yield (Scheme 3).
Scheme 3. Synthesis of Amantadine Analogues through Ritter Reaction and Wagner–Meerwein Rearrangement.
Reagents and conditions: (i) For 3a: CH3Li, Et2O, reflux, overnight, 91% yield; for 3b: CH3CH2Li, Et2O, reflux, overnight, 56% yield; for 3c: CH3CH2CH2MgCl, THF, reflux, overnight, 89% yield; for 10: (CH3)2CHMgBr, Et2O, reflux, overnight, 75% yield; for 17: CH3Li, Et2O, reflux, overnight, 51% yield. (ii) ClCH2CN, AcOH, H2SO4, rt, overnight, 89% for 6a; 89.5% for 6b; 91% for 6c; 38% for 14; and 93% from 17. (iii) Thiourea, ethanol, AcOH, reflux, overnight, 78% for 7a; 64% for 7b; 95% for 7c; 62% for 15; and 74% for 18. (iv) BrMg(CH2)5MgBr, THF, reflux, 24 h, 93% yield. (v) Urea, CF3CO2H, 115 °C, overnight, 43%. (vi) t-BuLi, Et2O, pentane, −78 °C to room temperature, 56% yield.
After successfully proving the viability of our approach, we synthesized a series of tertiary alcohols from ester 8. The alcohols were subsequently transformed into the corresponding 2,2-dialkylamantadine derivatives in high yields (Scheme 3). Remarkably, while the reaction of ester 8 with methyllithium, ethyllithium, and propylmagnesium chloride led to the expected tertiary noradamantyl alcohols in good yields, addition of an excess of isopropylmagnesium bromide to 8 led to a secondary alcohol, 10. Similarly, the reaction of 8 with pentamethylenebis(magnesium bromide) did not furnish 13, but 1-(3-noradamantyl)-5-hexen-1-ol, 9, in 93% yield. The reductive behavior of organometallic reagents on steric encumbered ketones is well documented, and these alcohols may arise from the reduction by a magnesium species of the initially obtained alkyl noradamantyl ketones.20 We finally succeeded in the synthesis of the cyclohexanol 13 using the known iodide 12 as the starting material.21 The reaction of 12 with t-butyllithium followed by addition of cyclohexanone led to the expected alcohol in 56% yield. Alcohol 13 smoothly underwent the Ritter reaction to the chloroacetamide 14, which was cleaved with thiourea to amine 15 (Scheme 3). Of note, from 10, we directly obtained the rearranged amine 11 using an excess of urea in trifluoroacetic acid at 115 °C in 43% yield. This one-step procedure for the synthesis of the amine from the alcohol seems to be general, as the application of this procedure to the alcohol 6c led to the corresponding amine 7c in 31% yield. However, the yield for the one-step procedure was much lower than the overall yield obtained using the cloroacetonitrile method.
Interestingly, our approach was also successfully implemented in the ring expansion of a bisnoradamantane derivative to the corresponding noradamantane amine. Thus, reaction of known ester 16 with excess of methyllithium followed by the application of Jirgensons' conditions to the alcohol 17 led to the noradamantane derivative 18 in good yield (Scheme 3).22
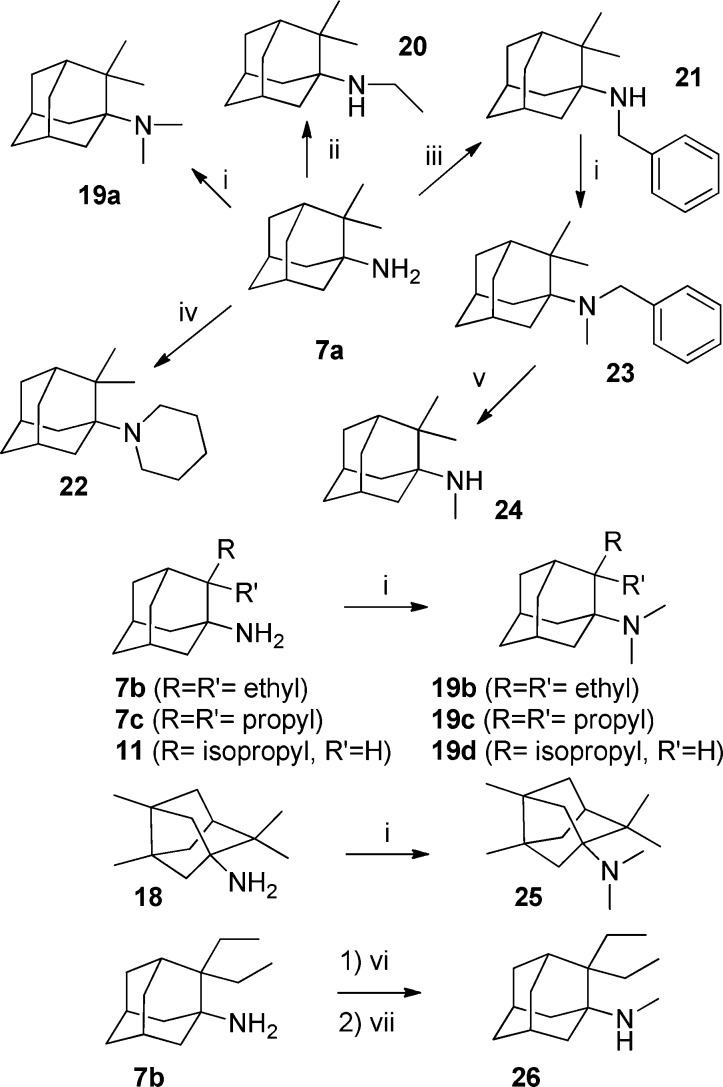
To gain further insight into the structure–activity relationship SAR, a small series of N-alkylated and N,N-dialkylated derivatives were synthesized from adamantylamine 7a, using standard amine chemistry (Scheme 4). Thus, from 7a, reductive alkylation with formaldehyde, acetaldehyde, or benzaldehyde and NaCNBH3 led to amines 19a, 20, and 21, respectively, in high yield. Several attempts to synthesize the N,N-diethylderivative of 7a, either from 7a or from 20, met with failure, probably as a consequence of the steric hindrance. Alkylation of 7a with 1,5-dibromopentane led to piperidine derivative 22 in low yield. Reductive methylation of secondary amine 21 with formaldehyde and NaCNBH3 led, in 80% yield, to the tertiary amine 23, which on catalytic hydrogenation quantitatively furnished secondary amine 24. Similarly, N,N-dimethylderivatives of amines 7b, 7c, 11, and 18 were also synthesized, in high yields, by reductive alkylation with formaldehyde and NaCNBH3. Finally, secondary amine 26 was synthesized by treatment of 7b with methyl chloroformiate followed by reduction with LiAlH4 in 92% overall yield (Scheme 4).
Scheme 4. Synthesis of N- and N,N-Substituted 2,2-Dialkylamantadines and Related Compounds.
Reagents and conditions: (i) H2CO, NaBH3CN, methanol, room temperature, overnight; 84% for 19a; 31% for 19b; 83% for 19c; 68% for 19d; 73% for 23; and 96% for 25. (ii) CH3CHO, NaBH3CN, methanol, rt, overnight, 84% yield. (iii) C6H5CHO, NaBH3CN, methanol, room temperature, overnight, 83% yield. (iv) 1,5-Dibromopentane, NaI, Et3N, anh. DMF, 60 °C, 26 h, 22% yield. (v) H2, Pd/C, ethanol, 38 atm, 100 °C, 24 h, 80% yield. (vi) ClCO2CH3, Et3N, Et2O, room temperature, overnight. (vii) LiAlH4, THF, reflux, 20 h, 62% yield overall.
The structure of all new compounds was confirmed by elemental analysis and/or accurate mass measurement, IR, 1H NMR, 13C NMR, and mass spectral data. The amines were fully characterized as their corresponding hydrochloride or tartrate salts. Moreover, the structure of chloroacetamide 14 was established by X-ray crystallography.23
Antiviral cell culture assays were performed to determine the antiviral activity of all of the synthesized compounds against a broad panel of DNA and RNA viruses. None of the compounds displayed activity against the enveloped DNA viruses herpes simplex virus or vaccinia virus; the enveloped RNA viruses feline coronavirus, parainfluenza-3 virus, respiratory syncytial virus, vesicular stomatitis virus, sindbis virus, or Punta Toro virus, or the nonenveloped RNA viruses Coxsackievirus B4 and Reovirus-1.
In influenza virus-infected Madin–Darby canine kidney (MDCK) cells, several compounds displayed low micromolar activity against the influenza A/H1N1 subtype, but no compound was active against the influenza A/H3N2 subtype (Table 1). The antiviral data obtained by microscopic inspection of the viral cytopathic effect (CPE) at day 3 postinfection were confirmed by a colorimetric cell viability assay.24 As anticipated, all compounds proved to be inactive against influenza B virus, which is known to be insensitive to amantadine and rimantadine.
Table 1. Antiviral Activity in Influenza Virus-Infected MDCKa Cells.
| antiviral EC50b (μM) |
||||||||
|---|---|---|---|---|---|---|---|---|
| influenza A/H1N1 |
influenza
A/H3N2 |
influenza B |
cytotoxicity (μM) |
|||||
| compd | CPE | MTS | CPE | MTS | CPE | MTS | MCCc | CC50d |
| 7a | 15 ± 3 | 10 ± 3 | >100 | >100 | >100 | >100 | 167 ± 33 | 77 ± 20 |
| 7b | 2.0 ± 0.0 | 1.7 | >100 | >100 | >100 | >100 | ≥100 | >100 |
| 7c | >100 | >100 | >100 | >100 | >100 | >100 | 6.0 ± 4.0 | 2.9 ± 1.4 |
| 11 | 5.8 | 1.2 ± 0.1 | >100 | >100 | >100 | >100 | 5.3 ± 2.1 | 8.6 ± 3.5 |
| 15 | 1.1 ± 0.1 | 0.9 ± 0.2 | >100 | >100 | >100 | >100 | 6.0 ± 4.0 | 4.7 ± 1.1 |
| 18 | 4.6 ± 2.4 | 4.5 ± 2.4 | >100 | >100 | >100 | >100 | ≥100 | >100 |
| 19a | 4.0 ± 0.0 | 4.8 ± 0.1 | >100 | >100 | >100 | >100 | 110 ± 90 | 68 ± 26 |
| 20 | >100 | >100 | >100 | >100 | >100 | >100 | 200 ± 0 | 85 ± 3 |
| 21 | >100 | >100 | >100 | >100 | >100 | >100 | 4 | 1.1 |
| 22 | >100 | >100 | >100 | >100 | >100 | >100 | 4 | 0.8 |
| 23 | >100 | >100 | >100 | >100 | >100 | >100 | 4 | 0.7 |
| 24 | 6.0 ± 1.0 | 10.0 ± 0 | >100 | >100 | >100 | >100 | 110 ± 90 | 70 ± 28 |
| 19b | 4.0 ± 2.5 | 9.6 | >100 | >100 | >100 | >100 | ≥100 | >100 |
| 26 | 4.0 ± 2.5 | >100 | >100 | >100 | >100 | ≥100 | >100 | |
| 19c | 6.0 ± 1.9 | 6.7 ± 0.8 | >100 | >100 | >100 | >100 | ≥100 | >100 |
| 25 | 5.8 ± 2.1 | 5.7 ± 2.4 | >100 | >100 | >100 | >100 | ≥100 | >100 |
| 19d | 5.1 ± 3.2 | 6.0 ± 1.2 | >100 | >100 | >100 | >50 | >100 | ≥100 |
| amantadine | 53 ± 11 | 3.4 ± 1.7 | >100 | >100 | 500 | >100 | ||
| rimantadine | 63 ± 18 | 0.17 ± 0.08 | >100 | >100 | ≥100 | >100 | ||
| ribavirin | 7.9 ± 0.6 | 8.2 ± 1.4 | 7.7 ± 1.2 | 7.0 ± 0.4 | 8.0 ± 1.7 | 8.1 ± 3.4 | >100 | ≥20 |
MDCK: Madin–Darby canine kidney cells.
Virus strains: A/PR/8/34 (A/H1N1), A/HK/7/87 (A/H3N2), and B/HK/5/72. The EC50 represents the 50% effective concentration, or compound concentration producing 50% inhibition of virus replication, as determined by microscopic scoring of the CPE, or by the MTS cell viability test.
MCC: minimum cytotoxic concentration or compound concentration producing minimal alterations in cell morphology.
CC50: 50% cytotoxic concentration, as determined by the MTS cell viability test. Values shown are the mean ± SEM of 2–5 determinations.
Analysis of the data in Table 1 revealed the following trends. First, with the single exception of the dipropyl derivative 7c, all of the primary amines were more potent than amantadine and rimantadine against the A/PR/8/34 strain of influenza A/H1N1. Although the more potent compound was the spiroderivative 15 (EC50 = 1.1 μM), the highest selectivity (i.e., ratio of cytotoxic to antiviral concentration) was noted with compound 7b (EC50 = 2.0 μM; ratio of CC50 to EC50 > 50). Second, while the introduction of one or two methyl groups into the primary amine was beneficial (i.e., 7c vs 19c) or practically neutral with regard to antiviral activity (i.e., 7b vs 19b or 26, 18 vs 25, and 11 vs 19d), the introduction of larger groups was clearly deleterious for the activity, leading largely to inactive, cytotoxic compounds.
The antiviral effect of three compounds displaying good selectivities, 7b (a primary amine), 19b (a tertiary amine), and 26 (a secondary amine), was further evaluated against a broader panel of influenza A/H1N1 and A/H3N2 viruses, including two amantadine sensitive A/H1N1 strains (A/Ned/378/05 and A/FM/1/47) and X-31, a chimeric strain containing the H3 and N2 proteins of A/Aichi/2/68 and the other proteins (including M2) from the amantadine-resistant A/PR/8/34 strain. As shown in Table 2, the three compounds behave similarly, showing activity against the three A/H1N1 strains and being inactive against the three A/H3N2 strains.
Table 2. Activity against a Broader Panel of Influenza A/H1N1 and A/H3N2 Viruses.
| antiviral
activity (EC50 in μM)a |
|||||||
|---|---|---|---|---|---|---|---|
| A/H1N1 subtype |
A/H3N2 subtype |
||||||
| compd | A/PR/8/34 | A/Ned/378/05 | A/FM/1/47 | A/HK/7/87 | A/Ishikawa/7/82 | A/X-31 | cytotoxicity (MCC in μM)b |
| 7b | 1.2 ± 0.5 | 4.7 ± 1.3 | 7.0 ± 0.0 | >100 | >100 | >100 | 100 |
| 19b | 0.70 ± 0.39 | 23 ± 3 | 43 ± 12 | >100 | >100 | >100 | ≥100 |
| 26 | 5.0 ± 2.5 | 8.7 ± 0.3 | 9.3 ± 0.3 | >100 | >100 | >100 | 100 |
| amantadine | 53 ± 11 | 2.0 ± 0.0 | 5.1 ± 2.4 | 3.4 ± 1.7 | 11 ± 8 | 61 ± 12 | 500 |
| rimantadine | 63 ± 18 | 0.50 ± 0.22 | 1.9 ± 1.1 | 0.17 ± 0.08 | 0.45 ± 0.15 | 7.0 ± 1.7 | ≥100 |
| oseltamivir carboxylate | 21 ± 9 | 1.5 ± 0.8 | 16 ± 2 | 29 ± 5 | 3.0 ± 1.0 | 0.11 ± 0.05 | >100 |
| zanamivir | 6.0 ± 1.3 | 1.7 ± 0.5 | 5.0 ± 1.6 | 1.2 ± 0.9 | 8.7 ± 3.8 | 0.16 ± 0.05 | >200 |
| ribavirin | 8.7 ± 0.7 | 7.6 ± 1.0 | 10 ± 1 | 8.6 ± 0.4 | 8.8 ± 0.3 | 8.8 ± 0.2 | ≥100 |
The EC50 represents the compound concentration producing 50% inhibition of virus replication, as determined by microscopic scoring of the CPE.
MCC: minimum cytotoxic concentration or compound concentration producing minimal alterations in cell morphology. See ref (24) for a description of the virus strains. Values shown are the mean ± SEM of 2–5 determinations.
It is well-known that the target of amantadine and rimantadine is the influenza A virus M2 channel protein and that a single S31N mutation in M2 is sufficient to render the virus resistant to both drugs.25−29 As most of the currently circulating strains of influenza A virus of the A/H3N2 or A/H1N1 subtype carry the S31N mutation in M2, there is an urgent need for the development of novel anti-influenza drugs that are effective against the most common amantadine-resistant mutants.7,30 The influenza A/PuertoRico/8/34 strain that we used has an M2 protein carrying two substitutions associated with amantadine resistance (i.e., S31N and V27T), and this strain was particularly sensitive to our new amines.
From the results in Table 2, it appears that the target of these new amantadine analogues is not the M2 protein, as the compounds displayed antiviral activity against all of the studied A/H1N1 strains, regardless of whether they carried a wt, amantadine-sensitive, or a mutated, amantadine-resistant M2 protein. Moreover, the new derivatives are inactive against all A/H3N2 strains studied, also regardless of carrying a wt or a mutated M2 protein.
To sum up, we have synthesized, fully characterized, and evaluated a series of novel 2,2-disubstituted amantadine analogues. Six compounds (7b, 18, 19a, 19b, 19d, and 26) were endowed with EC50 values up to 1 order of magnitude lower than that of amantadine, while not being cytotoxic. Three of the new amines displayed low micromolar activities against several A/H1N1 strains, including the amantadine-resistant A/PR/8/34 strain, while being inactive against A/H3N2 strains. The mechanism of action of these compounds is currently under study.
Acknowledgments
E.V. and L.N. acknowledge the technical assistance from L. Persoons and W. van Dam.
Glossary
Abbreviations
- CPE
cytopathic effect
- MDCK
Madin–Darby canine kidney
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- wt
wild-type
Supporting Information Available
Experimental procedures for the synthesis and characterization of novel compounds, a structural figure with probability ellipsoids and the CIF file from the X-ray of compound 14, antiviral assays in influenza virus-infected MDCK cells, and cytotoxicity assays. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors.
This work was funded by the Spanish Ministerio de Ciencia e Innovación (FPU Fellowship to E.T., Grant CTQ2011-22433 to S.V.), the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO No. 9.0188.07 to L.N.), and the Geconcerteerde Onderzoeksacties (GOA/10/014 to L.N.).
The authors declare no competing financial interest.
Supplementary Material
References
- Palese P.; Shaw M. L.. Orthomyxoviridae: The Viruses and Their Replication. In Fields Virology, 5th ed.; Knipe D. M., Howley P. M., Eds.; Lippincott Williams & Wilkins: Philadelphia, 2007; Vol. 2, pp 1647–1689. [Google Scholar]
- Alves Galvão M. G.; Rocha Crispino Santos M. A.; Alves da Cunha A. J. Amantadine and rimantadine for influenza A in children and the elderly. Cochrane Database Syst. Rev. 2012, CD002745. [DOI] [PubMed] [Google Scholar]
- Jefferson T.; Demicheli V.; Di Pietrantonj C.; Rivetti D. Amantadine and rimantadine for influenza A in adults. Cochrane Database Syst. Rev. 2006, CD001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K. Antivirals targeting influenza A virus. J. Med. Chem. 2012, 55, 6263–6277. [DOI] [PubMed] [Google Scholar]
- Bright R. A.; Medina M. J.; Xu X.; Pérez-Oronoz G.; Wallis T. R.; Davis X. M.; Povinelli L.; Cox N. J.; Klimov A. I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: A cause for concern. Lancet 2005, 366, 1175–1181. [DOI] [PubMed] [Google Scholar]
- Bright R. A.; Shay D. K.; Shu B.; Cox N. J.; Klimov A. I. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. J. Am. Med. Assoc. 2006, 295, 891–894. [DOI] [PubMed] [Google Scholar]
- Deyde V. M.; Xu X. Y.; Bright R. A.; Shaw M.; Smith C. B.; Zhang Y.; Shu Y. L.; Gubareva L. V.; Cox N. J.; Klimov A. I. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 2007, 196, 249–257. [DOI] [PubMed] [Google Scholar]
- Moscona A. Global transmission of oseltamivir-resistant influenza. N. Engl. J. Med. 2009, 360, 953–956. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T.; Fry A. M.; Gubareva L. V. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antiviral Ther. 2012, 17, 159–173. [DOI] [PubMed] [Google Scholar]
- Duque M. D.; Torres E.; Valverde E.; Barniol M.; Guardiola S.; Rey M.; Vázquez S.. Inhibitors of the M2 channel of influenza A virus. In Recent Advances in Pharmaceutical Sciences; Muñoz-Torrero D., Ed.; Transworld Research Network: Kerala (India), 2011; Chapter 2. [Google Scholar]
- Stoelting D. T.; Shiner V. J. Jr. Solvolysis of 1-(3-Noradamantyl)ethyl Sulfonates. J. Am. Chem. Soc. 1993, 115, 1695–1705. [Google Scholar]
- Edwards S.; Marquardt F.-H. Molecular rearrangements in the course of Ritter reactions. J. Org. Chem. 1974, 39, 1963–1963. [Google Scholar]
- Colombo M. I.; Bohn M. L.; Rúveda E. A. The mechanism of the Ritter reaction in combination with Wagner-Meerwein rearrangements. J. Chem. Educ. 2002, 79, 484–485. [Google Scholar]
- Sasaki T.; Eguchi S.; Oyobe T. Reaction of isoprenoids. XI. Stereochemistry of the Ritter reactions products of camphene with unsaturated nitriles. Bull. Chem. Soc. Jpn. 1970, 43, 1252–1254. [Google Scholar]
- Welniak M. Novel rearrangement of tertiary fenchyl alcohols with sulfuric acid in acetonitrile. Pol. J. Chem. 2002, 76, 37–44. [Google Scholar]
- Schneider G.; Hackler L.; Sohar P. Steroids. Part 33. Ritter reaction on steroids. 3. Ring expansion of steroid oxetanes into dihydrooxazines. Tetrahedron 1985, 41, 3377–3386. [Google Scholar]
- Kovalskaya S. S.; Kozlov N. G.; Dikusar E. A. Transformations of 2-(phenylethynyl)isocamphanol under acid catalysis. Russ. J. Org. Chem. 2007, 43, 674–678. [Google Scholar]
- Moss R. A.; Sauers R. R.; Sheridan R. S.; Tian J.; Zuev P. S. Carbon tunneling in the ring expansion of noradamantylchlorocarbene. J. Am. Chem. Soc. 2004, 126, 10196–10197. [DOI] [PubMed] [Google Scholar]
- Jirgensons A.; Kauss V.; Kalvinsh I.; Gold M. R. A practical synthesis of tert-alkylamines via the Ritter reaction with chloroacetonitrile. Synthesis 2000, 1709–1712. [Google Scholar]
- Brückner R.Organic Mechanisms. Reactions, Stereochemistry and Synthesis; Springer-Verlag: Berlin, 2010; pp 429–430. [Google Scholar]
- Sosnowski J. J.; Rheingold A. L.; Murray R. K. Jr. J. Org. Chem. 1985, 50, 3788–3791. [Google Scholar]
- Camps P.; Lukach A. E.; Rossi R. A. Synthesis of several halobisnoradamantane derivatives and their reactiveity through the SRN1 mechanism. J. Org. Chem. 2001, 66, 5366–5373. [DOI] [PubMed] [Google Scholar]
- CCDC-893166 (14) contains the supplementary crystallographic data for this paper. These data can be obtained free of from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- Vanderlinden E.; Vanstreels E.; Boons E.; ter Veer W.; Huckriede A.; Daelemans D.; Van Lommel A.; Roth E.; Sztaricskai F.; Herczegh P.; Naesens L. Intracytoplasmic trapping of influenza virus by a lipophilic derivative of aglycoristocetin. J. Virol. 2012, 86, 9416–9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J.; Wolstenholme A. J.; Skehel J. J.; Smith M. H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985, 4, 3021–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L. H.; Holsinger L. J.; Lamb R. A. Influenza virus M2 protein has ion channel activity. Cell 1992, 69, 517–528. [DOI] [PubMed] [Google Scholar]
- Wang J.; Qui J. X.; Soto C.; DeGrado W. F. Structural and dynamic mechanisms for the function and inhibition of the M2 proton channel from influenza A virus. Curr. Opin. Struct. Biol. 2011, 21, 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balannik V.; Carnevale V.; Fiorin G.; Levine B. G.; Lamb R. A.; Klein M. L.; DeGrado W. F.; Pinto L. H. Functional studies and modeling of pore-lining residue mutants of the influenza a virus M2 ion channel. Biochemistry 2010, 49, 696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M.; DeGrado W. F. Structural basis for proton conduction and inhibition by the influenza M2 protein. Protein Sci. 2012, 21, 1620–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrahan P.; Arkin I. T. Resistance characteristics of influenza to amino-adamantyls. Biochim. Biophys. Acta, Biomembr. 2011, 1808, 547–553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.