Abstract
Introduction:
The legibility of medication labelling is a concern for all Canadians, because poor or illegible labelling may lead to miscommunication of medication information and poor patient outcomes. There are currently few guidelines and no regulations regarding print standards on medication labels. This study analyzed sample prescription labels from Ontario, Canada, and compared them with print legibility guidelines (both generic and specific to medication labels).
Methods:
Cluster sampling was used to randomly select a total of 45 pharmacies in the tri-cities of Kitchener, Waterloo and Cambridge. Pharmacies were asked to supply a regular label with a hypothetical prescription. The print characteristics of patient-critical information were compared against the recommendations for prescription labels by pharmaceutical and health organizations and for print accessibility by nongovernmental organizations.
Results:
More than 90% of labels followed the guidelines for font style, contrast, print colour and nonglossy paper. However, only 44% of the medication instructions met the minimum guideline of 12-point print size, and none of the drug or patient names met this standard. Only 5% of the labels were judged to make the best use of space, and 51% used left alignment. None of the instructions were in sentence case, as is recommended.
Discussion:
We found discrepancies between guidelines and current labels in print size, justification, spacing and methods of emphasis.
Conclusion:
Improvements in pharmacy labelling are possible without moving to new technologies or changing the size of labels and would be expected to enhance patient outcomes.
Knowledge into Practice.
We found discrepancies between current prescription medication labels and print guidelines.
Designing labels according to print guidelines is needed to move from a pharmacy-centred approach to a more patient-centred approach.
Improving legibility of prescription labels is a simple way to enhance patients’ understanding of their medications.
Mise En Pratique Des Connaissances.
Nous avons relevé des écarts entre les étiquettes actuelles des médicaments sur ordonnance et les lignes directrices publiées.
Il est nécessaire de concevoir les étiquettes conformément aux lignes directrices de manière à passer d’une démarche axée sur la pharmacie à une approche axée sur le patient.
Une manière simple de renforcer la compréhension du traitement par les patients consiste à améliorer la lisibilité des étiquettes d’ordonnance.
Introduction
Patient-centred care is a professional obligation to take responsibility for an individual patient’s needs. This includes making sure patients understand their medications and how to take them. In that regard, one area that has received little attention is the legibility of medication labels.
The total number of people at risk of visual impairment in Canada is high and increases sharply with age. The 2006 Statistics Canada Participation and Activity Limitations Survey (PALS) identified 816,250 Canadians aged 15 years and over as having a self-reported vision difficulty.1 In the same year, Maberley et al.2 estimated that 0.7% of the total population had visual impairment visual acuity less than 6/12, which means that the minimum size of letters that they can recognize is twice that which a person with normal vision can recognize) and 0.24% are legally blind (6/60 or less—the minimum size of letters that they can recognize is 10 times that which a person with normal vision can recognize).2 These percentages rise to 9.8% of 75- to 84-year-olds and 18% of those 85 years and older, respectively.
Other measures of vision (besides visual acuity) are affected by aging in the absence of ocular disease. While high-contrast visual acuity is 2 times poorer in those aged 90 or older compared with younger adults, low-contrast acuity is nearly 4 times poorer.3 Contrast sensitivity and visual acuity for low contrast in low light are 6 times worse.3 The speed of reading is substantially slower in older adults and even among those who still have good high-contrast acuity.4 All these factors may affect the reading of print, as medicine labels may lose their contrast with time, and lighting in an individual’s home may be less than optimal.
As a result, the legibility of medication labelling is indeed a concern, as poor or illegible labelling together with poor vision may lead to misunderstandings of how to take medication.5,6 Poor vision may also result in increased anxiety about taking medications and increased dependence on others for medication management.6 Additionally, older adults are at increased risk for medication mistakes because they take more medications than younger persons.7-9 An increase in the number of medications used is associated with increased medication mistakes10 and decreased medication recall.11
Although there are guidelines for general print legibility from nongovernmental organizations (NGOs) and specifically for medication labels from some pharmaceutical and health organizations (Table 1), they may not be applied consistently to medication labels. For example, in Ontario, there are no legal requirements regarding the legibility of print, although the content of what must be included on the label is specified.12
Table 1.
General print guidelines for people with vision loss and for prescription labelling
| General guidelines for print accessibility | Guidelines for legibility of prescription labelling | |||||||
|---|---|---|---|---|---|---|---|---|
| CNIB (Canada) | RNIB (UK) | ACB (US) | USP (US) | ASCP (US) | MPAG (Sweden) | NPSA (UK) | AFB (US) | |
| Font style and point size | Sans-serif font Minimum 12, 14, 18 points |
Sans-serif font Minimum 12, 14 points |
Sans-serif font Minimum 18 points |
Sans-serif font Minimum 12 points |
Sans-serif font Minimum 18 points for vision loss |
Times New Roman font 9 points or more |
Font such as Arial Minimum 12 points |
Sans-serif font Largest font possible, 18 points for vision loss |
| Case | Not all upper case | Not all upper case | Not all upper case | Sentence case | Sentence case | Avoid upper case | Avoid overuse of upper case | Not all upper case |
| Bolding | For emphasis | Use sparingly | Use bold | Use bold for emphasizing important information | Use bold for most important information | Use bold for most important information | Use bold for most important information | |
| Italics | Not recommended | Italics harder to read | Do not use | Do not use | Do not use | |||
| Paper type | High contrast Nonglossy |
High contrast Nonglossy |
High contrast Nonglossy |
|||||
CNIB, Canadian National Institute for the Blind13; RNIB, Royal National Institute of Blind People14; ACB, American Council of the Blind15; USP, United States Pharmacopeia16; ASCP, American Society of Consultant Pharmacists17; MPAG, Medical Products Agency Guidelines (Sweden)18; NPSA, National Patient Safety Agency (UK)19; AFB, American Foundation for the Blind20
Surprisingly, there has been a dearth of studies addressing this important issue. Latham et al.21 assessed 24 prescription labels from 6 pharmacy chains in the United Kingdom and compared them to the National Safety Patient Agency, UK, Design for Patient Safety guidelines.19 The investigators found that none of the labels met the guidelines. Chubaty et al.22 compared health information leaflets to guidelines from Canada, the United Kingdom and the United States and found that only 33% met guidelines.
We therefore sought to better understand these issues by analyzing and comparing a larger sample of current prescription labels from Ontario, Canada, to available print legibility guidelines (both generic and specific to medication labels) from different organizations worldwide. The purpose was to sample the range of print characteristics of medication labels and to determine the percentage of different labels that meet the guidelines, rather than to determine a strict percentage that a patient might encounter.
Methods
This study was reviewed and received clearance from the Office of Research Ethics at the University of Waterloo.
Pharmacy label collection
Cluster sampling was used to randomly initially select 50 pharmacies from a total of 127 pharmacies in the tri-cities of Kitchener, Waterloo and Cambridge, Ontario. This was considered a good percentage (39%) of the total sample and feasible to complete in the time available. Each city was divided into 6 clusters, 4 of which were randomly selected. We sampled proportionally according to the percentage of pharmacies in each city (27 from Kitchener, 10 from Waterloo and 13 from Cambridge). To obtain the desired 50, all independent pharmacies and at least one randomly selected pharmacy from each pharmacy chain were invited to participate. Thus we ensured that the chains (including chains, food stores/mass merchandisers, banners and franchises) were represented and not excluded by random selection. However, they were not represented proportionally. This was in order to sample as widely as possible the range of labels that are available. A declining chain pharmacy was replaced by another of the same chain, in the same cluster or city or from another city (selected in this order). Similarly, a declining independent pharmacy was replaced by another randomly selected independent pharmacy from the same city or from another city if needed. Each participating pharmacist was contacted by a phone call, during which the purpose of the study was explained. This was followed by a more detailed letter of information, and pharmacists signed a consent form before taking part. There was no deception; that is, the pharmacists were told that the purpose was to study the legibility of the labels. They were asked to provide a regular sample prescription label based on the same fictitious prescription that was provided to them. They were also asked to provide a large-print label, if possible. An example of a regular-print label is shown in Figure 1, which shows the prescription information.
Figure 1.

Example of a regular-print prescription label, showing the prescription information*
*The pharmacy logo and identifying information have been covered.
Label analysis
The print characteristics of patient-critical information were compared against the recommendations for print accessibility by nongovernmental organizations and for prescription labels by pharmaceutical and health organizations (Table 1). For the purpose of this study, the patient name, instructions and trade and generic drug names were included as patient-critical information.23
First, the type of print of patient-critical information was identified as being either sans-serif or serif font. A serif font is one where the letters have small flourishes at the end of their strokes, whereas sans-serif fonts (without serifs) have simpler letters without these flourishes (Figure 2). As most of the guidelines are not specific and suggest clear, nondecorative, plain fonts such as Arial or Verdana (Table 1), the typeface was analyzed by comparison with printed fonts from Microsoft Word.
Figure 2.

Illustration of serif and sans-serif fonts
The print size of each patient-critical piece of information was recorded. Since print sizes change with font style, and also because most recommendations suggest Arial or similar, we determined the size by comparing with printed samples of Arial font in different point sizes (points are a common measure of print size). This was done by holding up the label and printouts against a light or by measuring with a ruler. The 3 labels that did not use Arial font were written in capital letters, so the heights were compared from the lowest point to the highest point of the letters. One of these labels was printed in Telidon dot font (in which the width is greater than the height); thus, comparing by height alone would not be representative. We therefore took an average of the height and the width compared with the Arial samples for this particular label. Any information (other than the pharmacy information and logo) was noted if it was larger than the instructions.
Components that were bolded were noted (excluding the pharmacy information and logo) for the following components: prescription number, patient name, instructions, trade drug name, generic drug name, strength, physician name, dosage form, pharmacist initials and refills. Bolding was defined as print of the same size that was composed of thicker strokes than the standard print. The number of patient-critical pieces of information (as defined above) that were bolded was calculated as a percentage of the total pieces of information that were bolded for each label. The percentage of patient-critical information that was highlighted was similarly determined. Highlighting was defined as there being a background colour (including grey) behind the line of print.
Left justification of the patient-critical information was noted if present. Information printed in italics was also recorded. Spacing was measured subjectively by judging whether the information could have been spaced out better without changing the print size. The print colour (excluding pharmacy information and logo) was noted, as well as whether it was high contrast or not. High contrast was defined as the print being black against a white background. The paper finish (glossy or nonglossy) was defined as whether the paper background noticeably showed any specular reflections or not. Descriptive analyses were undertaken for the sample label analysis. We planned to have all these print characteristics remeasured for 20% of the labels by a second investigator who was not informed of the first results.
Results
Sample labels
Of the total number of 96 pharmacies contacted, 45 pharmacies provided us with labels (response rate: 47%): 25 from Kitchener, 9 from Waterloo and 11 from Cambridge. Sixteen (35.6%) were chains and food stores/mass merchandisers, 14 (31%) were banners and franchises24 and 15 (33.3%) were independents. Forty-one pharmacies indicated which dispensing software program they used, as follows: Kroll 44% (n = 18), Nexxsys 34% (n = 14),14 Assyst Rx, Health Watch and Connexus 5% (n = 2 each)2 and Other 7% (n = 3).3 Only 3 pharmacists were able to supply a large-print version, 2 of which were from the same pharmacy chain.
The results of the label analysis are shown in Table 2. Over 90% of labels followed the guidelines for font style, contrast, print colour and nonglossy paper. Ninety-three percent (42 labels) used Arial or a sans-serif font resembling Arial, while 4.4% (2 labels) used a font resembling Univers 65 bold. One label (2.2%) was printed with a dot matrix printer, which created a low-contrast result, and the font resembled Telidon. The instructions on all the labels were printed in upper case. For the drug and patient names, 58% and 44% of labels were printed in upper case, respectively.
Table 2.
Comparison of the print of sample prescription labels with print recommendations for prescription labels
| Characteristic | Recommendations by guidelines | Number (%) of sample prescription labels that followed guidelines (total n = 45) |
|---|---|---|
| Serif | Sans-serif | 45 (100) |
| Font style | Arial | 42 (93) Arial or resembling Arial 2 (4) Univers 65 Bold 1 (2) Telidon dot |
| Contrast | High contrast | 44 (98) |
| Print colour | Black | 44 (98) |
| Paper | Nonglossy | 43 (95) |
| Case | Sentence case | 0% instructions 20 (44) drug name 26 (58) patient name |
| Bolding | For emphasis | 20 (44) bolded instructions, drug name and patient name |
| Alignment | Left justification20,23,24 | 23 (51) |
| Spacing | 25%-30%,22 or 40% of the text size or 1.5 × spacing20 | 2 (4) made the best use of spacing |
| Highlight | For emphasis | 13 (29) used highlighting: 2 (4) highlighted the patient name, 11 (24) used grey highlighting, none highlighted drug name or instructions. |
The majority (95.6%) of the labels used bolding for emphasis of some text on the label, but it was not always the patient-critical information that was bolded. Forty-four percent of the labels bolded all patient-critical information, 84.4% bolded the instructions and 84.4% bolded either the generic or trade drug name (note that these were not the same labels that made up the percentages), while 55.6% bolded the patient name. About half (51.1%) of the labels were left-justified (as opposed to right justified or centred). Just 5% of the labels made the best use of spacing, using either the recommended spacing of 25%-30%22 or 40% of the print size or 1.5 spacing between lines.20 Highlighting was used in 29%13of the labels, but only 4.4%2 used yellow highlighting. The rest used some form of grey highlighting. Highlighting was mainly used to emphasize information other than the patient-critical information. Only 2 highlighted the patient name and none highlighted the drug name or directions. Of the 13 labels that used highlighting, 10 highlighted the prescription number and refills. The use of italics was found in 82.2% of the labels for information other than the pharmacy logo.
Print size is shown graphically in Figure 3 for the 3 most important components. Forty-four percent of the instructions on the labels met the minimum guideline of 12-point type. None of the labels used 14-point type or larger. None of the drug names or patient names were 12-point type or larger. The prescription number was larger than the instructions for 13 labels (29%). The prescription number and the patient name were larger than the instructions for 2 labels (4.4%). The rest of the labels had the instructions as the largest component.
Figure 3.
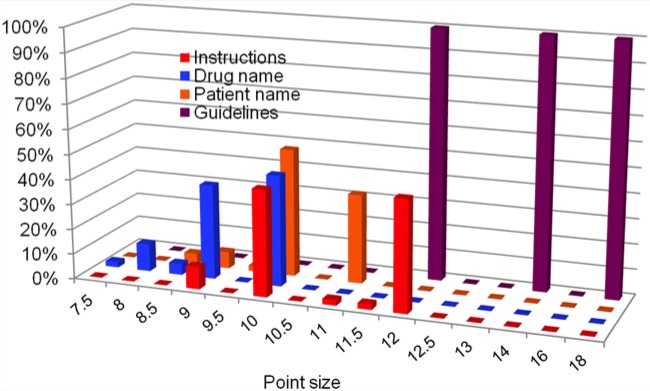
Print sizes of sample labels for instructions, drug name and patient name compared with guidelines from various nongovernmental organizations
Guidelines considered from USP, United States Pharmacopeia16; ASCP, American Society of Consultant Pharmacists17; NPSA, National Patient Safety Agency (UK)19; AFB, American Foundation for the Blind20; CNIB, Canadian National Institute for the Blind13; RNIB, Royal National Institute of Blind People14; ACB, American Council of the Blind.15
Of the 3 large-print labels, all used a sans-serif font, 2 of which resembled Univers 65 Bold and 1 of which resembled Arial. The large-print labels were nonglossy and the print was of high contrast. Two of the large-print labels had 13.5-point print for the instructions. All 3 large-print labels were written in upper case and were not left-justified. All 3 labels had bolding but it was not used to strictly highlight the patient-critical information. None of the large-print labels had highlighting.
When the print characteristics for 10 labels (22%) were rechecked by the second investigator, there was 100% agreement (Kappa = 1.0) for all parameters except for spacing and contrast, for which there was 90% agreement (Kappa = 0.78).
Discussion
We found that current medication labels do conform to guidelines regarding sans-serif font, style, high contrast and nonglossy paper. However, they have several deficiencies that could lead to confusion and poor patient outcomes. The medication label can be thought of as an extension of pharmacist’s care and, as such, should meet the individual needs of patients, many of whom have visual impairment.
Our findings are consistent with the results of Latham et al.21 Sans-serif fonts may be more legible by allowing improved horizontal movement, which is important for adults with low vision.25 Use of high-contrast and nonglossy paper is important for older adults, considering the loss of low-contrast acuity, low-lighting visual acuity and the increased sensitivity to glare with age.3 Widespread implementation of these printing options should be straightforward. However, some pharmacists may tape the label to the vial, which would effectively turn a nonglossy label into a glossy version. Thus, the percentage of glossy labels in practice may be higher than documented here. There was one label that had low contrast attributable to use of a dot-matrix printer, which could easily be updated.
More discrepancies between guidelines and current labels were found in print size, spacing and methods of emphasis such as bolding or highlighting patient-critical information. This was similar to Latham et al.’s study21 using UK labels, which found discrepancies in print size, centre justification, bolding, highlighting and branding. Similarly, Chubaty et al.22 found that only one-third of medication information leaflets met print-size recommendations, and only 19% used appropriate spacing.
Recommendations suggest the use of sentence case (where you only capitalize the first letter of the first word in a line or heading—just as you would in a sentence), and none of the labels met this criteria for the instructions. The use of capital letters may reduce the clarity of the label and is considered to be particularly difficult to read by those with visual impairments.26 The various nongovernmental organizations (Table 1) support limited use of capital letters, although not all stress this strongly (e.g., CNIB). It has largely been assumed, rather than demonstrated, that sentence case is more legible.26,27 It was suggested that capital letters are less visually pleasing and harder to read due to the block appearance, which eliminates the unique shape of each word.27 However, the use of capital letters may result in faster reading when comparing equivalent size in point print in sentence case for both people with and without visual impairment.26 The optimum use of sentence case versus capitals is, therefore, still not confirmed.
Bolding was used in 95.6% of the labels, but was not used to strictly emphasize the patient-critical information, as recommendations would suggest. All of the labels that used bolding had at least one of the patient-critical components bolded; however, none of the labels bolded only the patient-critical information. Similarly, highlighting was found in some of the labels for the prescription number and refills section, which is not considered to be the most important information for the patient. Furthermore, use of grey highlighting lowers the contrast of the print. It may be more beneficial to limit bolding and highlighting to patient-critical information. If bolding and highlighting capabilities exist within the software used by pharmacists, this could be easily achieved.
It is noteworthy that neither italics nor underlining is recommended as a method for emphasis by common guidelines,13-15,17,20 yet 82% of the labels used italics in some form or another. The guidelines that mention italics suggest avoiding their use completely, and so it may be best practice to only use italics for information that is not critical to patients.
Print size was an area for potential improvement for all labels in our study. Only 47% of labels met the 12-point font size guideline for instructions, with a mean font size of 10.9 points. Two of the large-print labels met the 12-point guideline at 13.5-point print for instructions. All other essential components of the labels were below the guideline of 12-point font size (the most frequent guideline), with both the American Society of Consultant Pharmacists and the American Foundation for the Blind recommending 18-point font size for those with vision loss. This is important, because the larger the font size, the greater the percentage of the population that would be able to read it properly. As 18-point font size may not be feasible with the current standard label size, a compromise of 14- or 16-point font size might be possible. Perhaps a medication label guideline should specify that print should be made as large as possible, depending on the amount of text that is required, with a minimum font size of 12 points for patient-critical information (Table 1).
Print may also be made more legible with better use of spacing and minimal distracters.28 Pharmacy-centred information such as the logo, address and contact information is also important but may be distracting and can use up valuable space on the label.28 Most labels in our study did not use space on the label optimally, and our impression was that pharmacy logos were the largest and most eye-catching feature. Consistent with our findings, Shrank et al.29 showed that 84% of pharmacies in several large cities in the United States displayed the pharmacy logo as the most prominent feature. Those investigators also noted that the mean print size for the logo was larger than the mean print sizes for any of the other components. A parsimonious way to increase space on the label for patient-critical information is to decrease the size of the pharmacy logo. Improved use of spacing, larger font size and bolding for emphasis for patient-critical information could then easily be achieved for most labels. Although Shrank et al. suggest that there is little direct evidence that improved labels increase safety or adherence in studies that have been conducted so far and that many errors are due to lack of comprehension rather than legibility, they do mention that improving the label format can increase legibility and understanding.30-32 Prescription labels often serve as the only or “last line” source of medication instructions,28 so it is generally agreed that labels should be as clear and legible as possible (Box 1).33

Limitations
One limitation of this study is that the sample of prescription labels was taken from a group of medium-sized cities in Ontario and therefore may not be fully generalizable to all of Ontario or Canada. A second limitation is that our sampling ensured that chains were represented, but not proportionally. We were more interested in assessing the range and percentage of different labels that meet the guidelines rather than in determining the strict likelihood that a patient would encounter a particular size of print on a label. Third, we cannot be sure whether the failure to follow guidelines is due to the software capabilities or the choice of each pharmacy. We attempted to contact the software suppliers, with little success. Further study is required to determine this. Last, it is possible that the pharmacists modified the way that they printed the labels, as they knew we were studying label legibility, even though we specifically asked them not to do this. However, we think this is unlikely, as only 3 provided a large-print label. If pharmacists had been trying to influence the appearance of the results, it might have been expected that more would provide large-print labels. Also, in many cases the pharmacist printed the label while the researcher was waiting, so probably would not have taken the time to modify the print characteristics.
Conclusion
This study demonstrates discrepancies between print guidelines and certain print characteristics in many medication labels, in particular for font size, use of case, bolding, justification and spacing. These are characteristics that might be modified, and result in greater legibility, without moving to new technologies or even larger labels. Changes in the printing software may be needed to move from a pharmacy-centred approach to a more patient-centred approach. Additionally, the development of Canadian guidelines or regulations for print characteristics on medication labels may assist more patients to read the important information independently and may increase their health and safety. ■
References
- 1. Statistics Canada. The 2006 Participation and Activity Limitation Survey: disability in Canada. Available: www.statcan.gc.ca/bsolc/olc-cel/olc-cel?catno=89-628-X&CHROPG=1&lang=eng (accessed Jan. 14, 2014).
- 2. Maberley DA, Hollands H, Chuo J, Tam G, et al. The prevalence of low vision and blindness in Canada. Eye 2006;20:341-6 [DOI] [PubMed] [Google Scholar]
- 3. Haegerstrom-Portnoy G. The Glenn A. Fry Award Lecture 2003: vision in elders—summary of findings of the SKI Study. Optom Vis Sci 2005;82:87-93 [DOI] [PubMed] [Google Scholar]
- 4. Lott LA, Schneck ME, Haegerström-Portnoy G, et al. Reading performance in older adults with good acuity. Optom Vis Sci 2001;78:316-24 [DOI] [PubMed] [Google Scholar]
- 5. Hellier E, Edworthy J, Derbyshire N, Costello A. Considering the impact of medicine label design characteristics on patient safety. Ergonomics 2006;49(5-6):617-30 [DOI] [PubMed] [Google Scholar]
- 6. American Foundation for the Blind. Access to drug labels survey report; 2008. Available: www.afb.org/section.aspx?FolderID=3&SectionID=3&TopicID=135&DocumentID=4520 (accessed June 14, 2013).
- 7. Reason B, Terner M, Moses McKeag A, et al. The impact of polypharmacy on the health of Canadian seniors. Fam Pract 2012;29:427-32 [DOI] [PubMed] [Google Scholar]
- 8. Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008;300:2867-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Linton A, Garber M, Fagan NK, Peterson MR. Examination of multiple medication use among TRICARE beneficiaries aged 65 years and older. J Manag Care Pharm 2007;13:155-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meredith S, Feldman PH, Frey D, et al. Possible medication errors in home healthcare patients. J Am Geriatr Soc 2001;49:719-24 [DOI] [PubMed] [Google Scholar]
- 11. O’Hare F, Jeganathan VS, Rokahr CG, et al. Readability of prescription labels and medication recall in a population of tertiary referral glaucoma patients. Clin Exp Ophthal 2009;37:849-54 [DOI] [PubMed] [Google Scholar]
- 12. Service Ontario. Drug and Pharmacies Regulation Act R.S.O. 1990, Chapter H.4 c. 33, Sched. 6, s. 51. 2009. Available: http://www.e-laws.gov.on.ca/html/statutes/english/elaws_statutes_90h04_e.htm (accessed April 4, 2014).
- 13.Canadian National Institute for the Blind. Clear print design standard. Available: www.cnib.ca/en/services/resources/clearprint/Pages/default.aspx (accessed June 5, 2013).
- 14.Royal National Institute of Blind People. Clear print. Available: www.rnib.org.uk/professionals/accessibleinformation/text/Pages/clear_print.aspx (accessed June 5, 2013).
- 15.American Council of the Blind. Best practices and guidelines for the large print documents used by the low vision community. Available: http://acb.org/node/750 (accessed June 5, 2013).
- 16.The United States Pharmacopeial Convention. Recommendations on prescription container labeling. Available: www.usp.org/usp-nf/notices/retired-compendial-notices/recommendations-prescription-container-labeling (accessed June 5, 2013).
- 17.American Society of Consultant Pharmacists. Guidelines for prescription labeling and consumer medication information for people with vision loss. Available: www.ascpfoundation.org/programs/visuallyimpaired.cfm (accessed June 5, 2013).
- 18.Läkemedelsverket Medical Products Agency. Guideline to Medical Products Agency’s regulation on labelling and package leaflets for medicinal products. Available: www.lakemedelsverket.se/english/All-news/NYHETER---2009/Guideline-in-English--labelling-and-package-leaflets-for-medicinal-products/ (accessed June 5, 2013).
- 19.National Safety Patient Agency. Design for patient safety: a guide to the design of dispensed medicines. Available: www.nrls.npsa.nhs.uk/resources/patient-safety-topics/medication-safety/?entryid45=59829 (accessed June 5, 2013).
- 20.American Foundation for the Blind. Guidelines for prescription labeling and consumer medication information for people with vision loss. Available: www.afb.org/section.aspx?FolderID=3&SectionID=3&TopicID=403&SubTopicID=256&DocumentID=4064 (accessed June 5, 2013).
- 21. Latham K, Waller S, Schaitel J. Do best practice guidelines improve the legibility of pharmacy labels for the visually impaired? Ophthal Physiol Opt 2011;31:275-82 [DOI] [PubMed] [Google Scholar]
- 22. Chubaty A, Sadowski CA, Carrie AG. Typeface legibility of patient information leaflets intended for community-dwelling seniors. Age Ageing 2009;38:441-7 [DOI] [PubMed] [Google Scholar]
- 23.National Association of Boards of Pharmacy. Report of the Task Force on Uniform Prescription Labeling Requirements. Available: www.nabp.net/news/assets/08TF_Uniform_Presc_Labeling_Req.pdf (accessed Sep. 14, 2011).
- 24. Taro Pharmaceuticals and McKesson Canada. Tenth pharmacy trends report 2003. Available: http://216.150.136.82/chronic_lobby_category.asp?siteID=273&catId=1637&sub1=2 (accessed Oct. 16, 2013).
- 25. Connolly GK. Legibility and readability of small print: effects of font, observer age and spatial vision. Calgary (AB): University of Calgary; 1998 [Google Scholar]
- 26. Arditi A, Cho J. Letter case and text legibility in normal and low vision. Vis Res 2007;47:2499-505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tinker M. Legibility of print. Ames (IO): Iowa State University Press; 1963 [Google Scholar]
- 28. Wolf MS, Davis TC, Shrank W, et al. To err is human: patient misinterpretations of prescription drug label instructions. Patient Educ Couns 2007;67:293-300 [DOI] [PubMed] [Google Scholar]
- 29. Shrank WH, Agnew-Blais J, Choudhry NK, et al. The variability and quality of medication container labels. Arch Intern Med 2007;167:1760-5 [DOI] [PubMed] [Google Scholar]
- 30. Shrank W, Avorn J, Rolon C, Shekelle P. Effect of content and format of prescription drug labels on readability, understanding, and medication use: a systematic review. Ann Pharmacother 2007;41:783-801 [DOI] [PubMed] [Google Scholar]
- 31. Shrank WH, Gleason PP, Canning C, et al. Can improved prescription medication labeling influence adherence to chronic medications? An evaluation of the Target pharmacy label. J Gen Intern Med 2009;24:570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shrank WH, Parker R, Davis T, et al. Rationale and design of a randomized trial to evaluate an evidence-based prescription drug label on actual medication use. Contemp Clin Trials 2010;31:564-71 [DOI] [PubMed] [Google Scholar]
- 33. Shrank WH, Patrick A, Gleason PP, et al. An evaluation of the relationship between the implementation of a newly designed prescription drug label at Target pharmacies and health outcomes. Med Care 2009;47:1031 [DOI] [PMC free article] [PubMed] [Google Scholar]


