Abstract
For many years, clinical and non-clinical investigations have investigated cortical bone structure in an attempt to address questions related to normal bone development, mineralisation, pathologies and even evolutionary trends in our lineage (adaptations). Research in the fields of medicine, materials science, physical anthropology, palaeontology, and even archaeobiology has contributed interesting data. However, many questions remain regarding the histomorphological and histochemical variations in human cortical bone during different stages of life. In the present work, we describe a study of long bone cortex transformations during ontogeny. We analysed cross-sections of 15 human humeri histomorphologically and histochemically from perinatal to adult age, marking and quantifying the spatial distribution of bone tissue types using GIS software and analysing the mineral composition and crystallinity of the mineralised cortex using Raman spectroscopy and X-ray diffraction. Our results allowed us to propose that human cortical bone undergoes three main ‘events’ through ontogeny that critically change the proportions and structure of the cortex. In early development, bone is not well mineralised and proportionally presents a wide cortex that narrows through the end of childhood. Before reaching complete maturity, the bone mineral area increases, allowing the bone to nearly reach the adult size. The medullary cavity is reduced, and the mineral areas have a highly ordered crystalline structure. The last event occurs in adulthood, when the ‘oldest’ individuals present a reduced mineralised area, with increasing non-mineralised cavities (including the medullary cavity) and reduced crystalline organisation.
Keywords: compartmentalisation, cortical bone, histology, humerus, Raman spectroscopy
Introduction
When attempting to understand bone mineralisation, it is critical to know not only how bone grows and is organised in living people but also its behaviour during accommodation and repair after fractures, surgical interventions, during normal mass loss in the adult life (i.e. osteopenia), and in pathologies that actively destroy mineralised bone (i.e. osteoporosis) (Boskey, 2007; Morgan & Bouxsein, 2008). Bones are assembled from biomolecules and cells. However, the development of a biomineralised tissue requires differentiated morphology, structure, compartmentalisation, and chemical composition (Schinke & Amling, 2007). Moreover, bones have a unique mechanism of growth, which is dependent on the relationship with the surrounding tissues (i.e. muscles and ligaments), a specific pattern of vascularisation, and a particular mechanism of repair, replacement, and reconstruction (Enlow, 1963, 1968; Currey, 2002; Seeman, 2008). All areas of mineralised bone are configured as a composite material that integrates an organic phase primarily composed of collagen fibres (Paschalis et al. 2001) and an inorganic component that is composed of hydroxyapatite (calcium-phosphate minerals; Tadic & Epple, 2004). Most cells embedded in this mineralised matrix have the same embryological source (i.e. mesoderm), although neural crest cells are also implicated in bone tissue development (e.g. neurocranium or skull vault). The mineralised matrix and cells become organised via two different modes of ossification, one using a cartilage precursor, known as ‘endochondral ossification’, and one in which the mineralised area becomes differentiated directly over the mesenchyme cells, known as ‘intramembranous ossification’ (Karaplis, 2008; Gosman, 2012). Unfortunately, although the ossification processes are relatively well known, bone development is still poorly understood because bone formation occurs in a more complex setting that must be understood as a whole. In this sense, apart from being properly mineralised, bones need to reach large sizes and adjust their shape according to the body's ontogenetic changes (Maggiano, 2012; Gosman et al. 2013). Therefore, after the first mineralisation, bones must be continuously macro- and microscopically reconfigured, changing their design and chemical composition in mineralised and non-mineralised tissues by continuously modelling and remodelling.
Modelling concerns the most important morphological variations throughout the life of a bone. In the case of long bones, modelling involves increases in length and width through epiphyseal elongation, reduction of the metaphysis, and cortical drift. These three processes contribute to bone growth simultaneously (Enlow, 1963, 1968; Maggiano, 2012) and are accompanied by bone tissue-type transformations which reconfigure the cortex area from an initial mineralised scaffold of woven bone (WB; in which collagen fibres are randomly distributed and mineral organisation is low) to progressively more orderly bone with a parallel disposition of collagen fibres (lamellar bone) that is deposited more slowly with highly organised mineral areas (Currey, 2002). During epiphyseal elongation, the long bone growth plates, situated between the epiphyses and the metaphyses, produce chondrocytes that proliferate and become hypertrophic (Gosman, 2012). In the metaphyseal areas, the existence of bone deposition and resorption on contralateral surfaces permits metaphyseal reduction and the consequent diaphysis elongation (i.e. the ‘V’ principle; Enlow, 1963, 1968). Deposition and resorption of complementary surfaces also produce cross-sectional geometrical variations in the shaft (i.e. cortical drift), changing its shape and thickness and varying asymmetrically during ontogeny (Enlow, 1963, 1968; Ruff, 2000; McFarlin, 2006; Goldman et al. 2009; Maggiano, 2012; Gosman et al. 2013). Lamellar bone constitutes the new bone areas in the cortical expansion. Fibrolamellar bone (FLB; lamellar bone that is quickly deposited and in which a large amount of vascular blood vessels become trapped as primary osteons) is found in the outer and medial cortex (subperiosteal area), while endosteal bone (EB), which is also lamellar but is less vascularised, is formed in the inner cortex (endosteal area) and sometimes interrupts the FLB lamination. This differential bone deposition implies the progressive destruction of young tissues and the deposition of new bone in other areas. Therefore, when a bone cross-section is observed, there can be different types of mineralised tissues and other structures that interact (cortical stratification; Enlow, 1963), which reflect events that occurred in the individual's life.
Bone remodelling modifies mineralised tissues, reconfiguring the microstructure. Unlike modelling, remodelling involves the resorption and deposition of new bone at the same location (Robling et al. 2006; Stout & Crowder, 2012). This process requires the cooperative and synchronised action of osteoclasts and osteoblasts (BMU; basic multicellular units) that produce new bone (Haversian bone, HB) in a cutting cone that perforates the cortical bone longitudinally and transversely (Seeman, 2008; Little et al. 2011). After chemical and/or mechanical stimulation, the number of osteoclasts increases and bone resorption occurs, causing irregular perforations in the cortical area called ‘resorption spaces’. Non-mineralised tissues, mainly blood vessels and nerves, colonise resorption spaces, and osteoblasts begin to generate bone that becomes centripetally deposited in the concentric lamina, filling the gap and forming an osteon (secondary osteon). Bone lamellae are deposited until there is no space left. Collagen fibres in bone lamellae are rotated about four degrees for every new lamina (Sedlin & Frost, 1963). This feature enables the differentiation of some types of secondary osteons (Haversian bone type I, II, and III) based on several types of collagen fibre orientation (transverse, longitudinal, and mixed) (Bromage et al. 2003; Stout & Crowder, 2012). Bone remodelling begins in the early months of development (Burton et al. 1989) and continues throughout life. Whether the mechanism is stochastic or targeted has been discussed for many years (Baltadjiev, 1995) but, regardless, it is clear that osteonal accumulation progressively replaces all or almost all primary fibrolamellar bone. Eventually, all newly created osteons remove evidence of previously existing osteons, and, as a result, osteon density ceases to increase with age (Stout & Crowder, 2012).
Currently, it is largely accepted that alterations or variations during early phases of growth can increase the possibility of being affected by pathologies such as bone fragility and osteoporosis, which alter biomineralised structures during adult life (Cooper et al. 2000; Seeman, 2002; Goldman et al. 2009). The problem is that during normal development, as we have described above, changes in bone shape and size during growth blur or even erase the morphological and histological characteristics established during the initial growth phases. The histomorphological features of young bone are not visible in adult bone due to the effects of modelling and remodelling (Bocquet & Bergot, 1977; Currey, 2002; Robling et al. 2006; Goldman et al. 2009). If evidence of earlier stages disappears with increasing age, we must ask how we can approach the study of bone histology variation through life or the origin of these pathologies. We cannot study bone histology using bones from living people (i.e. biopsies). However, we can use postmortem remains (bones from archaeological sites or from autopsies) to infer data (Ruff, 2000). In this sense, the analyses of archaeological bone, in which skeletal samples are normally larger than forensic ones, could potentially help to generate new knowledge in the understanding of growth in living populations.
In previous studies developed using osteoarchaeological material, we suggested that multi-parametric analysis of the cortical bone structure could provide considerable information that would permit a better understanding of the variations in size, shape, and chemical composition through life. Variations in mineralised and non-mineralised cortical tissue compartmentalisation are accompanied by mineral variations that modify bone structure locally (Cambra-Moo et al. 2012; Nacarino Meneses et al. 2012). Here, we present new studies on a sample of 15 humeral cross-sections from individuals at perinatal to adult ages. Our main objectives in this case were to explore (i) the compartmentalisation and spatial distribution of mineralised and non-mineralised tissues using a methodology based on GIS software; (ii) the spatial configuration of the different bone mineralised tissue typologies through ontogeny (inspired by the work developed by McFarlin, 2006); and (iii) the mineral variations in the cortex through Raman spectroscopy, X-ray diffraction, and thermogravimetric analysis.
Materials and methods
Materials
We selected a total of 15 left human humeri (Fig. 1) from the Santa María de la Soledad ossuary excavated in the town of Almansa, Castilla-La Mancha, Spain (dated from the 12th to 18th centuries). All of the humeri were carefully documented and macroscopically reviewed to avoid taphonomic or pathological alterations that could alter the study. Anthropometric measurements were also obtained to get the maximum information from each individual. Because this type of burial does not allow us to obtain complete individuals (only isolated bones are available), age-at-death and sex determinations were impossible. However, to classify the bones, we performed an age-at-death estimation using different methodologies to increase the confidence in the results (Ubelaker, 1978; Buikstra & Ubelaker, 1994; Scheuer & Black, 2000; White & Folkens, 2005; Klepinger, 2006). The 15 humeri were classified into the following four age groups: four perinatal humeri (PH; up to 1 year old), three infant humeri (IH; 1–13 years old), two juvenile humeri (JH; 13–20 years old), and six adult humeri (AH; more than 20 years old). PH1 and PH2 were <0.5 years old, while PH3 and PH4 were between 0.5 and 1 year old. IH5 and IH6 ranged from 1.5 to 3.5 years old, and IH7 ranged from 4.5 and 6.5 years old. Finally, a range between 10 and 18 years old was established for JH8 and between 14 and 20 years old for JH9.
Figure 1.
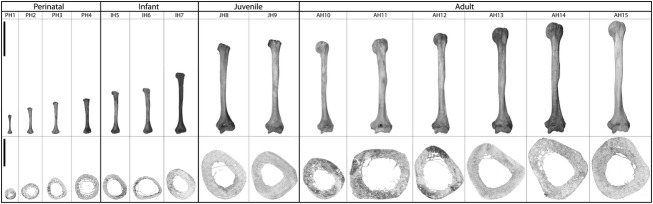
Complete left humeri (top row) and cross-sections after image processing (bottom row). The black bar in the left column represents the scale bar, 10 cm in the upper row and 10 mm in the bottom row.
Histomorphological analysis
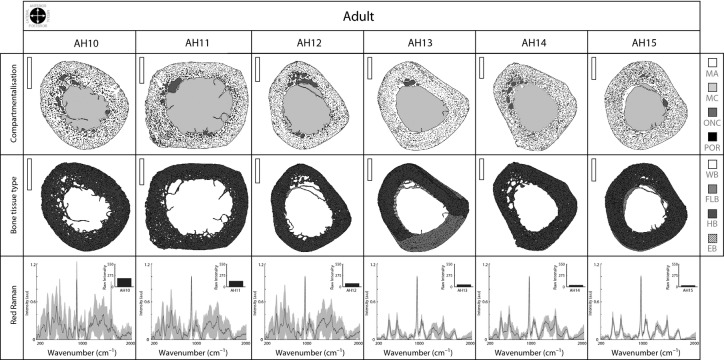
After photographing and measuring the bones, they were manually trimmed and finely polished to obtain mid-shaft thin sections for observation and imagine by transmission (BX61 microscope equipped with a DP70 camera both from Olympus, Hamburg, Germany) and polarised light (DM2500P microscope, Leica, Wetzlar, Germany) microscopy. We mapped each bone section using Geographical Information Systems (GIS) software (arcgis 9.3, Esri, Redlands, CA, USA) as previously described (Cambra-Moo et al. 2012). Using GIS software, we drew a total of 19 793 polygons (see top and middle rows in Figs 5), representing the following different cross-section compartments: mineralised area (MA), medullary cavity (MC), and ‘vascularisation’ (including the porosity, POR, which represents the secondary osteon lumens, and other non-mineralised areas, ONC, which represent resorption spaces and large vascular canals). Furthermore, and taking into account the studies developed by McFarlin (2006), we mapped the different types of bone tissue in the mineralised area of the different ontogenetic stages (i.e. WB, woven bone; FLB, fibrolamellar bone; HB, Haversian bone; and EB, endosteal bone; see middle rows in Figs 5). Table 1 summarises the data obtained from the histomorphological analysis of the different humeri cross-sections.
Figure 5.
Adult bone cross-section analysis. Details as in previous figure. Scale bar: 5 mm (vertical white bar in the upper left corner in the top and middle rows).
Table 1.
Histomorphological data from humeri. Compartmentalisation (MA, mineralised area; MC, medullary cavity; ONC, other non-mineralised cavities; POR, porosity) and bone tissue types (WB, woven bone; FLB, fibrolamellar bone; EB, endosteal bone; HB, Haversian bone). Note that vascularisation refers to all non-mineralised areas except the medullary cavity. Empty cells have zero value.
| Individual | Maximum Length (mm) | Section Area (mm2) | Compartmentalisation | Bone Tissue Types | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mineralised | Non-mineralised | |||||||||
| Vascularisation | MC (%) | |||||||||
| MA (%) | ONC (%) | POR (%) | WB (%) | FLB (%) | EB (%) | HB (%) | ||||
| PH1 | 55.43 | 13.48 | 76.93 | 4.45 | 2.67 | 15.95 | 97.69 | 2.31 | ||
| PH2 | 75.86 | 30 | 63.05 | 11.70 | 1.69 | 23.56 | 95.39 | 4.61 | ||
| PH3 | 92.80 | 40.61 | 47.89 | 9.51 | 0.98 | 41.62 | 11.83 | 63.39 | 24.78 | |
| PH4 | 101.84 | 63.35 | 50.62 | 9.03 | 1.28 | 39.07 | 3.90 | 45.77 | 8.76 | 41.57 |
| IH5 | 123.74 | 59.47 | 51.93 | 4.72 | 0.91 | 42.44 | 1 | 12.46 | 19.73 | 66.81 |
| IH6 | 131.76 | 68.99 | 41.66 | 2.38 | 1.07 | 54.89 | 18.82 | 28.29 | 52.89 | |
| IH7 | 177 | 87.75 | 64.27 | 7.66 | 1.01 | 27.06 | 24.03 | 12.15 | 63.82 | |
| JH8 | 263 | 200.82 | 69.64 | 3.41 | 1.25 | 25.70 | 8.68 | 12.65 | 78.67 | |
| JH9 | 272.50 | 209.13 | 74.89 | 0.50 | 1.35 | 23.26 | 39.09 | 13.54 | 47.37 | |
| AH10 | 267.50 | 167.61 | 64.47 | 6.83 | 2.22 | 26.48 | 100 | |||
| AH11 | 275 | 336.77 | 53.30 | 7.13 | 1.34 | 38.23 | 100 | |||
| AH12 | 291 | 249.02 | 55.35 | 5.67 | 1.44 | 37.54 | 2.05 | 97.95 | ||
| AH13 | 306.50 | 298.31 | 65.07 | 1.79 | 1.24 | 31.90 | 32.37 | 5.95 | 61.68 | |
| AH14 | 321 | 335.83 | 62.13 | 6 | 1.46 | 30.41 | 0.55 | 99.45 | ||
| AH15 | 327 | 336.31 | 67.17 | 3.33 | 1.85 | 27.65 | 2.80 | 5.36 | 91.84 | |
Histochemical analyses
Red Raman spectroscopy
Histomorphological analysis of each section was complemented with mineral analysis of cortical bone. Vibrational spectroscopy provides considerable information for the study of biomineralised tissues (including archaeological bone) (Carden & Morris, 2000). Raman analysis was carried out at three points (i.e. outer, middle, and inner cortex) of four different areas across all the humeri sections (i.e. anterior, posterior, medial, and lateral), establishing a total of 12 spectra for each humerus. Humeri blocks, after being finely polished with a 20-μm diamond cloth and different grit sanding papers (Buehler®, Lake Dluff, IL, USA), were observed with a Raman microscope (inVia Raman microscope, Renishaw®, Wotton under the Edge, Gloucestershire, UK) using a 785-nm diode laser to reduce the fluorescence effect and obtain better spectrum signals from the analyses (described in Bertoluzza et al. 1997; Ager et al. 2005; Ashok et al. 2011). Raman Processing software was employed in ‘adaptive min-max’ polynomial fit mode to subtract the background fluorescence and to progressively construct a baseline for each spectrum (Smith & Rehman, 1995; Cao et al. 2007). This fluorescence (raw Raman intensity data) was quantified by integrating the area under the raw spectra using originpro 8.5 software (OriginLab Corporation©, Northampton, MA, USA) (see the upper right corner in bottom rows from Figs 5). The mean spectra (12 points over each bone section), without fluorescence and with their standard deviation values, were plotted for each individual and compared with the human hydroxyapatite control described in Smith & Rehman (1995).
X-Ray diffraction (XRD) and thermogravimetric (TGA) analyses
We also performed XRD and TGA analyses on several exemplars from each age group (PH2, IH7, JH9, AH13) to corroborate/complement the Raman analyses. XRD provided information related to the whole bone crystallinity of each cortex and could be used to determine whether changes in the Raman intensity values are directly related not only to the molecules’ vibrational changes but also to changes in the level of mineralised tissue organisation. After smoothing the outer bone surface using different grit sandpaper (to improve its flatness), we used a new bone section to obtain XRD data with a Bruker D8 advanced diffractometer (Bruker®, Billerica, MA, USA), and the results were analysed with eva software (Bruker®). To achieve a representative sampling of the whole bones, we used the same section in the TGA analyses. The analysis was carried out at 10°C min–1 up to 1400 °C in air and enabled the calculation of the percentage of organic compounds that remained in the humeri. The results confirmed that the intensity of the spectra (fluorescence) is independent of the organic compounds present on bone.
Results
Mineralised area is progressively reduced during perinatal development
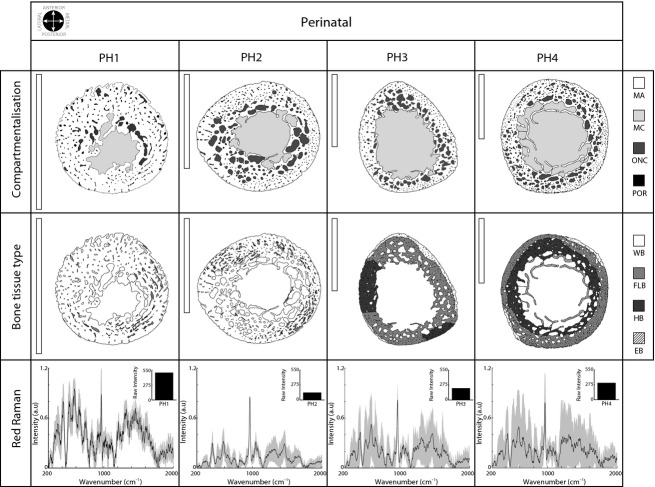
We found large differences in the perinatal individuals in terms of bone compartmentalisation (top row in Fig. 2 and Table 1). PH1 was characterised by a thick mineralised cortex that was poorly vascularised (7.12%, ONC + POR) and had a small medullary cavity (15.95%). In contrast, PH2, PH3, and PH4 presented a progressively reduced mineralised cortex (approximately 26% < PH1) and a larger medullary cavity (that increased up to nearly 40%). The highest vascularisation was in PH2 (13.39%), where the ONCs surround the medullary cavity, unlike PH1, where ONCs were concentrated in the lateral and medial cortex. PH3 and PH4 also presented a large number of ONCs concentrated in the anterior, posterior, and medial side of the cortex (top row in Fig. 2).
Figure 2.
Perinatal bone cross-sections. The top row shows compartmentalisation, the middle row shows different bone tissues, and the bottom row shows Raman spectroscopy data. The line plot represents the mean Raman spectrum values after background fluorescence subtraction, and the grey colour represents the standard deviation among the 12 points sampled in each individual. The black bar plots in the upper right corner (bottom row) show raw Raman intensity values after signal processing (fluorescence subtracted). Scale bars: 5 mm (vertical white bar, upper left corner in top and middle row). See Table 1 for abbreviations.
With regard to the bone tissue type distribution (middle row, Fig. 2), perinatal individuals were characterised by the presence of woven bone (WB, white colour), which was only present at high levels in the PH1 and PH2 individuals (where WB occupies more than 95% of the total mineralised area, see Table 1). Small dark grey areas in PH1 and PH2 revealed emerging fibrolamellar bone (FLB, < 5%). In these small areas, and in association with ONC, parallel lamination was visible with a polarised light microscope (data not shown). WB was largely reduced in PH3 and PH4 (< 4% in PH4), and FLB occupied a large portion of the cortical bone. PH2 presented isolated secondary osteons; however, in PH3, a real HB (contiguous secondary Type II osteons covering over 20% of the cortex) was identified for the first time. In PH4, HB occupied over 40% of the total mineralised area, and endosteal bone (EB, lamellar bone in the inner border of the cortex) appeared for the first time. In contrast with PH1 and PH2, both PH3 and PH4 also presented a large increase (nearly two-fold) in the cross-sectional area.
The Raman signal was very complex in perinatal individuals (bottom row, Fig. 2). In PH1, the Raman signal had many gross peaks, and although the inorganic and organic phases of biomineralised human hydroxyapatite could be inferred, only the phosphate peak (ν1 PO43− at 957–962 cm−1) was clearly differentiated. In addition, the raw Raman intensity value (i.e. fluorescence subtracted, see upper right corner in bottom row, Fig. 2) was extremely high in PH1. In PH2, PH3, and PH4, the Raman mean signal seemed to be better defined (more peaks of hydroxyapatite were partially observable; ν2 PO43− at 422–454 cm−1 and ν3 PO43− at 578–617 cm−1; and ν1 CO32− at 1065–1071 cm−1). However, the disparity between the points sampled for each section was higher (standard deviation values in Raman plot, Fig. 2). The organic phase of the bone was distinguished in PH2 (Amide III at 1243–1269 cm−1; CH2 at 1447–1452 cm−1; and amide I at 1595–1720 cm−1).
Cortical bone incorporates more bone tissue types during infancy but the mineral component is still poorly defined
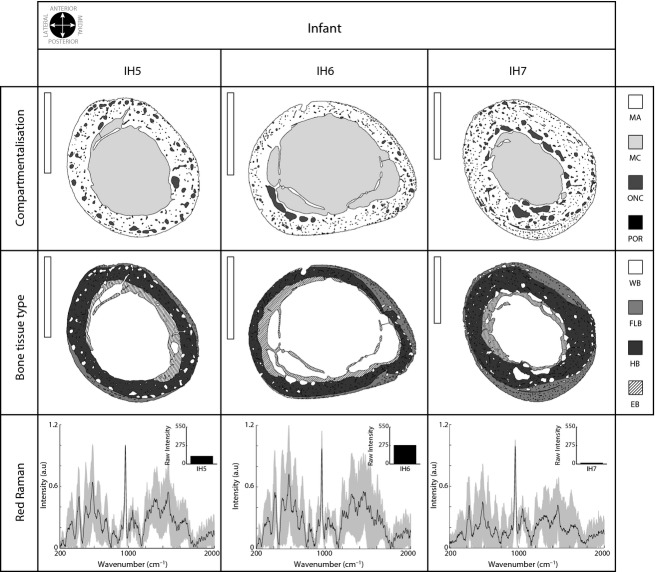
In infant individuals, the cross-section size, despite variability, was larger than of the perinatal individuals (more than 80 mm2 in IH7). The differences in compartmentalisation were concentrated in the medullary cavity (increasing up to 54.89% in IH6; see Table 1 and top row in Fig. 3). In this group, vascularity was lower (mean value 5.91%), and ONCs were regularly distributed in the cortex, with the largest ones aligned in different areas of the cortex (see Fig. 3). The largest individual (IH7) presented the most differentiated pattern. The cross-section was greater than in IH5 and IH6 and presented a thicker mineral area (over 60%) and a clearly reduced medullary cavity (under 30%).
Figure 3.
Infant bone cross-section analysis. See Fig. 2 for details. Scale bar: 5 mm (vertical white bar in the upper left corner in the top and middle rows).
WB was drastically reduced and disappeared entirely in IH6. In IH5, small WB areas were recognised in the anterior area of the external cortex, but they did not represent more than 1%. FLB and HB occupied almost all the cortical area, establishing different annular zones surrounding the medullary cavity (FLB externally and HB internally, Fig. 3, middle row). EB reached the largest values in IH6 (28.29%) and was reduced in IH7 (12.15%).
The Raman signal in infant bone was similar to that described in larger perinatal individuals. In general, the mean Raman spectrum presented many peaks corresponding to hydroxyapatite and the organic components of bone. The overall signal presented high variability among the sampled points in the cortex (bottom row, Fig. 3), and the raw intensity values (fluorescence) of IH5 and IH6 were similar to those described in the largest perinatal. In contrast, IH7 presented a slightly more defined signal and lower fluorescence values (upper right corner in IH7 Raman signal, bottom row, Fig. 3).
Vascularisation is minimal in juveniles, whereas mineralisation is clearly defined
The size of the cross-section was notably increased in juvenile individuals (the mean was more than twice that observed in the infants). The mineral tissue reached the largest proportion (mean value 72.26%), and the medullary cavity was reduced in comparison with infant individuals (under 26%) (see Table 1 and top row in Fig. 4). Juvenile bone exhibited changes in shape, and the bone section seemed to be reoriented. The most evident characteristic seemed to be the extremely low vascularisation of this group. Although JH8 presented ONC alignment in the external cortex (top row Fig. 4), JH9 presented the least vascularisation (under 2%) and had almost no ONC.
Figure 4.
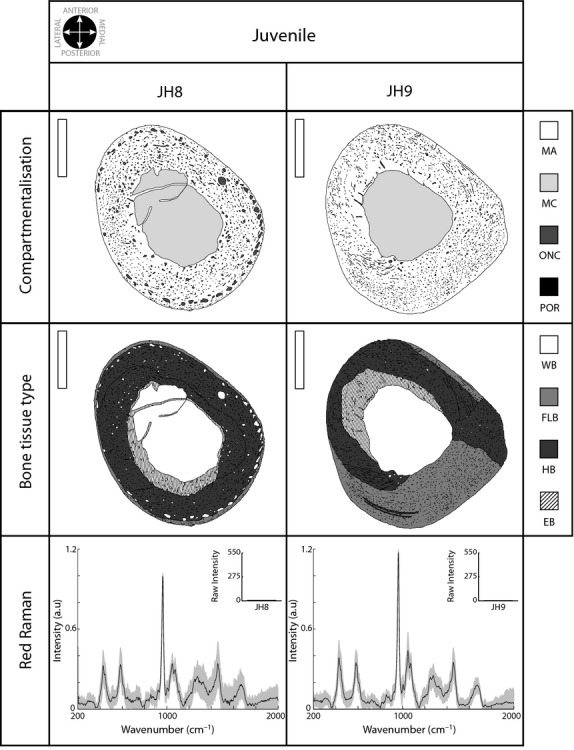
Juvenile bone cross-section analysis. Details as in previous figures. Scale bar: 5 mm (vertical white bar in the upper left corner in the top and middle rows).
Considering the bone tissue typologies (middle row Fig. 4), and despite the variability found between individuals, the juvenile group was characterised by a relative increase in FLB and HB area compared with the infant group (FLB near 40% in JH9 and HB up to 78.67% in JH8). EB asymmetrically occupied the inner area of the cortical bone; it was lateral and posterior in JH8 and lateral and anterior in JH9. However, in both individuals, the overall extent of EB was similar to that of the largest infant individual (near 13%).
The mineral content was well organised in juveniles. The Raman spectrum was extremely well defined (bottom row in Fig. 4) in contrast to the younger individuals. Only JH8 presented large differences between the points sampled in the cortex (standard deviation in the bottom row, Fig. 4). Continuing the tendency shown in IH7, the juveniles had extremely low Raman intensity values (fluorescence subtracted, see the upper right corner in the bottom row of Fig. 4).
Mineral definition varies dramatically among adults, allowing the definition of two different patterns
Despite the differences in the cross-sectional area between adult individuals (from 167.61 mm2 in AH10 to 336.31 mm2 in AH15, see Table 1), bone sections maintained similar proportions with regard to the various compartments. Differences between individuals were 14% (from 53.30% to 67.17%) for the mineralised area and 12% for the medullary cavity (from 26.48% to 38.23%). Vascularisation was similar in all humeri (more than 7%) except for AH13 and AH15, which did not exceed this value. As observed in Fig. 5 (top row), ONC seemed to be concentrated in the anterior part of the bone sections, except for the AH11 individual, who presented a more quadrangular section shape (the other adults maintained the cross-sectional configuration described in juveniles).
In adult individuals, HB was largely predominant and was almost entirely composed of type II and type III osteons (middle row, Fig. 5). However, FLB largely persisted in AH13 (32.37%) and to a minor extent in AH15 (2.8%) and AH14 (0.55%). Most of the EB completely disappeared, but a remnant of EB (under 6%) was identified in AH12, AH13, and AH15.
Raman analysis of the bone samples from adult individuals clearly identified two main patterns (bottom row, Fig. 5), one in which the spectrum was similar to that described in infant individuals and another that was similar to those described in juveniles. The AH10, AH11, and AH12 individuals presented a poorly defined spectrum and were characterised by high variability between points sampled across the bone section (bottom row, Fig. 5). In contrast, the AH13, AH14, and AH15 individuals presented a better-defined spectrum in which the peaks were easily individualised. In these individuals, the raw Raman intensity values (fluorescence) were also very low (see bar plots in bottom row, Fig. 5).
Discussion
The macro- and microstructure of bone reflect the effects of non-mechanical (e.g. gene expression, hormonal regulation, dietary calcium, paracrine effects, sex, age, pathologies) and biomechanical factors (e.g. bending and torsion loads) during biological development (Frost, 1999; Currey, 2002; Pearson & Lieberman, 2004; Little et al. 2011; Stout & Crowder, 2012). Over the years, animal models and postmortem human samples (forensic and archaeological) have been used by palaeontologists, anthropologists, bioarchaeologists, and researchers from biomedical disciplines to analyse the histomorphological changes in bone growth from clinical or non-clinical perspectives. Here, we provide an approach to assess bone development that combines the spatial distribution of the mineralised and non-mineralised compartments, the extension and distribution of histological typologies, and mineral variations within the cortex through cross-sectional analysis.
In this study, we examined the human left humeri because it could be considered to be less influenced by weight or mechanical load (Trinkaus et al. 1994; Ruff, 2000). In general, few studies have histomorphologically characterised the humeral cortical bone. However, surprisingly, previous works have conducted anthropological analyses with living and archaeological populations, as well as with other Homo species (Ruff et al. 1993; Trinkaus et al. 1994). Our sample could not be considered a real ontogenetic series because the skeletal remains belong to an archaeological deposit and could therefore introduce uncontrollable bias. However, taking into account the high quality of preservation of the skeletal remains (TGA results show that over 33% of the organic components of all individuals was preserved) and that our results show consistent similarities with projected results of previous studies, we believe that these remains offer information that could be used to address histomorphological and histochemical variation in cortical bone during development and contribute data to new investigations on the global understanding of long bone growth.
The histomorphological characteristics of the perinatal cortex have not been fully described. Baltadjiev (1995) quantified osteon density in tibiae during gestation (150 fetuses, 6–9 months), describing how low values characterise the late phases of intrauterine life. Our results agreed with those, although in our case we found that vascularisation increased quickly with early postnatal growth (up to PH4). Moreover, it seems that the cross-section size increase establishes important changes in the compartmentalisation of the perinatal cortex (upper plot, Fig. 6). The mineralised area (MA) was drastically reduced (26.31%, Table 1), and the medullary cavity increased (MC, 23.12%; see Fig. 6). Accompanying this transformation, cortical bone, broadly occupied by woven bone (WB), was completely replaced by a new fibrolamellar bone (FLB, over 60% in PH3, bottom plot in Fig. 6). The massive presence of WB indicates unorganised, rapidly growing bone that is not mineralogically defined (gross peaks in Raman spectrum; Carden & Morris, 2000) and does not have a well-defined or disordered crystalline structure, as demonstrated by the lower values in the X-ray plot (Fig. 7), the high standard deviation in the Raman spectrum (Fig. 2 bottom row), and the high raw Raman intensity values (fluorescence subtracted, bar plot in Fig. 2, bottom row) (see Fig. 2). The progressive appearance of FLB (more slowly deposited bone; Currey, 2002) is related to the increase in bone size and the shape transformations in the cortex.
Figure 6.
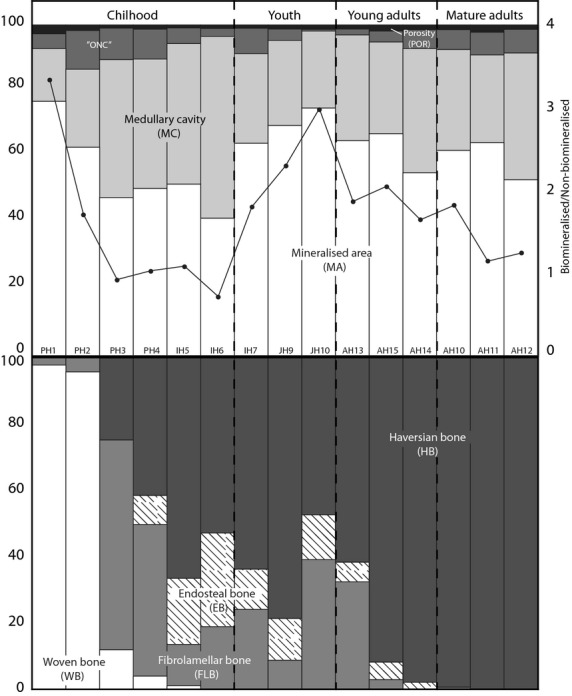
Compartmentalisation (upper) and bone tissue typology (bottom) variation during growth. Adult individuals appear ordered, supporting our proposal (see Discussion for details). In the upper plot, the black line represents the ratio between the mineralised (MA) and non-mineralised areas (MC + vascularisation). The bottom plot represents the percentages of different bone tissues, establishing the difference between modelling (bottom left) and remodelling (upper right).
Figure 7.
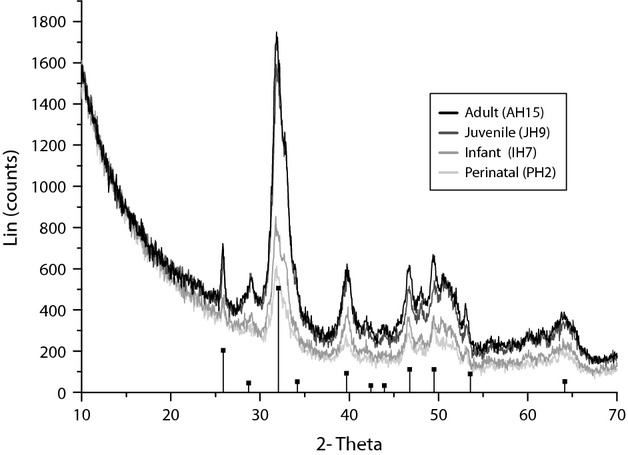
X-Ray diffraction (XRD) characterisation of the different ontogenetic groups. Note the gradual crystalline increase from perinatal to juvenile and adult individuals. Vertical lines represent hydroxyapatite (HDA) peak characterisation.
There are more data regarding cross-sectional changes during the infant and juvenile periods, although such data are difficult to analyse. Despite the fact that they did not include data on humeri, in 1970 Frisancho et al. (1970) studied 6972 metacarpal radiographs from 8–30-year-old people and showed that during childhood the cortex becomes thicker and the medullary cavity becomes larger, and in adolescence, the periosteal (FLB) and endosteal bone is added. This growth is relatively greater in males. Ruff et al. (1994) investigated the robustness and asymmetry of humeri during ontogeny (Ruff & Jones, 1981; Ruff et al. 1993; Trinkaus et al. 1994). The authors proposed that ontogeny involves a continuous increase in cross-sectional size principally due to periosteal apposition from childhood to adulthood, a medullary cavity expansion (endosteal resorption) during childhood and early adolescence, and a decrease in the medullary cavity due to endosteal apposition from mid-adolescence through early adulthood. Bass et al. (2002) analysed the influence of exercise during growth on the increase in bone mineral content. In contrast to Ruff et al. (1994), who collected radiographic data, Bass et al. (2002) used dual energy X-ray absorptiometry (DXA) and magnetic resonance imaging (MRI) in loaded and non-loaded arms from 47 competitive female tennis players (8–17 years). Bass et al. (2002) noted that the bone mineral content was related to activity during this period and that higher values of bone mineral content may correspond to the loaded arms in prepubescent players. Recently, in a study similar to ours, Goldman et al. (2009) exhaustively described the histology of 14 complete femur cross-sections (2–19 years old). The authors described how, during this period, cortical bone largely increased in size and mainly consisted of FLB and HB, with endosteal bone (EB) appearing during this period (largely asymmetric and could reach the outer cortex in several areas). These results agree with those proposed by Maggiano (2012) in which the existence of EB marks late adolescence and may continue into the fourth decade of life (in men). In our case, infant individuals presented a progressively diminished mineralised area that is almost occupied by HB (remodelled FLB) and EB and a large medullary cavity, which implies a continuation of the trend established in perinatal individuals (Fig. 6, upper plot) and positively correlates with data from other authors (Ruff et al. 1994). Nevertheless, the largest infant individual (IH7) presented a dissimilar pattern in which the medullary cavity was reduced, as described in the juvenile sample. This is the pattern that Ruff et al. (1994) described in juvenile individuals, consisting of a thicker mineralised cortex (mineral areas occupy a mean of 72.26% of the total cortex) and a narrower medullary cavity (mean value of 24.48%). This change is accompanied by a well mineralised cortex in which peaks are extremely well defined (Fig. 7), even more than in adults. This result also corroborates data presented by Akkus et al. (2003) in which Raman spectroscopy identified different tissue types in femoral sections of individuals ranging in age from 17 to 73. In general agreement with our results, the authors proposed that the juvenile primary cortex has a lower mineral content than the adult tissue, although the mineral crystallinity was better defined in juveniles than in older adults. Ager et al. (2005), using deep-ultraviolet Raman spectroscopy to evaluate how aging effects the organic component of the humeral cortical bone, also found that the Amide I band of cortical bone varies in height and location (moves to higher energy) with age.
Bocquet & Bergot (1977) analysed 504 adult skeletons (radiographs) from an archaeological collection and proposed a general pattern in which the humeral cortical bone becomes progressively reduced in thickness but the section diameter becomes wider as the individual ages. Furthermore, the authors concluded that these variations are concentrated in two periods in women (approximately 40–50 and 70 years old) and in one period in men (approximately 60 years old). Laval-Jeantet et al. (1983) used microradiographs and the ash weight of 89 humeri from cadavers (42–90 years old) to determine that cortical porosity increases with age in both sexes (4–10%). The authors also proposed that mineral density is linked to porosity and decreases markedly with age (less in men). In light of our results, we can discuss these conclusions. As we commented in the Results section, variations in compartmentalisation are minimal among adult individuals (see Table 1). However, the density of vascular cavities does differ among them; AH10, AH11, AH12, and AH14 present the highest values (over 7%) and almost completely remodelled cortex (HB occupies nearly 100% of the total cortical area); in contrast, as occurred in juveniles, AH13 and AH15 retain FLB and EB in their cortex and presented small values of vascularisation (under 5.5%). Moreover, these two patterns agree with the histochemical data; AH10, AH11, and AH12 presented less crystallographic order (bottom row, Fig. 5). In contrast, AH13, AH14, and AH15 had similar, well defined chemical compositions similar to those described in juveniles (bottom row, Fig. 5).
One may ask whether these data could be used to affirm that the AH10, AH11, and AH12 individuals are ‘older’. Given that mineral density decreases with age and is linked to an increase in porosity (Laval-Jeantet et al. 1983; Bousson et al. 2001; Bergot et al. 2009), we speculate that this could be a ‘mature adult’ configuration. Future studies in which age-at-death estimation and sex determination could be improved not only for adult individuals (e.g. using documented samples) will help us test our proposal (shown in Fig. 6 where adult individuals are reorganised) and, more importantly, determine whether the surprising similarities between our curve (the ratio between the mineralised and non-mineralised occupied area, upper plot, Fig. 6) and the general growth velocity curve proposed for humans (Tanner, 1990), are real or are just artefacts.
Conclusions
Bone is a complex tissue that not only supports the body but also provides information about the nutritional status and/or health of the individual. The most important difference that distinguishes bone from other tissues is mineralisation. However, biomineralisation cannot be observed as a discrete event; it is the effect of accumulated modelling and remodelling processes over time. As our results suggest, the mineral and non-mineral area distribution (compartmentalisation) is highly related to bone tissue typology allocation and the chemical composition and structure of the cortical bone. Our analyses of bone cross-sections show that, starting from an immature cortical bone in which the medullary area progressively increases, the cortical bone is modelled to a juvenile cortex in which mineralised tissue occupies almost the entire cross-section. After the modelling declines, remodelling transforms the juvenile cortex, which is highly crystalline, into a more variable structure in which the mineralised area is replaced by non-mineralised tissues. Although more analyses are needed to provide a better understanding of cortical histomorphological and histochemical variations, our results offer new insights into modelling and remodelling that must be strengthened with further investigations.
Acknowledgments
This research was supported and performed within projects CGL2009-10766 (Ministerio de Ciencia e Innovación), CICYT-MAT2010-17753, MICINN-12-HAR2011-23106 and CGL2012-35199 (Ministerio de Economía y Competitividad). The authors would especially like to thank the Instituto de Cerámica y Vidrio (iCV-CSIC) for the use of their technical facilities. We are deeply grateful to Miguel Ángel Fernández Rodríguez for technical support and Daniel Heredia Doval for comments during the first steps of this investigation.
Author contributions
O.C.M., C.N.M., M.A.R.B., and A.G.M. designed the project and supervised the experiments. O.C.M., C.N.M., M.A.R.B., O.G.G., J.R.P., M.D’.A., and M.C.M. performed the analyses. O.C.M., C.N.M., S.R.V., M.A.R.B., and A.G.M. analysed the results and wrote the paper.
References
- Ager JW, III, Nalla RK, Breeden KL. Deep-ultraviolet Raman spectroscopy study of the effect of aging on human cortical bone. J Biomedical Opt. 2005;10:1–8. doi: 10.1117/1.1924668. [DOI] [PubMed] [Google Scholar]
- Akkus O, Polyakova-Akkus A, Adar F, et al. Aging of microstructural compartments in human compact bone. J Bone Miner Res. 2003;18:1012–1019. doi: 10.1359/jbmr.2003.18.6.1012. [DOI] [PubMed] [Google Scholar]
- Ashok PC, Praveen BB, Dholakia K. Near infrared spectroscopic analysis of single malt Scotch whisky on an optofluidic chip. Opt Express. 2011;19:22982–22992. doi: 10.1364/OE.19.022982. [DOI] [PubMed] [Google Scholar]
- Baltadjiev G. Micromorphometric characteristics of osteons in compact bone of growing tibiae of human fetuses. Acta Anat. 1995;154:181–185. doi: 10.1159/000147767. [DOI] [PubMed] [Google Scholar]
- Bass SL, Saxon L, Daly RM, et al. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res. 2002;17:2274–2280. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- Bergot C, Wu Y, Jolivet E, et al. The degree and distribution of cortical bone mineralization in the human femoral shaft change with age and sex in a microradiographic study. Bone. 2009;45:435–442. doi: 10.1016/j.bone.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Bertoluzza A, Brasili P, Castri L, et al. Preliminary results in dating human skeletal remains by Raman spectroscopy. J Raman Spectrosc. 1997;28:185–188. [Google Scholar]
- Bocquet J-P, Bergot C. 1977. pp. 359–369. Évolution de l'os cortical de l'humérus en fonction de l’âge Bull Mém Soc Anthropol Parist.4 (série XIII)
- Boskey AL. Osteoporosis and osteopetrosis. In: Epple M, Baeuerlein E, editors. Handbook of Biomineralization. Medical and Clinical Aspects. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2007. pp. 59–80. [Google Scholar]
- Bousson V, Meunier A, Bergot C, et al. Distribution of intracortical porosity in human midfemoral cortex by age and gender. J Bone Miner Res. 2001;16:1308–1317. doi: 10.1359/jbmr.2001.16.7.1308. [DOI] [PubMed] [Google Scholar]
- Bromage TG, Goldman H, McFarlin SC. Circularly polarized light standards for investigations of collagen fiber orientation in bone. Anat Rec B New Anat. 2003;274:157–168. doi: 10.1002/ar.b.10031. [DOI] [PubMed] [Google Scholar]
- Buikstra JE, Ubelaker DH. 1994. Standards for data collection from human skeletal remains. Fayetteville: Arkansas Archaeological Survey Research Series No 44.
- Burton P, Nyssen-Behets C, Dhem A. Haversian bone remodeling in human fetus. Acta Anat. 1989;135:171–175. doi: 10.1159/000146748. [DOI] [PubMed] [Google Scholar]
- Cambra-Moo O, Nacarino Meneses C, Rodríguez Barbero MA, et al. Mapping human long bone compartmentalization during ontogeny: a new methodological approach. J Struct Biol. 2012;178:338–349. doi: 10.1016/j.jsb.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Cao A, Pandya AK, Serhatkulu GK, et al. A robust method for automated background subtraction of tissue fluorescence. J Raman Spectrosc. 2007;38:1199–1205. [Google Scholar]
- Carden A, Morris MD. Application of vibrational spectroscopy to the study of mineralized tissues (review) J Biomedical Opt. 2000;5:259–298. doi: 10.1117/1.429994. [DOI] [PubMed] [Google Scholar]
- Cooper C, Javaid MK, Taylor P, et al. The fetal origins of osteoporotic fracture. Calcif Tissue Int. 2000;70:391–394. doi: 10.1007/s00223-001-0044-z. [DOI] [PubMed] [Google Scholar]
- Currey JD. Bones. Structure and Mechanics. Princeton: Princeton University Press; 2002. [Google Scholar]
- Enlow D. Principles of Bone Remodeling. An Account of Post-Natal Growth and Remodeling Process in Long Bone Bones and the Mandible. Springfield, IL: Charles C Thomas; 1963. [Google Scholar]
- Enlow D. The Human Face. An Account of the Postnatal Growth and Development of the Craniofacial Skeleton. New York: Hoeber Medical Division, Harper & Row; 1968. [Google Scholar]
- Frisancho AR, Garn SM, Ascoli W. Subperiosteal and endosteal bone apposition during adolescence. Hum Biol. 1970;42:639–664. [PubMed] [Google Scholar]
- Frost HM. An approach to estimating bone and joint loads and muscle strength in living subjects and skeletal remains. Am J Hum Biol. 1999;11:437–455. doi: 10.1002/(SICI)1520-6300(1999)11:4<437::AID-AJHB4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Goldman HM, McFarlin SC, Cooper DML, et al. Ontogenetic patterning of cortical bone microstructure and geometry at the human mid-shaft femur. Anat Rec. 2009;292:48–64. doi: 10.1002/ar.20778. [DOI] [PubMed] [Google Scholar]
- Gosman JH. Growth and development. Morphology, mechanisms, and abnormalities. In: Crowder C, Stout S, editors. Bone Histology. An Anthropological Perspective. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2012. pp. 23–44. [Google Scholar]
- Gosman JH, Hubbell ZR, Shaw CN, et al. Development of cortical bone geometry in the human femoral and tibial diaphysis. Anat Rec. 2013;296:774–787. doi: 10.1002/ar.22688. [DOI] [PubMed] [Google Scholar]
- Karaplis AC. Embryonic development of bone and regulation of intramembranous and endochondral bone formation. In: Bilezikian JP, Raisz LG, Martin J, editors. Principles of Bone Biology. 3rd edn. San Diego: Academic Press, Elsevier; 2008. pp. 53–84. [Google Scholar]
- Klepinger LL. Fundamental of Forensic Anthropology. Hoboken, NJ: Wiley-Liss; 2006. [Google Scholar]
- Laval-Jeantet A-M, Bergot C, Carroll R, et al. Cortical bone senescence and mineral bone density of the humerus. Cacif Tissue Int. 1983;35:268–272. doi: 10.1007/BF02405044. [DOI] [PubMed] [Google Scholar]
- Little N, Rogers B, Flannery M. Bone formation, remodeling and healing. Surgery. 2011;29:141–145. [Google Scholar]
- Maggiano CM. Making the mold. A microstructural perspective on bone modeling during growth and mechanical adaptation. In: Crowder C, Stout S, editors. Bone Histology. An Anthropological Perspective. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2012. pp. 45–90. [Google Scholar]
- McFarlin SC. Ontogenetic Variation in Long Bones Microstructure in Catarrhines and its Significance for Life History Research. New York: The City University of New York; 2006. [Google Scholar]
- Morgan EF, Bouxsein ML. Biomechanics of bone and age-related fractures. In: Bilezikian JP, Raisz LG, Martin J, editors. Principles of Bone Biology. 3rd edn. San Diego: Academic Press; 2008. pp. 29–51. [Google Scholar]
- Nacarino Meneses C, Cambra-Moo O, Rodríguez Barbero MA, et al. Aportaciones de la Paleohistología humana al estudio de biomaterials (Contributions of human palaeohistology to the study of biomaterials) Bol Soc Esp Ceram V. 2012;51:313–320. [Google Scholar]
- Paschalis EP, Verdelis K, Doty SB, et al. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- Pearson OM, Lieberman DE. The aging of Wolff's ‘Law’: ontogeny and responses to mechanical loading in cortical bone. Yearbook Phys Anthropol. 2004;47:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- Ruff CB. Biomechanical analyses of archeological human skeletons. In: Kazenberg M, Saunders SR, editors. Biological Anthropology of the Human Skeleton. New York: Wiley-Liss Inc; 2000. pp. 71–102. [Google Scholar]
- Ruff CB, Jones HH. Bilateral asymmetry in cortical bone of the humerus and tibia-sex and age factors. Hum Biol. 1981;53:69–86. [PubMed] [Google Scholar]
- Ruff CB, Trinkaus E, Walker A, et al. Postcranial robusticity in Homo I: temporal trends and mechanical interpretation. Am J Phys Anthropol. 1993;91:21–53. doi: 10.1002/ajpa.1330910103. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Walker A, Trinkaus E. Postcranial robusticity in Homo. III: ontogeny. Am J Phys Anthropol. 1994;93:35–54. doi: 10.1002/ajpa.1330930103. [DOI] [PubMed] [Google Scholar]
- Scheuer L, Black S. Developmental Juvenile Osteology. San Diego: Academic Press; 2000. [Google Scholar]
- Schinke T, Amling M. Mineralization of bone: an active or passive process? In: Epple M, Baeuerlein E, editors. Handbook of Biomineralization. Medical and Clinical Aspects. Weinheim: Wiley-VCH, Verlag GmbH & Co. KGaA; 2007. pp. 3–18. [Google Scholar]
- Sedlin ED, Frost HM. Variations in rate of human osteon formation. Can J Biochem Phys. 1963;41:19–22. [PubMed] [Google Scholar]
- Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- Seeman E. Modeling and remodeling. The cellular machinery responsible for the gain and loss of bone's material and structural strength. In: Bilezikian JP, Raisz LG, Martin J, editors. Principles of Bone Biology. 3rd edn. San Diego: Academic Press, Inc; 2008. pp. 3–28. [Google Scholar]
- Smith R, Rehman I. Fourier transform Raman spectroscopic studies of human bone. J Mater Sci. 1995;5:775–778. [Google Scholar]
- Stout S, Crowder C. Bone remodeling, histomorphology, and histomorphometry. In: Crowder C, Stout S, editors. Bone Histology. An Anthropological Perspective. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2012. pp. 1–22. [Google Scholar]
- Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004;25:987–994. doi: 10.1016/s0142-9612(03)00621-5. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Fetus into Man. Physical Growth from Conception to Maturity. Cambridge, MA: Harvard University Press; 1990. [Google Scholar]
- Trinkaus E, Churchill SE, Ruff CB. Postcranial robusticity in Homo. II: humeral bilateral asymmetry and bone plasticity. Am J Phys Anthropol. 1994;93:1–34. doi: 10.1002/ajpa.1330930102. [DOI] [PubMed] [Google Scholar]
- Ubelaker DH. Human Skeletal Remains: Excavation, Analysis, Interpretation. Washington, USA: Taraxacum; 1978. [Google Scholar]
- White TD, Folkens PA. The Human Bone Manual. London: Academic Press, Elsevier; 2005. [Google Scholar]