Abstract
Tooth crown patterning is governed by the growth and folding of the inner enamel epithelium (IEE) and the following enamel deposition forms outer enamel surface (OES). We hypothesized that overall dental crown shape and covariation structure are determined by processes that configurate shape at the enamel–dentine junction (EDJ), the developmental vestige of IEE. This this hypothesis was tested by comparing patterns of morphological variation between EDJ and OES in human permanent maxillary first molar (UM1) and deciduous second molar (um2). Using geometric morphometric methods, we described morphological variation and covariation between EDJ and OES, and evaluated the strength of two components of phenotypic variability, canalization and morphological integration, in addition to the relevant evolutionary flexibility, i.e. the ability to respond to selective pressure. The strength of covariation between EDJ and OES was greater in um2 than in UM1, and the way that multiple traits covary between EDJ and OES was different between these teeth. The variability analyses showed that EDJ had less shape variation and a higher level of morphological integration than OES, which indicated that canalization and morphological integration acted as developmental constraints. These tendencies were greater in UM1 than in um2. On the other hand, EDJ and OES had a comparable level of evolvability in these teeth. Amelogenesis could play a significant role in tooth shape and covariation structure, and its influence was not constant among teeth, which may be responsible for the differences in the rate and/or period of enamel formation.
Keywords: developmental constraints, evolvability, geometric morphometrics, morphological variability, odontogenesis
Introduction
Dental morphological characteristics such as cusps, accessory cusps, and ridges on the occlusal surface have been used extensively in studies of hominoid evolution and phylogenetic relationships (Miller, 1918; Simons & Pilbeam, 1972; Dean, 2000; Pilbrow, 2006; Matsumura et al. 2011). Tooth crown morphology is determined by two developmental processes (Avishai et al. 2004; Skinner & Gunz, 2010; Smith et al. 2011). The first process is the growth and folding of the inner enamel epithelium (IEE) during the bell stage. This morphogenesis (= tooth crown patterning) is governed by interactions between the IEE and underlying mesenchymal tissues. The final configuration of the IEE is preserved as the enamel–dentine junction (EDJ). The second process is biomineralization by the enamel-forming ameloblasts and dentine-forming odontoblasts. Ameloblasts are derived from the IEE cells and odontoblasts from the dental papilla cells. Enamel formation starts at the cusp tips, and proceeds apically to complete the outer enamel surface (OES).
Recent micro-CT dental analyses have revealed that crown morphological traits of the completed EDJ are modified or masked by the process of enamel deposition (Skinner et al. 2009, 2010; Ortiz et al. 2012), and that the extent of modification varies, depending, in part if not totally, on the enamel thickness (Ortiz et al. 2012). This raises a concern about whether shared derived features and homoplastic features of similarity at the OES can be properly discriminated (Hunter & Jernvall, 1995; Collard & Wood, 2000; Finarelli & Clyde, 2004). Additionally, by examining enamel thickness variation and its heritability in pedigreed baboon molars, Hlusko et al. (2004) showed that enamel thickness could change rapidly under moderate or low selective pressure over evolutionarily short periods, increasing the potential for homoplasy. Although the OES morphology is directly related to dental functions such as occlusion and feeding and thus is a direct target of natural selection, the morphology of EDJ has been considered to be more conservative evolutionarily and a more reliable representation of the phenotype for estimating phylogenetic relationships (Kraus, 1952; Korenhof, 1960; Smith et al. 1997, 2000; Sasaki & Kanazawa, 1999; Olejniczak et al. 2007).
So far researchers have explored to what extent enamel formation influences the crown morphology by comparing EDJ with OES (Kraus, 1952; Korenhof, 1960, 1961; Nager, 1960; Sakai & Hanamura, 1971; Skinner et al. 2008, 2009; Ortiz et al. 2012). However, these studies mainly have focused on discrete dental traits. Although a few studies have tried to evaluate general morphological differences between EDJ and OES quantitatively using intercusp distance (Smith et al. 1997) or surface complexity (Skinner et al. 2010), the complex dental crown topography of EDJ and OES has not been clarified in detail. Examining morphological variation and covariation between EDJ and OES will enable us to understand the effects of morphological change caused by enamel formation.
Additionally, given the different developmental backgrounds between the EDJ and OES, it is likely that the patterns of phenotypic variability differ between these structures. Phenotypic variability is defined as the tendency or potential of an organism to vary (Wagner & Altenberg, 1996; Wagner et al. 1997; Willmore et al. 2007). Therefore, it determines the potential range or distribution of morphological variation, and ultimately affects the tempo and mode of evolutionary change. Recent literature about phenotypic variability has focused closely on canalization and morphological integration (Wagner & Altenberg, 1996; Hallgrímsson et al. 2002, 2009; Willmore et al. 2007). Canalization is generally considered the property of an organism that limits phenotypic variation by buffering developmental processes against both environmental and genetic perturbations (Wagner et al. 1997; Willmore et al. 2007). Morphological integration refers to the tendency for different characters to covary as a result of common underlying developmental factors (Hallgrímsson et al. 2002), which constrains the production of phenotypic variation (Wagner & Altenberg, 1996; Chernoff & Magwene, 1999). Canalization and morphological integration are interrelated and can act as developmental constraints (Alberch, 1982; Maynard Smith et al. 1985; Hallgrímsson et al. 2002). Since the morphological integration framework is directly connected to the rate and direction of evolutionary change (Cheverud, 1996; Wagner & Altenberg, 1996), some studies have focused on quantification of the intervening effect of morphological integration on evolutionary trajectory (Lande, 1979; Lande & Arnold, 1983). The resultant data have led to recent studies that evaluated evolvability (the ability of a population or species to respond to selection: Hansen, 2003) using the simulation of evolutionary responses to selection (Marroig et al. 2009; Villmoare et al. 2011; Lewton, 2012; Grabowski, 2013). The relationships and interactions among developmental processes, variability and variation, mediated by the feedback loop of natural selection, are critically involved in evolutionary change (Willmore et al. 2007). Comparison of the pattern of variability between EDJ and OES helps to infer how the production of morphological variation is regulated in each of these components.
In this study, we explore the relationship between the crown morphology and odontogenesis through quantitative analyses of the EDJ and OES morphology. We hypothesized that overall dental crown shape and covariation structure are determined by processes that configurate shape at the EDJ. If this hypothesis is rejected, a significant role of enamel formation for patterning of crown morphological variation must be presumed. To test this hypothesis, we described morphological variation and covariation between EDJ and OES and revealed how much variation in the OES shape is explained by the EDJ shape variation. Consequently, we evaluated the strength of two components of phenotypic variability, canalization and morphological integration, in addition to the relevant evolutionary flexibility.
Canalization
If EDJ shows larger variation, this means that more variable morphology is created during the early phase of the tooth development, and subsequent enamel formation acts as a stabilizing developmental process that buffers the deviation from mean shape. On the other hand, if OES shows larger variation, this indicates that enamel formation brings about not only homogeneous enamel distribution above the EDJ after the morphogenesis, but also some modification of the OES associated with the increased variation.
Morphological integration
During either morphogenesis or the enamel formation process, some developmental factors may produce higher morphological integration of one of these structures (whether EDJ or OES). Combined with the results regarding canalization, this analysis will help to determine what factors play important roles in generating or reducing morphological variance.
Evolutionary flexibility
In relation to the above two components of phenotypic variability, we specifically compared how the developmental constraints exert an influence on the ability of the response to selection in EDJ vs. OES.
This study focused on EDJ and OES shape variation of the permanent maxillary first molar (UM1) and deciduous second molar (um2). Although UM1 and um2 share similar patterns of occlusal morphology that are elaborated through the same developmental processes, their developmental timing, speed, and duration are different (Nanci, 2013). The differences between UM1 and um2 will provide a better understanding of the relationship between odontogenesis and crown morphological variability.
Materials and methods
The samples used in this study comprised fully formed but unworn UM1 and um2 crowns obtained from archaeological sites in Japan. The total sample (57 UM1 and 48 um2) consisted of samples from the Jomon (14500–300 BC; n = 8 and 5), Medieval (13th–15th century AD; n = 13 and 8), and Edo (17th–19th century AD; n = 36 and 35) periods. Although the total sample was from a mixture of populations from different periods and regions, the aim of this study was to investigate differences and patterns of variability produced by a common tooth formation process of the Holocene human, and therefore mixing these samples does not violate the objective of this study. To maximize sample size, no discrimination between right and left teeth was made, but only a single tooth was used from each individual. All specimens were regarded as left side. Right molar images were transformed into the mirror image using imagej software (NIH, USA). Sex was unknown for most of the samples, since they were taken from juvenile individuals.
Each specimen was μCT scanned (ScanXmateA080S, Comscantecno, Japan) with a pixel size and slice interval of 31–32 μm (80 kV, 125 μA). To facilitate tissue segmentation, the image stack for each tooth was filtered using a median filter followed by a Kuwahara filter, and enamel and dentine tissues were segmented by the seed region growing method in imagej. Triangular mesh models of the 3D EDJ and OES of each specimen were reconstructed using analyze 6.0 (Mayo Clinic, USA) with the marching cube method. Subsequent procedures were done using the software rapidform 2004 (INUS Technology, Korea).
We treated the EDJ and the OES as biologically corresponding structures in order to compare variability between them directly, and digitized (semi)landmarks on both of them as follows. We digitized main cusp tips (paracone, protocone, metacone, and hypocone) at the OES and the dentine horn tips at the EDJ, and the lowest points on the ridges at both the OES and the EDJ, connecting the two cusps as landmarks. Each ridge on both the OES and the EDJ was divided into eight sections by the cusp tips and the lowest points, respectively. For each section, a given number of semi-landmarks was digitized equi-distantly, as illustrated in Fig. 1. The number of semi-landmarks on the EDJ and the OES were determined to satisfy two criteria, namely, that each corresponding section in the EDJ and the OES had the same number of (semi)landmarks, and that the contributions of the section between the (semi)landmarks to the curve were approximately equal to each other (Skinner et al. 2009; Skinner & Gunz, 2010). The dataset consisted of four configurations (UM1EDJ, UM1OES, um2EDJ and um2OES), each with eight landmarks and 48 semi-landmarks.
Figure 1.
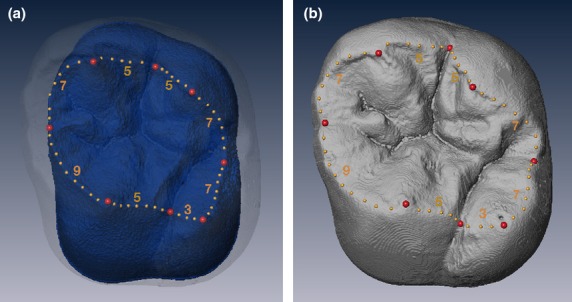
Digital image of permanent maxillary first molar crown (lingual view). (a) EDJ ridge curve digitized on the EDJ surface. (b) OES ridge curve digitized on the OES. Red circles are landmarks, and yellow circles are semi-landmarks. Numbers appended to each section of the ridge curve refer to the equally spaced interpolated semi-landmarks.
Semi-landmarks are not considered to be homologous landmarks unless they are slid (Bookstein, 1997). The minimum bending energy algorithm (Bookstein, 1997; Gunz et al. 2005) was adopted. This data processing was performed by W. Y. using mathematica 8 (http://www.wolfram.com). Each homologous set of landmarks was converted to shape coordinates by generalized Procrustes analysis (GPA; Rohlf & Slice, 1990), which was performed using MorphoJ version 1.05d (Klingenberg, 2011).
Morphometric analysis
Covariation between EDJ and OES was analyzed using 2B-PLS. This method compares two morphological datasets by using a singular value decomposition of the cross-covariation matrix, finds new pairs of axes that account for the maximum amount of covariance between both datasets, and visualizes the main associated morphological changes. The RV coefficient was used to evaluate the strength of multivariate correlations between datasets. This coefficient is a multivariate analogue of the squared correlation coefficient (Escoufier, 1973; Klingenberg, 2008). The significances of both the correlation between the scores for each pair of PLS axes and RV coefficient were evaluated by means of resampling tests with 1000 random permutations. These procedures were carried out with MorphoJ software (Klingenberg, 2011).
A principal component analysis of Procrustes shape coordinates was used to extract the main patterns of morphological variation across EDJ and OES in both UM1 and um2. Using the first few PC scores of EDJ and OES, we performed a regression analysis between these two structures to test whether shape variation of OES can be predicted by that of EDJ.
The difference in multivariate morphological change vector from EDJ to OES between UM1 and um2 was assessed by calculating the length and direction of shape change using a residual randomization procedure outlined in Collyer & Adams (2007). The length of a vector describes the overall amount of morphological change and the direction of a vector describes the way in which multiple traits covary. Observed vector lengths and directions were compared with 999 random permutations plus the observed value to assess significance.
Variability analysis
Among-individual phenotypic variation is the most common measurement of canalization. Canalization is generally inferred from a reduction of the observed phenotypic variance. Here we quantified both size and shape variance within each of the four configurations. For size, the centroid size (CS) of each configuration was calculated. The coefficient of variation (CV) of the LogCS was used to compare size variation, and tested as suggested by Sokal & Braumann (1980). For comparison of shape variability among configurations, the square root of the sum of the squared distances between Procrustes-transformed coordinates of each cusp and its landmark mean configuration was used as the measure of shape variation. To test whether there was a significant difference of variability between the EDJ and the OES within the same tooth class, a nonparametric Kruskal–Wallis test and multiple-comparison test were performed.
To compare the overall strength of morphological integration, we followed Wagner (1984) in using the variance of the eigenvalues for the variance–covariance matrix as the measure of integration. This measure of integration captures whether shape variance can be explained by a small number of principal components, or whether variance is more evenly distributed across principal components. The former case would be considered more integrated and the latter less integrated. The variance of eigenvalues (VE) was compared between the EDJ and the OES within the same tooth using bootstrap resampling methods (Manly, 1997). For each of the EDJ and the OES, the original data matrix was bootstrapped 1000 times, a variance–covariance matrix was derived from each bootstrap sample, and VE was calculated from each of the 1000 variance–covariance matrices. For each of the 1000 VE replicates, the difference between the EDJ and the OES was calculated. This created a distribution of differences in VE replicates that was then zero-centered. Each of the zero-centered differences was then compared with the observed difference in VE between the EDJ and the OES. The two-tailed P-value was calculated as the number of times the difference from the zero-centered distribution was equal to or greater than the observed difference, divided by the number of bootstrap replicates (Manly, 1997).
The ability of EDJ and OES morphology to respond to selection was evaluated using mean flexibility (f) (Marroig et al. 2009), which is derived from Lande's (1979) multivariate selection equation:
where G is the genetic covariance matrix, β is a selection vector, and Δz is the response vector. Here the phenotypic covariance matrix P is substituted for G because previous studies established a structural similarity between them (e.g. Cheverud, 1996; Porto et al. 2009). The covariance matrix for each of EDJ and OES was subjected to 1000 randomly generated selection vectors and the angle between the selection and response vectors was calculated for each time. The mean cosine of angles in 1000 repeats is called the mean flexibility (Marroig et al. 2009), which describes the degree to which the response and selection vectors are aligned in multivariate space. Response and selection vectors that are parallel (i.e. when the cosine of the angle between them is 1.0) indicate a structure that is more responsive to selection, i.e. more evolvable. A larger angle between the response and selection vectors is indicative of less evolvability. In general, high levels of evolvability measures, such as evolutionary flexibility, tend to be associated with low levels of integration measures (e.g. VE). Pairwise comparisons of evolutionary flexibility between EDJ and OES within the same tooth class were performed as described for VE; the distribution of vector correlations obtained from the covariance matrix and 1000 random selection vectors for EDJ and OES were compared using the difference of means test and accompanied by a two-tailed P-value. All statistical analyses were performed using r version 2.13.1 (R Development Core Team, 2011).
Results
Morphometric analysis
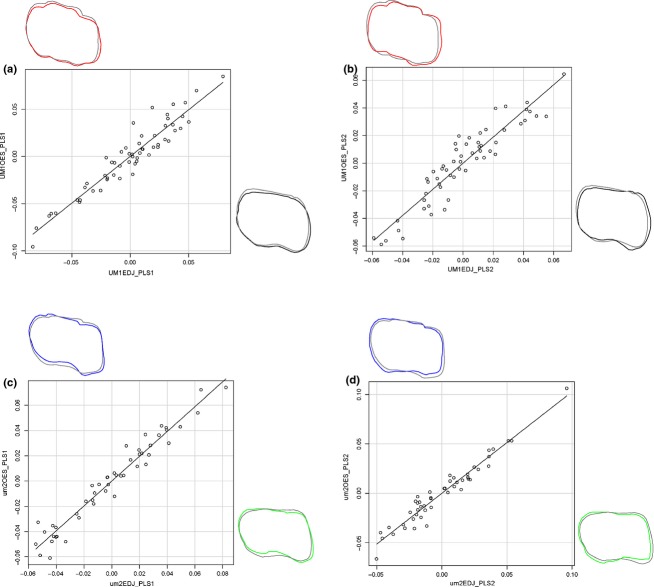
Covariation between EDJ and OES is higher in um2 (RV = 0.914; P < 0.001) than in UM1 (RV = 0.794; P < 0.001). 2B-PLS analysis in UM1 revealed that the first axis explained 49.43% of total shape covariance and that corresponding shape change mainly involves the contraction of buccal side and expansion of distolingual cusp (hypocone) for both EDJ and OES (Table 1, Fig. 2a). The second axis also revealed that EDJ and OES showed a similar shape change, with a contraction of the mesiobuccal cusp (paracone) and a contraction of the distal side (Fig. 2b). In um2, the first singular axis of correspondence to the comparison of EDJ and OES revealed a correlated reduction of mesiolingual-distobuccally and expansion of mesiobuccal-distolingually (Fig. 2c). The second axis also revealed significant shape change of reduction of mesial cusps and reduction of distal cusps for both EDJ and OES (Fig. 2d).
Table 1.
Results of PLS analyses between EDJ and OES corresponding to UM1 and um2.
| UM1 | um2 | |||||
|---|---|---|---|---|---|---|
| % Total Cov. | Correlation coefficient | P-valuea | % Total Cov. | Correlation coefficient | P-valuea | |
| 1 | 49.43 | 0.951 | < 0.001 | 43.14 | 0.974 | < 0.001 |
| 2 | 17.39 | 0.933 | < 0.001 | 25.11 | 0.970 | < 0.001 |
| 3 | 14.65 | 0.908 | < 0.001 | 17.76 | 0.954 | < 0.001 |
| 4 | 10.22 | 0.879 | < 0.001 | 6.52 | 0.948 | < 0.001 |
Randomiztion iterations: 1000.
Figure 2.
Scatter plots representing the first and second pairs of PLS axes between EDJ and OES within the same tooth class. (a) PLS1 UM1, (b) PLS2 UM1, (c) PLS1 um2, (d) PLS2 um2. Shape deformation corresponding to each axis is provided to the left of x-axes or above y-axes. Each shape deformation is represented in colored line whose scale factor used for is 0.1 and mean shape is represented with a gray line.
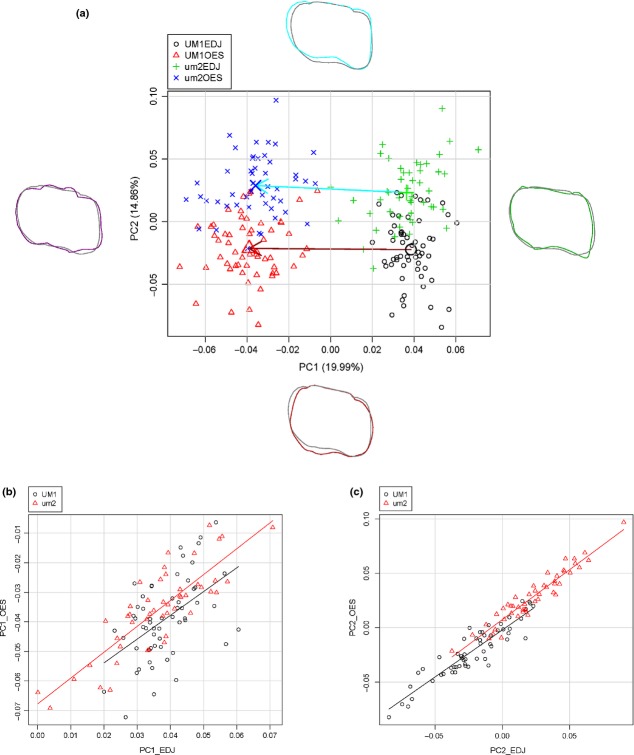
In the PCA, the first two principal components account for 34.85% of the total variation (Table 2 Fig. 3a). Positive scores of PC1 are associated with relatively high and sharp cusp tips and lingually located hypocone. Its negative values correspond to relatively gentle and inner located cusp tips with deep intercuspal grooves. Positive PC2 scores are associated with mesial expansion and contraction of protocone and negative ones with mesial contraction with lingually expanded protocone. PC1 corresponds to the distinction between EDJ and OES, whereas PC2 separates between UM1 and um2. Figure 3(b,c) illustrates the regressions of first two PCs for EDJ and OES in both teeth. The adjusted R-squared value was lower in UM1 than that in um2 for both PC1 (0.249 vs. 0.700) and PC2 (0.842 vs. 0.907), which indicated that the OES shape variation was better predicted by EDJ shape variation in um2 than in UM1.
Table 2.
Results of principal component analysis with the total sample.
| Eigenvalue | % Explained variance | % Cumulative variance | |
|---|---|---|---|
| 1 | 0.0016 | 19.99 | 19.99 |
| 2 | 0.0012 | 14.86 | 34.85 |
| 3 | 0.0009 | 11.80 | 46.64 |
| 4 | 0.0007 | 9.08 | 55.73 |
| 5 | 0.0005 | 6.86 | 62.58 |
| 6 | 0.0005 | 6.68 | 69.26 |
| 7 | 0.0004 | 5.31 | 74.58 |
| 8 | 0.0002 | 3.14 | 77.71 |
| 9 | 0.0002 | 2.90 | 80.61 |
| 10 | 0.0002 | 2.35 | 82.96 |
Figure 3.
Principal component plots for shape variation between EDJ and OES of both UM1 and um2. (a) Plots of PC1 vs. PC2 scores. Variance explained by PC1 and PC2 is 34.85% of total variance. Shape deformation corresponding to the positive or negative loadings of each axis is provided to the left and right for x-axes or the above and bottom for y-axes. Each shape deformation is represented with a coloured line whose scale factor used for is 0.1 and mean shape is represented with a gray line. Arrows show morphological change vectors from mean shape represented in large symbols of EDJ to that of OES for each tooth class. (b) Relationship between EDJ and OES for PC1 in both UM1 and um2. The slope and intercept of the regression line for UM1 are 0.804 and −0.070, respectively (r = 0.51, P < 0.001). The slope and intercept of the regression line for um2 are 0.876 and −0.068, respectively (r = 0.84, P < 0.001). (c) Relationship between EDJ and OES for PC2 in both UM1 and um2. The slope and intercept of the regression line for UM1 are 0.863 and −0.002, respectively (r = 0.92, P < 0.001). The slope and intercept of the regression line for um2 are 0.918 and 0.007, respectively (r = 0.95, P < 0.001).
The tooth specific morphological change vectors between EDJ and OES were not statistically different in length (ΔD = 0.004; P = 0.27). However, the angle between these vectors was significantly greater than expected by chance (θ = 27.62°; P < 0.001: Fig. 3a).
Variability analysis
Canalization
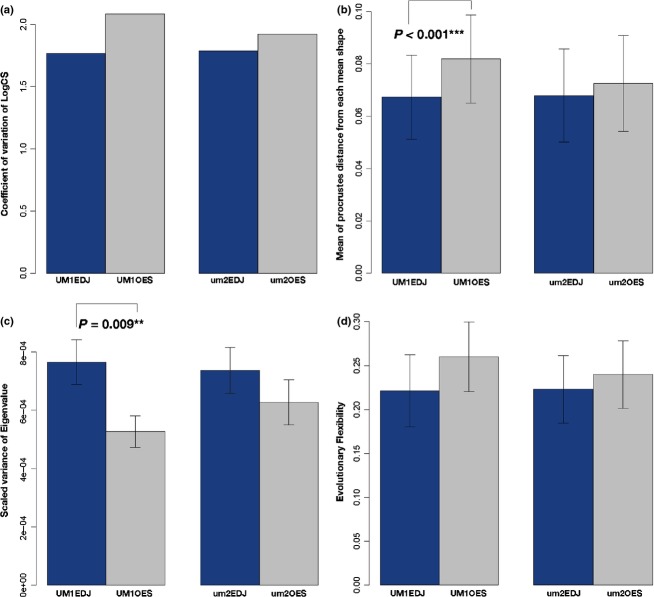
The coefficients of variation of the LogCS for each configuration (UM1EDJ, UM1OES, um2EDJ, and um2OES) was not significantly different from each other, although OES tended to be more variable than EDJ in both the UM1 and um2 tooth classes (Fig. 4a). On the other hand, shape variability was significantly different among these configurations, and pair-wise tests showed that only in UM1 was there a significant difference in shape variability between EDJ and OES (Fig. 4b).
Figure 4.
(a) Bar graph showing the size variation for four configurations (UM1EDJ, UM1OES, um2EDJ, and um2OES). Significance test for coefficient of variation for LogCS among them reveals that there is no significant difference (P > 0.05). (b) Bar graph showing mean of Procrustes distance from each mean shape for shape variance of four configurations (UM1EDJ, UM1OES, um2EDJ, and um2OES), and the error bars show standard deviations. The Kruskal–Wallis test reveals a significant difference among them (P < 0.001). A nonparametric multiple-comparison test between EDJ and OES within the same tooth class reveals that the difference is highly significant in UM1 (P < 0.001). (c) Bar graph showing the scaled variances of eigenvalue for morphological integration for four configurations (UM1EDJ, UM1OES, um2EDJ, and um2OES). The error bars shown are standard deviations obtained by resampling the original datasets with 1000 iterations. Bootstrap tests between EDJ and OES within the same tooth class reveal that the difference is highly significant only in UM1 (P = 0.009). (d) Bar graph showing the evolutionary flexibility for four configurations (UM1EDJ, UM1OES, um2EDJ, and um2OES). The error bars shown are standard deviations obtained by resampling the original datasets with 1000 iterations. Bootstrap tests between EDJ and OES within the same tooth class reveal that there is no significant difference (P > 0.05).
Morphological integration
The variance of the eigenvalue (VE) was significantly greater for EDJ than for OES in UM1, but not in um2 (Fig. 4c). The greater VEs for EDJ were seen in both UM1 and um2, indicating that EDJ was more integrated than OES.
Evolutionary flexibility
The mean cosine between the selection vector and the response vector for OES tended to be greater than that for EDJ, but a significant difference was not detected between them in either tooth class (Fig. 4d). This meant that there was no difference in the extent to which EDJ and OES would be influenced by the selection vector.
Discussion
Both UM1 and um2 showed significantly correlated shape changes between EDJ and OES corresponding to singular axes. Enamel formation does not alter the basic morphology of the dentine horn and EDJ ridges and corresponding features (cusp tips and ridges) on OES. Our results agree with results from previous studies that dental traits seen in EDJ can be observed at OES (Nager, 1960; Korenhof, 1961, 1982; Sakai & Hanamura, 1973; Corruccini, 1998; Sasaki & Kanazawa, 1999; Skinner et al. 2008, 2009), which supports the major role of the EDJ in the origin and degree of dental crown traits. However, this does not necessarily mean that tooth shape and covariation structure are predetermined by processes that configurate tooth shape at EDJ. Comparisons between um2 and UM1 revealed different influences of enamel formation on the OES morphology. In um2, OES shape variation is predicted better from the EDJ shape variation. Thus, multivariate covariation between EDJ and OES is higher than for UM1. This result suggests that morphological change caused by enamel formation is more stable and less vulnerable to random perturbations in um2. This could be attributed to the difference in the enamel thickness (Grine, 2005), the rate of enamel formation (Shellis, 1984), and/or the period of enamel formation (Liversidge & Molleson, 2004). Although the amount of overall morphological change induced by enamel formation does not differ between UM1 and um2, the direction of change described by traits covariation is significantly different. Given the different period of formation between UM1 and um2 (Nanci, 2013), it may be expected that they show similar directions of morphological change with different amounts of morphological change. However, the result is converse, suggesting a complex nature of crown enamel formation. For example, Grine (2005) noted that the difference in enamel thickness between the paracone tip and the protocone tip was greater in um2 rather than in UM1. The difference in patterns of enamel distribution between UM1 and um2 might affect the mode of covariation between EDJ and OES. Thus, enamel formation has a significant effect on patterns of morphological change, probably according to a tooth-specific developmental parameter, though it does not cause a drastic change in morphology during odontogenesis.
The lack of significant difference in size variation between EDJ and OES in both tooth classes examined here suggests that the strength of the influence of canalization on size is almost constant throughout the processes of morphogenesis and the subsequent period of enamel formation. A recent developmental study revealed that molar crown sizes were regulated by intrinsic factors from mesenchymal tissues (Cai et al. 2007) and adjacent molars during development (Kavanagh et al. 2007). Several dental metrics studies confirmed that tooth crown size was less variable than intercusp distance and/or cusp size owing to stronger genetic control (Townsend et al. 2003; Harris & Dinh, 2006), which would also be supported by experimental evidence that cusp density (intercusp distances) was likely to be polygenic (Harjunmaa et al. 2012). The present analysis of EDJ and OES at the dentine horns/cusp tips and ridges provided the insight that the size variation in intercusp distances might not be altered mostly by enamel formation. Additionally, the spatial relationship with the surrounding tissues, including the maxillary bone and/or other tooth germs, and the available space for tooth growth (Boughner, 2011), may be involved in the canalization of crown size during odontogenesis. The extent of the deviation from mean size in EDJ and OES was not significantly different, and therefore both EDJ and OES size differences among groups being compared can be used as a reliable measure of phylogenetic relatedness.
In the case of UM1, shape variation of OES was greater than that of EDJ. This result suggests that canalization of crown shape may be weakened during the process of enamel formation. Kraus & Jordan (1965) argued that early stages of tooth development were mediated by genes that are more evolutionarily stable than the genes associated with calcification. Hlusko's (2004) simulation model indicated that enamel thickness could change rapidly under appropriate selective pressure. The present result obtained at the cusp tips and ridges is in accord with these studies and implies that shape (e.g. intercusp topological relationship) variation is more susceptible to modifications resulting from enamel formation than is size variation, which might be likely to cause homoplasy that would confuse phylogenetic reconstructions (note here ‘size’ refers to the centroid size of the cuspal tips and ridges and not commonly used crown size proxies such as maximum mesiodistal × buccolingual dimensions).
The result of VE analysis showed that EDJ was more integrated than OES in UM1, although the same was not supported statistically in um2. Molar crown morphogenesis is a morphodynamic process in which inductive events and morphogenetic processes act at the same time, and it is regulated by interactions between the epithelial and underlying mesenchymal tissues. Cusp initiation and patterning in tooth germ is an iterative process that repeatedly utilizes the same set of genes and signaling pathways, which would lead to higher morphological integration in EDJ. On the other hand, the pattern of enamel formation is the end product of a sequence proceeding from ameloblast differentiation from the IEE cells, to secretion of enamel proteins including amelogenins and enamelins, and finally organization of the enamel crystallites into enamel rods or prisms (Boyde, 1964, 1989). Topological developmental parameters, such as the rate and the duration of enamel apposition and/or ameloblast extension and termination (Simmer et al. 2010), might impact the OES formation, which could lead to weaker morphological integration in OES.
It is predicted that stronger integration between traits acts as a limitation on producing phenotypic variation (Wagner & Altenberg, 1996). The results of the canalization and morphological integration analyses presented here are consistent with this prediction, i.e. the more strongly integrated EDJ shows smaller variability. The set of genes expressed during morphogenesis of the tooth are also used in different organs, including hair, pancreas, mammary gland, salivary gland, thymus, and vibrissae (Fincham et al. 2000; Jernvall & Jung 2000). Mutations in coding region that alter the function or activity of proteins are likely to have widespread and many potentially negative effects on development and fitness, and may thus be under considerable constraint (Carroll, 2008). Size and shape of EDJ are thus more likely to be stabilized to reduce the risks of negative pleiotropic side effects. The high level of integration in EDJ can be regarded as a relatively rigorous developmental constraint during odontogenesis. Meanwhile, the set of genes that contribute to enamel formation, such as amelogenin, enamelin, ameloblastin, and enamelysin genes, is highly specialized, and can easily modify the OES morphology during the enamel formation process. Morphological change of the OES, which has less developmental constraint, can easily be brought about by neutral evolution by non-natural selective genetic factors such as random genetic drift.
The observed pattern of morphological integration and the results of evolutionary flexibility analyses presented here are not consistent with those of previous studies, in which low levels of integration accompanied high levels of evolvability (Marroig et al. 2009; Porto et al. 2009; Lewton, 2012). The developmental constraints due to canalization and morphological integration act more strongly on the shape of EDJ than on that of OES in UM1, but there is no significant difference in the evolutionary flexibility between EDJ and OES. This may result from the relatively integrated covariance structure of each cusp (for both EDJ and OES). Since the secondary enamel knot that functions as a signaling center and regulates cusp formation at the future cusp tip acts as a ‘developmental module’ (Jernvall & Jung, 2000), it can directly affect the covariance structure of EDJ, and indirectly affect that of the overlying OES. In the case of the human tooth, if the crown covariance structure is divided into individual cusp units, this patterning cascade mode of cusp development facilitates the ability to respond to selective challenges (Jernvall & Jung, 2000) and enables a certain level of evolvability to be maintained at EDJ despite the existence of developmental constraints. The comparable level of evolutionary flexibility between EDJ and OES suggests that both of them can be utilized as an equally effective proxy for inferring phylogenetic relationships that would result from selective pressure.
Overall, the difference of each measurement (canalization, morphological integration, and evolutionary flexibility) between the EDJ and OES in the present study was greater in UM1 than in um2. The process of enamel formation is more likely to influence crown morphological variability and evolvability in UM1 than in um2, which can be explained by the duration and/or thickness of enamel formation. Compared with UM1, the enamel deposition period of um2 is shorter and the enamel thinner (Nanci, 2013). Therefore enamel formation may exert less influence on shape change in um2, possibly related to the conservation of primitive morphology, as discussed in previous studies (Dahlberg, 1945; Butler, 1956, 1971; Suzuki & Sakai, 1973; Saunders & Mayhall, 1982). Since not only morphology but also variability would be likely to differ between EDJ and OES, a tooth crown that has a longer period of enamel formation and/or thicker enamel would require careful evaluation for phylogenetic studies.
This study compared patterns of canalization, morphological integration, and evolutionary flexibility between the EDJ and the OES in UM1 and um2 to explore their possible effects on phylogenetic reconstructions. Our results suggest that a tooth crown that has thicker enamel and/or a longer period of enamel formation can be more variable in shape at the OES, where similarity may be due to homoplasy. Recent advances in imaging techniques have made it possible to approach the details of developmental trajectories reflected in the teeth of fossil species (Avishai et al. 2004; Smith et al. 2011). Understanding the morphological variability and evolvability produced by the developmental process is an important step in validating phylogenetic hypotheses based on the OES morphology alone.
Conclusions
Both morphometric and variability analyses indicate that not only are tooth shape and covariation structure determined by processes that contribute to tooth shape at the EDJ, but amelogenesis can also play a significant role. The influence of enamel formation on morphological variation and patterns of variability is not constant among teeth, which may be responsible for the differences in the rate and/or period of enamel formation.
Acknowledgments
The authors thank Y. Kunimatsu, D. Shimizu, N. Morimoto, and other members of the Laboratory of Physical Anthropology, Kyoto University, for helpful discussions and comments, P. Gunz and P. Mitteroecker for permission to use software routines they developed, and K. Hirata, K. Shimatani, and K. Miyazawa for access to specimens under their care. We are also grateful to the Editor and to two anonymous reviewers for their constructive criticism. This study was supported in part by a JSPS Research Fellowship (11J00940), and by Grants for Excellent Graduate Schools, MEXT, Japan. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Author contributions
W.M. and W.Y. designed the research and performed the analysis. W.M., T.N., and M.A. collected the data. W.M., H.O., and M.N. wrote the manuscript.
References
- Alberch P. Developmental constraints in evolutionary processes. In: Bonner JT, editor. Development in Evo006Cution. Berlin: Springer-Verlag; 1982. pp. 313–332. [Google Scholar]
- Avishai G, Müller R, Gabet Y, et al. New approach to quantifying developmental variation in the dentition using serial microtomographic imaging. Microsc Res Tech. 2004;65:263–299. doi: 10.1002/jemt.20131. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med Image Anal. 1997;1:225–243. doi: 10.1016/s1361-8415(97)85012-8. [DOI] [PubMed] [Google Scholar]
- Boughner JC. Making space for permanent molars in growing baboon (Papio anubis) and great ape (Pan paniscus and P. troglodytes) mandibles: possible ontogenetic strategies and solutions. Anat Res Int. 2011;2011:16. doi: 10.1155/2011/484607. 484607, 2011. DOI: 10.1155/2011/484607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyde A. The structure and development of mammalian enamel. 1964. PhD dissertation. University of London.
- Boyde A. Enamel. In: Oksche A, Vollrath L, editors. Handbook of Microscopic Anatomy, Volume 6: Teeth. Berlin: Springer-Verlag; 1989. pp. 309–473. [Google Scholar]
- Butler PM. The ontogeny of molar pattern. Biol Rev. 1956;31:30–70. [Google Scholar]
- Butler PM. Growth of human tooth germs. In: Dahlberg AA, editor. Dental Morphology and Evolution. Chicago: University of Chicago Press; 1971. pp. 3–14. [Google Scholar]
- Cai J, Cho SW, Kim JY, et al. Patterning the size and number of tooth and its cusps. Dev Biol. 2007;304:499–507. doi: 10.1016/j.ydbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Carroll SB. Evo-devo and expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Chernoff B, Magwene PM. Morphological Integration: Forty Years Later. In: Olsen EC, Miller RL, editors. Morphological Integration. Chicago: University of Chicago Press; 1999. pp. 319–353. [Google Scholar]
- Cheverud JM. Developmental integration and the evolution of pleiotropy. Am Zool. 1996;36:44–50. [Google Scholar]
- Collard M, Wood B. How reliable are human phylogentic hypotheses? Proc Natl Acad Sci U S A. 2000;97:5003–5006. doi: 10.1073/pnas.97.9.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collyer ML, Adams DC. Analysis of two-state multivariate phenotypic change in ecological studies. Ecology. 2007;88:683–692. doi: 10.1890/06-0727. [DOI] [PubMed] [Google Scholar]
- Corruccini RS. The dentino-enamel junction in primate mandibular molars. In: Lukacs JR, editor. Human Dental Development, Morphology, and Pathology: A Tribute to Albert A. Dahlberg. Portland: University of Oregon Anthropological Papers; 1998. pp. 1–16. [Google Scholar]
- Dahlberg AA. The changing dentition of man. J Am Dent Assoc. 1945;32:676–690. [Google Scholar]
- Dean MC. Progress in understanding hominoid dental development. J Anat. 2000;197:77–101. doi: 10.1046/j.1469-7580.2000.19710077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoufier Y. Le traitement des variables vectorielles. Biometrics. 1973;29:751–760. [Google Scholar]
- Finarelli JA, Clyde WC. Reassessing hominoid phylogeny: evaluating congruence in the morphological and temporal data. Paleobiology. 2004;30:614–651. [Google Scholar]
- Fincham AG, Luo W, Moradian-Oldak J. Enamel biomineralization: the assembly and dissassembly of the protein extracellular organic matrix. In: Teaford MF, Meredith-Smith M, Ferguson MWJ, et al., editors. Development, Function and Evolution of Teeth. Cambridge: Cambridge University Press; 2000. pp. 37–61. [Google Scholar]
- Grabowski MW. Hominin obstetrics and the evolution of canstraints. Evol Biol. 2013;40:57–75. [Google Scholar]
- Grine FE. Enamel thickness of deciduous and permanent molars in modern Homo sapiens. Am J Phys Anthropol. 2005;126:14–31. doi: 10.1002/ajpa.10277. [DOI] [PubMed] [Google Scholar]
- Gunz P, Mitteroecker P, Bookstein FL. Semilandmarks in three dimensions. In: Slice DE, editor. Modern Morphometrics in Physical Anthropology. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 73–98. [Google Scholar]
- Hallgrímsson B, Willmore K, Hall BK. Canalization, developmental stability, and morphological integration in primate limbs. Am J Phys Anthropol. 2002;35:131–158. doi: 10.1002/ajpa.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrímsson B, Jamniczky H, Young NM, et al. Deciphering the palimpsest: studying the relationship between morphological integration and phenotypic covariation. Evol Biol. 2009;36:355–376. doi: 10.1007/s11692-009-9076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TF. Is modularity necessary for evolvability? Remarks on the relationship between pleiotropy and evolvability. Biosystems. 2003;69:83–94. doi: 10.1016/s0303-2647(02)00132-6. [DOI] [PubMed] [Google Scholar]
- Harjunmaa E, Kallonen A, Voutilainen M, et al. On the difficulty of increasing dental complexity. Nature. 2012;483:324–327. doi: 10.1038/nature10876. [DOI] [PubMed] [Google Scholar]
- Harris EF, Dinh DP. Intercusp relationships of the permanent maxillary first and second molars in American whites. Am J Phys Anthropol. 2006;130:514–528. doi: 10.1002/ajpa.20389. [DOI] [PubMed] [Google Scholar]
- Hlusko LJ. Integrating the genotype and phenotype in hominid paleontology. Proc Natl Acad Sci USA. 2004;101:2653–2657. doi: 10.1073/pnas.0307678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlusko LJ, Suwa G, Kono R, et al. Genetics and the evolution of primate enamel thickness: a baboon model. Am J Phys Anthropol. 2004;124:223–233. doi: 10.1002/ajpa.10353. [DOI] [PubMed] [Google Scholar]
- Hunter JP, Jernvall J. The hypocone as a key innovation in mammalian evolution. Proc Natl Acad Sci USA. 1995;92:10718–10722. doi: 10.1073/pnas.92.23.10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Jung HS. Genotype, phenotype, and developmental biology of molar tooth characters. Am J Phys Anthropol. 2000;43:171–190. doi: 10.1002/1096-8644(2000)43:31+<171::aid-ajpa6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kavanagh KD, Evans AR, Jernvall J. Predicting evolutionary patterns of mammalian teeth from development. Nature. 2007;449:427–432. doi: 10.1038/nature06153. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. Morphological integration and developmental modularity. Annu Rev Ecol Evol Syst. 2008;39:115–132. [Google Scholar]
- Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11:353–357. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- Korenhof CAW. Morphogenetical Aspects of the Human Upper Molar. Utrecht: Uitgeversmaatschappij Neerlandia; 1960. [Google Scholar]
- Korenhof CAW. The enamel–dentine border: a new morphological factor in the study of the (human) molar pattern. Proc Koninkl Nederl Acad Wetensch. 1961;64B:639–664. [Google Scholar]
- Korenhof CAW. Evolutionary trends of the inner enamel anatomy of deciduous molars from Sangiran (Java, Indonesia) In: Kurtén B, editor. Teeth: Form, Function and Evolution. New York: Columbia University Press; 1982. pp. 350–365. [Google Scholar]
- Kraus BS. Morphologic relationships between enamel and dentin surfaces of lower first molar teeth. J Dent Res. 1952;31:248–256. doi: 10.1177/00220345520310021001. [DOI] [PubMed] [Google Scholar]
- Kraus BS, Jordan RE. The Human Dentition before Birth. Philadelphia: Lea and Febiger; 1965. [Google Scholar]
- Lande R. Quantitative genetic analysis of multivariate evolution, applied to brain: body size allometry. Evolution. 1979;33:402–416. doi: 10.1111/j.1558-5646.1979.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Lewton KL. Evolvability of the primate pelvic girdle. Evol Biol. 2012;39:126–139. [Google Scholar]
- Liversidge HM, Molleson T. Variation in crown and root formation and eruption of human deciduous teeth. Am J Phys Anthropol. 2004;123:172–180. doi: 10.1002/ajpa.10318. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. London: Chapman & Hall; 1997. [Google Scholar]
- Marroig G, Shirai L, Porto A, et al. The evolution of modularity in the mammalian skull II: evolutionary consequences. Evol Biol. 2009;36:136–148. [Google Scholar]
- Matsumura H, Domett KM, O'Reilly DJW. On the origin of pre-Angkorian peoples: perspectives from cranial and dental affinity of the human remains from Iron Age Phum Snay, Cambodia. Anthropol Sci. 2011;119:67–79. [Google Scholar]
- Maynard Smith J, Burian R, Kauffman S, et al. Developmental constraints and evolution. Q Rev Biol. 1985;60:265–287. [Google Scholar]
- Miller GS. The Piltdown jaw. Am J Phys Anthropol. 1918;1:25–52. [Google Scholar]
- Nager G. Der Vergleich Zwischen dem Räumlichen Verhalten des Dentinkronenreliefs und dem Schmelzrelief der Zahnkrone. Acta Anat. 1960;42:226–250. [Google Scholar]
- Nanci A. Ten Cate's Oral Histology: Development, Structure and Function. 8th edn. St. Louis: Mosby Elsevier; 2013. [Google Scholar]
- Olejniczak AJ, Gilbert CG, Martin LB, et al. Morphology of the enamel–dentine junction in sections of anthropoid primate maxillary molars. J Hum Evol. 2007;53:292–301. doi: 10.1016/j.jhevol.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Ortiz A, Skinner MM, Bailey SE, et al. Carabelli's trait revisted: an examination of mesiolingual features at the enamel–dentine junction and enamel surface of Pan and Homo sapiens upper molars. J Hum Evol. 2012;63:586–596. doi: 10.1016/j.jhevol.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Pilbrow V. Population systematics of chimpanzees using molar morphometrics. J Hum Evol. 2006;51:646–662. doi: 10.1016/j.jhevol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Porto A, de Oliveira FB, Shirai LT, et al. The evolution of modularity in the mammalian skull I: morphological integration patterns and magnitudes. Evol Biol. 2009;36:118–135. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2011. Available at http://cran.R-project.org. [Google Scholar]
- Rohlf FJ, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. [Google Scholar]
- Sakai T, Hanamura H. A morphology study of enamel–dentin border on the Japanese dentition. Part V. Maxillary molar. J Anthropol Soc Nippon. 1971;79:297–322. [Google Scholar]
- Sakai T, Hanamura H. A morphology study of enamel–dentin border on the Japanese dentition. Part VII. General conclusion. J Anthropol Soc Nippon. 1973;81:87–102. [Google Scholar]
- Sasaki K, Kanazawa E. Morphological traits on the dentino-enamel junction of lower deciduous molar series. In: Mayhall J, Heikkinen T, editors. Dental Morphology 1998: Proceedings of the 11th International Symposium on Dental Morphology. Oulu: Oulu University Press; 1999. pp. 167–178. [Google Scholar]
- Saunders SR, Mayhall JT. Developmental patterns of human morphological traits. Arch Oral Biol. 1982;27:45–49. doi: 10.1016/0003-9969(82)90175-3. [DOI] [PubMed] [Google Scholar]
- Shellis RP. Variations in growth of the enamel crown in human teeth and a possible relationship between growth and enamel structure. Arch Oral Biol. 1984;29:697–705. doi: 10.1016/0003-9969(84)90175-4. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Papagerakis P, Smith CE, et al. Regulation of dental enamel shape and hardness. J Dent Res. 2010;89:1024–1038. doi: 10.1177/0022034510375829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons EL, Pilbeam D. Hominoid paleoprimatology. In: Tuttle R, editor. The Functional and Evolutionary Biology of Primates. Chicago: Aldine-Atherton; 1972. pp. 36–62. [Google Scholar]
- Skinner MM, Gunz P. The presence of accessory cusps in chimpanzee lower molars is consistent with a patterning cascade model of development. J Anat. 2010;217:245–253. doi: 10.1111/j.1469-7580.2010.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MM, Wood BA, Boesch C, et al. Dental trait expression at the enamel–dentine junction of lower molars in extant and fossil hominoids. J Hum Evol. 2008;54:173–186. doi: 10.1016/j.jhevol.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Skinner MM, Wood BA, Hublin JJ. Protostylid expression at the enamel-dentine junction and enamel surface of mandibular molars of Paranthropus robustus and Australopithecus africanus. J Hum Evol. 2009;56:76–85. doi: 10.1016/j.jhevol.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Skinner MM, Evans A, Smith T, et al. Brief Communication: contributions of enamel–dentine junction shape and enamel deposition to primate molar crown complexity. Am J Phys Anthropol. 2010;142:157–163. doi: 10.1002/ajpa.21248. [DOI] [PubMed] [Google Scholar]
- Smith P, Gomorri JM, Spitz S, et al. Model for the examination of evolutionary trends in tooth development. Am J Phys Anthropol. 1997;102:283–294. doi: 10.1002/(SICI)1096-8644(199702)102:2<283::AID-AJPA9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Smith P, Gomori JM, Shaked R. A computerized approach to reconstruction of growth patterns in hominid molar teeth. In: Mayhall J, Heikkinen T, et al., editors. Proceedings of the 11th International Symposium on Dental Morphology. Oulu: Oulu University Press; 2000. pp. 388–397. [Google Scholar]
- Smith P, Avishai G, Muller R. Computerized reconstruction of prenatal growth trajectories in the dentition: implications for the taxonomic status of Neandertals. In: Condemi S, Weniger G-C, et al., editors. Continuity and Discontinuity in the Peopling of Europe: One Hundred Fifty Years of Neanderthal Study. New York: Springer Science+Business Media B.V; 2011. pp. 165–173. [Google Scholar]
- Sokal RR, Braumann CA. Significance tests for coefficients of variation and variability profiles. Syst Zool. 1980;29:50–66. [Google Scholar]
- Suzuki M, Sakai T. Occlusal surface pattern of the lower molars and the second deciduous molar among living Polynesians. Am J Phys Anthropol. 1973;39:305–315. doi: 10.1002/ajpa.1330390221. [DOI] [PubMed] [Google Scholar]
- Townsend G, Richards L, Hughes T. Molar intercuspal dimensions: genetic input to phenotypic variation. J Dent Res. 2003;82:350–355. doi: 10.1177/154405910308200505. [DOI] [PubMed] [Google Scholar]
- Villmoare B, Fish J, Jungers W. Selection, morphological integration, and strepsirrhine locomotor adaptations. Evol Biol. 2011;38:88–99. [Google Scholar]
- Wagner GP. On the eigenvalue distribution of genetic and phenotypic dispersion matrices: evidence for a nonrandom organization of quantitative character variation. J Math Biol. 1984;21:77–95. [Google Scholar]
- Wagner GP, Altenberg L. Complex adaptations and the evolution of evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Booth G, Bagheri-Chaichian H. A population genetic theory of canalization. Evolution. 1997;51:329–347. doi: 10.1111/j.1558-5646.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Willmore KE, Young N, Richtsmeier JT. Phenotypic variability: its components, measurement and underlying developmental processes. Evol Biol. 2007;34:99–120. [Google Scholar]