Abstract
The aim of the present study was to demonstrate the location of the different members of the caveolin (cav) family in human muscle spindles. Twenty spindles of three human muscles (vastus medialis, ischiocavernosus, bulbospongiosus) from 12 cadavers were immunohistochemically stained for cav-1, cav-2, and cav-3, and the equatorial and polar regions evaluated. All layers of the outer and inner spindle capsule and all blood vessels within the spindle stained for cav-1 and cav-2. In the muscle spindle, intrafusal muscle fibres stained selectively for cav-3, but with a patchy appearance. Caveolinopathies may therefore also include changes in muscle spindle function.
Keywords: caveolin-1, caveolin-2, caveolin-3, immunohistochemistry, striated muscle
Introduction
The membrane protein caveolin (cav) associates with cholesterol and sphingolipids, leading to the formation of caveolae (Razani et al. 2002). Caveolae are small lipid- enriched membrane invaginations present in almost all adherent cells of the mammalian body, and are involved in signal transduction and endocytosis. Currently, three different members of the caveolin family are known: cav-1 (forming two splice variants; Fujimoto et al. 2000) and cav-2 (forming three isoforms; Scherer et al. 1995; Kogo et al. 2002) are most prominent in endothelial, fibrous, and adipose cells, whereas cav-3 is restricted to different kinds of muscle cells (Tang et al. 1996; Way & Parton, 1996). In skeletal muscle fibres, cav-3 is localised to the sarcolemma and T-tubule system, co-localising with dystrophin and associated glycoproteins (Song et al. 1996; Lapidos et al. 2004), with the dihydropyridine receptor (Couchoux et al. 2011), and with the ryanodine receptor (Whiteley et al. 2012).
Almost all skeletal muscles show specialised muscle fibres that act as sensors along the length of the muscle. In mammalians, these muscle spindles (Fig. 1A) are enclosed by a multi-laminar, fusiform and fluid-filled connective tissue capsule (Österlund et al. 2013). The capsule lamellae are formed by alternating layers of epithelial capsular sheet cells, surrounded by a basal lamina, and collagen fibers (Merrillees, 1960; Banker & Girvin, 1971). At its poles, the capsule is connected with endomysial tissue (Banker & Girvin, 1971). In addition to this outer capsule, an inner one is also described, investing directly the intrafusal muscle fibres and their sensory terminals (Ovalle & Dow, 1983). In the equatorial zone, this innermost endomysial/endoneurial layer separates the intrafusal fibers (axial compartment) from the periaxial space (Merrillees, 1960; Ovalle & Dow, 1983). Capillaries, small arterioles and nerves coursing between the capsule layers (Merrillees, 1960; Banker & Girvin, 1971) in human and avian spindles capillaries have been seen in the periaxial space (Cooper & Daniel, 1963; Ovalle, 1976). Myelinated nerves penetrate the capsule and enter the periaxial space (Merrillees, 1960; Banker & Girvin, 1971).
Figure 1.
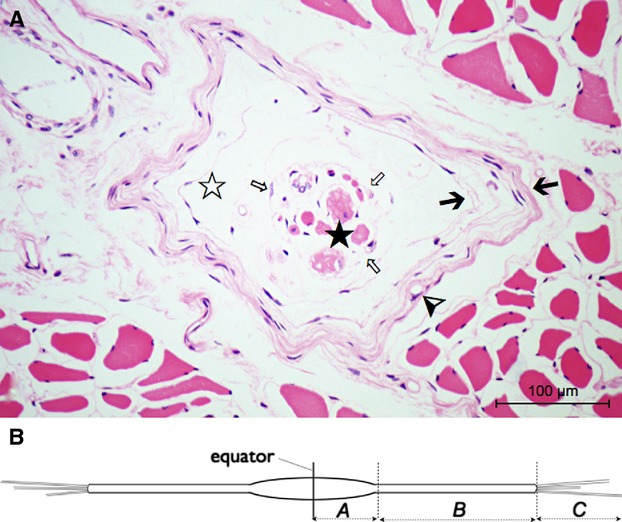
Components and regions of human muscle spindles. The micrograph (A) shows a cross-section of a spindle (H&E staining, cadaver no. 7, vastus medialis) at the equatorial level (region A). Besides the outer multi-laminate capsule (black arrows) there is an inner one forming a fine reticular network (white arrows) surrounding the intrafusal fibres, the axial compartment (black star). The fluid-filled periaxial space (white star) lies between the capsule elements. The thin innermost layer of the outer capsule seems to become continuous with the axial sheath. Capillaries (arrowhead) often course between the capsule's layers. (B) Schematic drawing of a single muscle spindle. Three spindle regions can be distinguished: Region A from the equator to the end of the periaxial space, B between the end of the periaxial space and the end of the capsule, C is the extracapsular part.
The spindles contain intrafusal muscle fibres, which are classified by mATPase activity, morphological and immunohistochemical properties into nuclear bag1, nuclear bag2, and nuclear chain fibres (Ovalle & Smith, 1972; Schiaffino & Reggiani, 2011; Österlund et al. 2013). They are characterised by a noncontractile central (equatorial) region (containing myonuclei – nuclei among the myofibrils – and cytoplasm) and two peripheral (polar) regions for active regulation of the central tension (Banker & Girvin, 1971; Barker & Banks, 1994; Proske, 1997). Barker and colleagues distinguished three regions of the muscle spindle that were defined as follows (Fig. 1B): region A from the equator to the end of the periaxial space, region B between the end of the periaxial space and the end of the capsule, and region C as extracapsular part (Barker & Banks, 1994).
As only little is known about the appearance of caveolins and their presumed function within the different structures of the muscle spindle – only Gossrau (1998) briefly mentioned cav-3 in muscle spindles of the gerbil – the aim of the present work was to describe the distribution of all three caveolin family members in human muscle spindles. The investigated muscles were taken from two different regions (perineal and femoral region) and functional systems (sexual and locomotion functions). This selection was made to test whether the staining behaviour for caveolin is general or has local differences.
Material and methods
Tissue preparation
Muscle specimens of different skeletal muscles (vastus medialis, ischiocavernosus, bulbospongiosus) were collected from 12 human cadavers. They were part of the donor program of the Departments of Anatomy in Dresden and Erlangen (both Germany) who had given their written consent during their lifetimes to use their bodies for the purpose of science and education after death. There were seven male and five female cadavers, aged 67–97 years (mean 81.3 ± 9.5 years) with no documented neuro- or myopathies in their medical history (Table 1). All cadavers were fixed 1–2 days postmortem with a mixture of formalin and alcohol and remained in that solution for at least 1 year. The specimens (approximately 1 cm3 in size) were washed several times in phosphate-buffered saline (PBS, pH 7.4, 0.01 m) and embedded in paraffin wax.
Table 1.
Specification of the human tissue donors and the investigated spindles.
| No. | Sex | Age | Investigated spindles |
|---|---|---|---|
| 1 | Male | 67 | 1 VM |
| 2 | Male | 71 | 3 VM |
| 3 | Female | 97 | 3 VM |
| 4 | Male | 89 | 1 BS |
| 5 | Female | 81 | 1 IC |
| 6 | Male | 84 | 2 VM |
| 7 | Male | 84 | 1 VM |
| 8 | Male | 68 | 3 VM |
| 9 | Female | 87 | 1 VM |
| 10 | Male | 90 | 1 VM |
| 11 | Female | 85 | 1 VM |
| 12 | Female | 73 | 1 BS, 1 IC |
BS, bulbospongiosus muscle; IC, ischiocavernosus muscle; VM, vastus medialis muscle.
Histology and immunohistochemistry
Serial sections (5 μm thick) of each specimen were performed and selected sections were stained with haematoxylin and eosin (H&E) to identify muscle spindles and their general morphology. A total of 20 muscle spindles were found and consecutive sections from the equatorial and polar zone (regions A & B) of the spindles were further processed for immunohistochemistry: these were dewaxed, rehydrated and irradiated with microwaves in 0.01 m sodium citrate buffer (pH 6.0) for 2 × 5 min at 800 W. The purpose of this pretreatment was to unmask the antigens. After washing in PBS, the sections were treated with 0.3% hydrogen peroxide for 10 min and blocked in normal mouse or rabbit serum for 15 min at 37 °C followed by washing in PBS. The primary antibodies (see below) were incubated overnight at 4 °C. After washing in PBS, an appropriate biotinylated secondary antibody was added and incubated for 15 min at 37 °C, followed by washing and incubation with the ABC complex for 15 min at 37 °C. Visualisation of peroxidase activity was realised by adding 3,3-diaminobenzidine for 8 min. The sections were counterstained with haematoxylin and embedded in DePeX (Serva, Heidelberg, Germany). For immunohistochemistry, commercially available VECTASTAIN® Elite ABC rabbit and mouse kits (PK 6101, PK 6102; Vector Laboratories Inc., Burlingame, CA, USA) were used.
In addition, selected slides were stained with Sirius red to show collagen fibres in the spindle capsule. The staining procedure was performed as described previously (Kasper et al. 2004).
Antibodies
The following primary antibodies were used for immunohistochemical staining: cav-1 (anti caveolin-1 IgG1, mouse, clone 2297, dilution 1 : 1600, BD Biosciences, Heidelberg, Germany), cav-2 (anti caveolin-2 IgG1, mouse, clone 65, dilution 1 : 100, BD Biosciences), cav-3 (anti caveolin-3 IgG1, mouse, clone 26, dilution 1 : 800, BD Biosciences), podocalyxin (anti podocalyxin, antibody 0601, polyclonal rabbit, dilution 1 : 4000, a kind gift from Dr M. G. Farquhar, San Diego, CA, USA), S46 (specific for MyHC-sto, monoclonal mouse IgG1, dilution 1 : 50, DSHB, University of Iowa, USA) and NF (anti-Human Neurofilament Protein IgG1, mouse, clone 2F11, dilution 1 : 1000, Dako, Glostrup, Denmark).
Analysis and data collection
The sections were examined in a Zeiss Jenamed2 microscope (Carl Zeiss AG, Oberkochen, Germany) and images were recorded using a Digital Sight DS-Fi1 camera (Nikon AG, Tokyo, Japan).
To visualise and spatialise morphological components of the muscle spindle, the following antibodies were used: podocalyxin for detection of endothelial cells of the spindle capillaries (Kasper et al. 2004; Furness & McNagny, 2006), NF for the spindle nerve (Schlaepfer, 1987), and S46 to identify nuclear bag fibres. S46 is specific for the myosin heavy chain isoform MyHC-sto (slow tonic) which occurs in avian slow tonic muscle fibres, and in humans only in intrafusal nuclear bag fibres or extraocular muscles (Bombardi et al. 2006; Sokoloff et al. 2007; Schiaffino & Reggiani, 2011).
Results
Cav-1
Staining for cav-1 was present in all endothelial and smooth muscle cells of vessels inside and outside the muscle spindle (Figs 2A and 3A,B). In addition, intense staining occurred in all layers of the spindle sheath along the entire corpuscle (regions A & B): the outer capsule containing perineural epithelium and the inner capsule a contiguous network of cells possessing long cytoplasmic processes that enclosed intimately in the intrafusal muscle fibres (Fig. 3A) and their equatorially localised sensory terminals. Hence, the axial sheath had an endomysial appearance. Immunoreactivity was also present in the perineural cells of the spindle nerve. Its perineurium was continuous with the outer capsule of the spindle (Fig. 3C,D). One spindle of an elderly cadaver (cadaver no. 3, 97 years, female) showed a significant increase in capsule thickness based on expansion of the space between the single layers (Fig. 3B). All cellular layers showed intense staining for cav-1. The collagen fibres in between the layers of capsular sheet cells in the outer capsule were estimated by staining with Sirius red (Fig. 4A).
Figure 2.
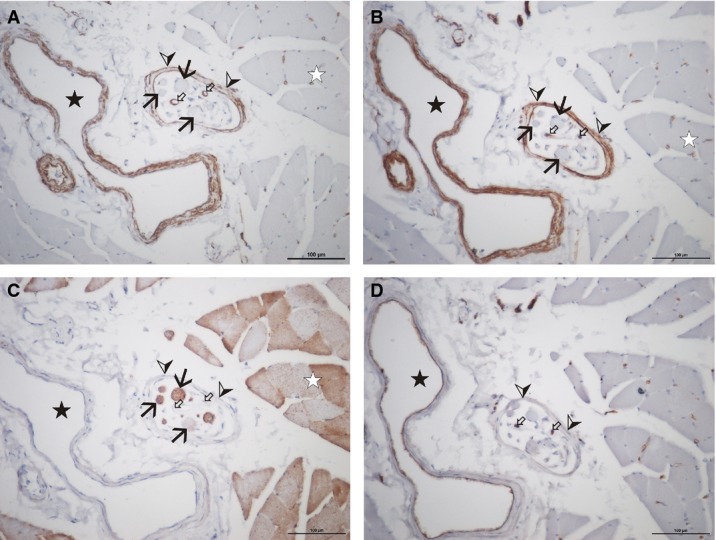
Low magnification of a muscle spindle (cadaver no. 1, vastus medialis), stained with antibodies against cav-1 (A), cav-2 (B), cav-3 (C), and podocalyxin (D). Note the immunoreactivity of the larger vessels (black star) and capillaries (small white arrows) for cav-1, cav-2 and podocalyxin, but not with cav-3. All layers of the spindle sheath (arrow heads) stain for cav-1 and cav-2, but not for cav-3 or podocalyxin. The intrafusal muscle fibres (black arrows) show only cav-3 positive staining. The staining intensity varies in a similar way to staining of the extrafusal muscle fibres (white star). Scale bar: 100 μm.
Figure 3.
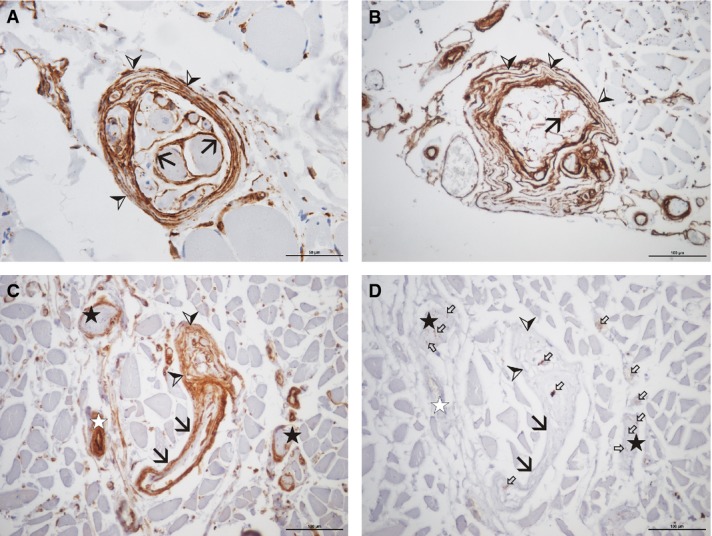
Muscle spindles of of different cadavers aged 71 (A), 97 (B), and 89 (C and D) years (A, cadaver no. 2 and B, cadaver no. 3, both vastus medialis; C and D, cadaver no. 4, bulbospongiosus), stained with antibodies against cav-1 (A-C) and neurofilament (D). Note the intense staining for cav-1 of all cellular layers of the muscle spindle sheath (arrowheads), regardless of the collagen content (‘normal’ appearance in A, thickened appearance in B). The sheaths around the individual intrafusal muscle fibres (the inner capsule) are intensely stained (black arrows in A and B). The perineural sheath of individual nerve bundles is intensely stained for cav-1 (stars in C and D). The sheath of the nerve fibres supplying the muscle spindle (black arrows in C and D) is continuous with the sheath of the muscle spindle (arrowheads). Small white arrows indicate single neurofilament positive nerve fibres. Scale bars: 50 μm (A), 100 μm (B–D).
Figure 4.
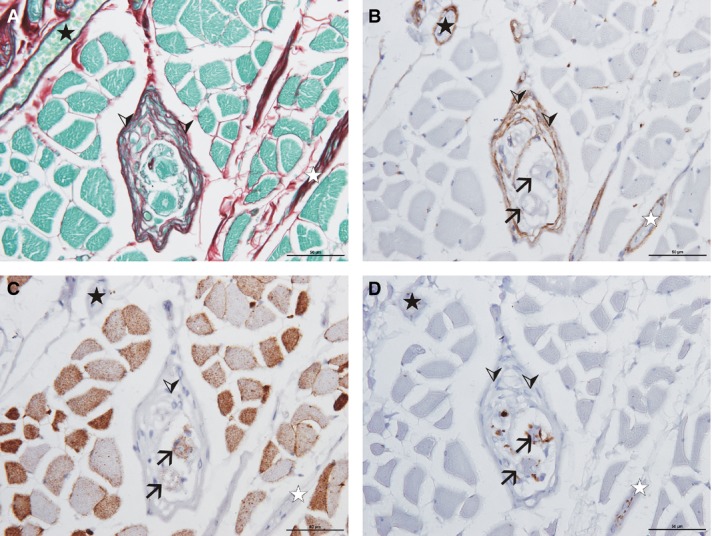
Micrographs of a muscle spindle (cadaver no. 5, ischiocavernosus), stained with Sirius red (A) and with antibodies against cav-2 (B), cav-3 (C), and neurofilament (D). Note the staining of the muscle spindle sheath (arrowheads) with cav-2. Staining for Sirius red shows the amount of collagen (red) and cytoplasmatic components (green) within the specific muscle spindle sheath. The intrafusal muscle fibres (black arrows) are sectioned at the equatorial level: the fibres are surrounded by numerous nerve fibres, the anulospiral endings (D); the staining for cav-3 (C) is present but on a low level. Note the anulospiral ending around the intrafusal muscle fibres (D). Black star: blood vessel; white star: nerve fibre bundle. Scale bar: 50 μm.
Staining of sections with podocalyxin (Fig. 2D) revealed co-localisation with cav-1 in the vessels, but podocalyxin was absent in all spindle sheath layers. Capillaries were identified in between the capsule layers as well as within the periaxial space. Immunoreactivity could not be detected in either the central or the peripheral parts of the intrafusal muscle fibre cav-1.
Cav-2
The staining for cav-2 was identical to cav-1 (Figs 2B and 4B).
Cav-3
Staining for cav-3 was present in extrafusal and intrafusal muscle fibres but was absent in the smooth muscle cells of the vascular wall and in the spindle capsule (Figs 2C and 4C,D). There was no difference in staining between nuclear bag and nuclear chain fibres, both fibres being differentiated by additional S46-staining (Fig. 5). The distribution of cav-3 within the muscle fibres was patchy: some fibres showed staining over the entire cytoplasm, whereas in other fibres the immunoreactivity was restricted to the sarcolemmal region or was not detectable. At the equatorial level the intensity of cav-3 reactivity was less (Fig. 4C) than in the juxta-equatorial and polar regions (Figs 2C and 5). There was no significant difference in staining between intrafusal and extrafusal muscle fibres or between intrafusal fibre types.
Figure 5.

High magnification of two muscle spindles (cadaver no. 3, vastus medialis), stained with antibodies against cav-3 (A, C) and S46 (B, D). Note that all intrafusal muscle fibres show cav-3 immunoreactivity, regardless of their fibre type (nuclear bag fibres marked with black arrows, nuclear chain fibres marked with arrowheads). Scale bar: 50 μm.
Discussion
Our study on the distribution of the different members of the caveolin family in human muscle spindles revealed a distinct localisation for cav-1/cav-2 to the spindle sheath and cav-3 to the intrafusal muscle fibres. This was shown in three different human skeletal muscles in both male and female subjects. There were no differences in caveolin staining properties in all muscle spindles investigated, pointing to a general finding independent of specific muscle function or location.
In the human skeletal muscle, all blood vessels and the perineural sheath stained for cav-1 and cav-2 similar to staining described in rat tissue (Voldstedlund et al. 2001). The outer capsule of the muscle spindle is continuous with the perineural sheath of the supporting nerves in rats and humans (Merrillees, 1960; Banker & Girvin, 1971; Ovalle & Dow, 1983; Barker & Banks, 1994) and therefore adopts similar functions. One of its main tasks is controlled transport to maintain a specific intracapsular environment (Ovalle & Dow, 1983). For that purpose, cav-1- and cav-2-mediated caveolae play a crucial role, and consequently these proteins are abundantly present in this tissue, as shown in this paper for the first time. The presence of caveolae within the muscle spindle capsule has been shown by ultrastructural images (Merrillees, 1960; Katto et al. 1987), consistent with our results.
Interestingly, the staining of cav-1 and cav-2 was not restricted to the outer muscle spindle capsule, but was also present in the inner layers continuous with the endomysium of the spindle fibres. As the endomysium of extrafusal muscle fibres contains only collagen fibrils and is not cav-1/cav-2-positive, the intrafusal muscle fibres reveal a specific sheath with cell processes similar to the endoneural complex containing collagen fibrils and Schwann cells, the latter known to express cav-1 (Mikol et al. 1999; Kawahara, 2004; Lee et al. 2008). Regarding the muscle spindle as a sensoric organ, parallels of caveolin function can be suggested to Ruffini bodies, where the presence of cav-1 was also described (Iizuka et al. 2009). In conclusion, the presence of cav-1 defines the spindle capsule as part of the cav-1-positive mesothelium (von Ruhland et al. 2004).
Spindles show mature morphology already early in life (Österlund et al. 2011) and only mild changes occur during aging, varying in different muscles of the body (Boyd-Clark et al. 2002; Kararizou et al. 2005). Among these changes is an increase in capsular thickness (Shaffer & Harrison, 2007); our study showed that this is not predominantly due to a change in cellular layers but rather to the content of extracellular material (collagen). Regardless of the collagen content, however, all cellular layers showed preserved cav-1 and cav-2 staining, indicating that aging per se does not change the above-mentioned function assigned to the caveolae.
The intrafusal muscle fibres showed cav-3 staining similar to that of the extrafusal fibres. In contrast to most of the literature published (Gossrau, 1998; Lo et al. 2011) the staining was not always restricted to the sarcolemmal region but showed immunoreactivity over the entire cytoplasm. This was also described for the heart muscle, and was patchy regardless of postmortem time or fixation (Volonte et al. 2008). We cannot exclude the possibility that the postmortem times or the long fixation might have affected the tissue and therefore the staining in the cytoplasm might represent an artefact. Further studies on human tissue might address this aspect. Also, the presence of noncaveolar caveolin with a separate function has to be taken into account (Lajoie et al. 2009).
The differences in cav-3 intensity between equatorial and polar regions is presumably due to the accumulation of myonuclei in spindle region A.
Due to the age of the cadavers used in this study, age-related factors also have to be taken into consideration for the intrafusal fibres. Here, the literature is quite inconsistent: some muscle spindles seem to have age-related changes in the number and size of the fibres, whereas others remain unchanged throughout life. Although modifications in myosin heavy chain content have been described (Shaffer & Harrison, 2007), the gross function appears to be constant. Therefore, the staining described in older individuals might be representative for all age groups. The general role for cav-3 in skeletal muscle components was confirmed by the fact that all fibres stained positive for cav-3, regardless of their specific type.
As caveolinopathies seem to play a role in musculo-skeletal diseases (Serra & Scotlandi, 2009; Gazzerro et al. 2010), our findings could inspire further research on the afferent (intrafusal) changes in such conditions.
Acknowledgments
The authors thank Silvia Bramke for staining assistance and the preparatory team from the Departments of Anatomy in Erlangen and Dresden for assistance in tissue sampling. The late donors are especially recognized for their individual support of science. No author has any conflicts of interest.
Author contributions
K.P. took tissue samples, performed the staining and evaluation, and prepared the manuscript. M.K. supported the staining and reviewed the manuscript. C.A.M. took tissue samples, supervised the staining and evaluation, and prepared the manuscript.
References
- Banker BQ, Girvin JP. The ultrastructural features of the mammalian muscle spindle. J Neuropathol Exp Neurol. 1971;30:155–195. doi: 10.1097/00005072-197104000-00001. [DOI] [PubMed] [Google Scholar]
- Barker D, Banks RW. The muscle spindle. In: Engel AG, Franzini–Armstrong C, editors. Myology. New York: McGraw-Hill; 1994. pp. 333–360. [Google Scholar]
- Bombardi C, Grandis A, Chiocchetti R, et al. Immunohistochemical localization of alpha(1a)-adrenoreceptors in muscle spindles of rabbit masseter muscle. Tissue Cell. 2006;38:121–125. doi: 10.1016/j.tice.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Boyd-Clark LC, Briggs CA, Galea MP. Muscle spindle distribution, morphology, and density in longus colli and multifidus muscles of the cervical spine. Spine. 2002;27:694–701. doi: 10.1097/00007632-200204010-00005. [DOI] [PubMed] [Google Scholar]
- Cooper S, Daniel PM. Muscle spindles in man; their morphology in the lumbricals and the deep muscles of the neck. Brain. 1963;86:563–586. doi: 10.1093/brain/86.3.563. [DOI] [PubMed] [Google Scholar]
- Couchoux H, Bichraoui H, Chouabe C, et al. Caveolin-3 is a direct molecular partner of the Cav1.1 subunit of the skeletal muscle L-type calcium channel. Int J Biochem Cell Biol. 2011;43:713–720. doi: 10.1016/j.biocel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Kogo H, Nomura R, et al. Isoforms of caveolin-1 and caveolar structure. J Cell Sci. 2000;113:3509–3517. doi: 10.1242/jcs.113.19.3509. [DOI] [PubMed] [Google Scholar]
- Furness SG, McNagny K. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol Res. 2006;34:13–32. doi: 10.1385/IR:34:1:13. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Sotgia F, Bruno C, et al. Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur J Hum Genet. 2010;18:137–145. doi: 10.1038/ejhg.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossrau R. Caveolin-3 and nitric oxide synthase I in healthy and diseased skeletal muscle. Acta Histochem. 1998;100:99–112. doi: 10.1016/S0065-1281(98)80009-3. [DOI] [PubMed] [Google Scholar]
- Iizuka N, Suzuki A, Nozawa-Inoue K, et al. Differential cell-specific location of Cav-1 and Ca(2+)-ATPase in terminal Schwann cells and mechanoreceptive Ruffini endings in the periodontal ligament of the rat incisor. J Anat. 2009;214:267–274. doi: 10.1111/j.1469-7580.2008.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kararizou E, Manta P, Kalfakis N, et al. Morphometric study of the human muscle spindle. Anal Quant Cytol Histol. 2005;27:1–4. [PubMed] [Google Scholar]
- Kasper M, Seidel D, Knels L, et al. Early signs of lung fibrosis after in vitro treatment of rat lung slices with CdCl2 and TGF-beta1. Histochem Cell Biol. 2004;121:131–140. doi: 10.1007/s00418-003-0612-6. [DOI] [PubMed] [Google Scholar]
- Katto Y, Okamura H, Yanagihara N. Electronmicroscopic study of muscle spindle in human interarytenoid muscle. Acta Otolaryngol. 1987;104:561–567. doi: 10.3109/00016488709128289. [DOI] [PubMed] [Google Scholar]
- Kawahara T. Caveolae localization and caveolin expressions in Schwann cells of mature rat spinal nerves. Kurume Med J. 2004;51:263–271. doi: 10.2739/kurumemedj.51.263. [DOI] [PubMed] [Google Scholar]
- Kogo H, Ishiguro K, Kuwaki S, et al. Identification of a splice variant of mouse caveolin-2 mRNA encoding an isoform lacking the C-terminal domain. Arch Biochem Biophys. 2002;401:108–114. doi: 10.1016/S0003-9861(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Lajoie P, Kojic LD, Nim S, et al. Caveolin-1 regulation of dynamin-dependent, raft-mediated endocytosis of cholera toxin-B sub-unit occurs independently of caveolae. J Cell Mol Med. 2009;13:3218–3225. doi: 10.1111/j.1582-4934.2009.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94:1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Park CH, Lee SJ, et al. Expression of caveolin-3 immunoreactivities in the developing sciatic nerve of the rat. Muscle Nerve. 2008;38:1021–1026. doi: 10.1002/mus.20973. [DOI] [PubMed] [Google Scholar]
- Lo HP, Bertini E, Mirabella M, et al. Mosaic caveolin-3 expression in acquired rippling muscle disease without evidence of myasthenia gravis or acetylcholine receptor autoantibodies. Neuromuscul Disord. 2011;21:194–203. doi: 10.1016/j.nmd.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Merrillees NC. The fine structure of muscle spindles in the lumbrical muscles of the rat. J Biophys Biochem Cytol. 1960;7:725–742. doi: 10.1083/jcb.7.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol DD, Hong HL, Cheng HL, et al. Caveolin-1 expression in Schwann cells. Glia. 1999;27:39–52. [PubMed] [Google Scholar]
- Österlund C, Liu JX, Thornell LE, et al. Muscle spindle composition and distribution in human young masseter and biceps brachii muscles reveal early growth and maturation. Anat Rec. 2011;294:683–693. doi: 10.1002/ar.21347. [DOI] [PubMed] [Google Scholar]
- Österlund C, Liu JX, Thornell LE, et al. Intrafusal myosin heavy chain expression of human masseter and biceps muscles at young age shows fundamental similarities but also marked differences. Histochem Cell Biol. 2013;139:895–907. doi: 10.1007/s00418-012-1072-7. [DOI] [PubMed] [Google Scholar]
- Ovalle WK. Fine structure of the avian muscle spindle capsule. Cell Tissue Res. 1976;166:285–298. doi: 10.1007/BF00220126. [DOI] [PubMed] [Google Scholar]
- Ovalle WK, Dow PR. Comparative ultrastructure of the inner capsule of the muscle spindle and the tendon organ. Am J Anat. 1983;166:343–357. doi: 10.1002/aja.1001660308. [DOI] [PubMed] [Google Scholar]
- Ovalle WK, Smith RS. Histochemical identification of three types of intrafusal muscle fibers in the cat and monkey based on the myosin ATPase reaction. Can J Physiol Pharmacol. 1972;50:195–202. doi: 10.1139/y72-030. [DOI] [PubMed] [Google Scholar]
- Proske U. The mammalian muscle spindle. Physiology. 1997;12:37–42. [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Caveolae. From cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- von Ruhland CJ, Campbell L, Gumbleton M, et al. Immunolocalization of caveolin-1 in rat and human mesothelium. J Histochem Cytochem. 2004;52:1415–1425. doi: 10.1369/jhc.4A6334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Tang Z, Chun M, et al. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270:16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Schlaepfer WW. Neurofilaments: structure, metabolism and implications in disease. J Neuropathol Exp Neurol. 1987;46:117–129. [PubMed] [Google Scholar]
- Serra M, Scotlandi K. Caveolins in the development and diseases of musculoskeletal system. Cancer Lett. 2009;284:113–121. doi: 10.1016/j.canlet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87:193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- Sokoloff AJ, Li H, Burkholder TJ. Limited expression of slow tonic myosin heavy chain in human cranial muscles. Muscle Nerve. 2007;36:183–189. doi: 10.1002/mus.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Scherer PE, Tang Z, et al. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, et al. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Voldstedlund M, Vinten J, Tranum-Jensen J. cav-p60 expression in rat muscle tissues. Distribution of caveolar proteins. Cell Tissue Res. 2001;306:265–276. doi: 10.1007/s004410100439. [DOI] [PubMed] [Google Scholar]
- Volonte D, McTiernan CF, Drab M, et al. Caveolin-1 and caveolin-3 form heterooligomeric complexes in atrial cardiac myocytes that are required for doxorubicin-induced apoptosis. Am J Physiol Heart Circ Physiol. 2008;294:H392–H401. doi: 10.1152/ajpheart.01039.2007. [DOI] [PubMed] [Google Scholar]
- Way M, Parton RG. M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 1996;378:108–112. doi: 10.1016/0014-5793(96)82884-5. [DOI] [PubMed] [Google Scholar]
- Whiteley G, Collins RF, Kitmitto A. Characterization of the molecular architecture of human caveolin-3 and interaction with the skeletal muscle ryanodine receptor. J Biol Chem. 2012;287:40302–40316. doi: 10.1074/jbc.M112.377085. [DOI] [PMC free article] [PubMed] [Google Scholar]


