Abstract
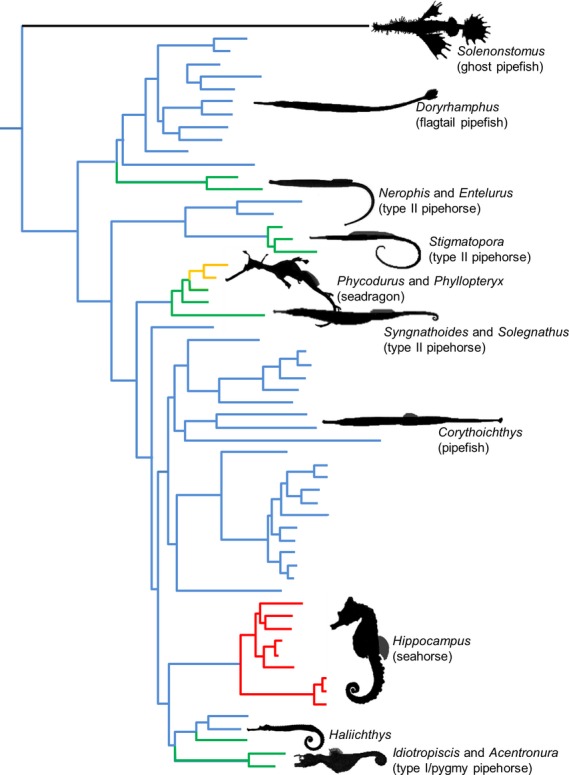
Seahorses and pipehorses both possess a prehensile tail, a unique characteristic among teleost fishes, allowing them to grasp and hold onto substrates such as sea grasses. Although studies have focused on tail grasping, the pattern of evolutionary transformations that made this possible is poorly understood. Recent phylogenetic studies show that the prehensile tail evolved independently in different syngnathid lineages, including seahorses, Haliichthys taeniophorus and several types of so-called pipehorses. This study explores the pattern that characterizes this convergent evolution towards a prehensile tail, by comparing the caudal musculoskeletal organization, as well as passive bending capacities in pipefish (representing the ancestral state), pipehorse, seahorse and H. taeniophorus. To study the complex musculoskeletal morphology, histological sectioning, μCT-scanning and phase contrast synchrotron scanning were combined with virtual 3D-reconstructions. Results suggest that the independent evolution towards tail grasping in syngnathids reflects at least two quite different strategies in which the ancestral condition of a heavy plated and rigid system became modified into a highly flexible one. Intermediate skeletal morphologies (between the ancestral condition and seahorses) could be found in the pygmy pipehorses and H. taeniophorus, which are phylogenetically closely affiliated with seahorses. This study suggests that the characteristic parallel myoseptal organization as already described in seahorse (compared with a conical organization in pipefish and pipehorse) may not be a necessity for grasping, but represents an apomorphy for seahorses, as this pattern is not found in other syngnathid species possessing a prehensile tail. One could suggest that the functionality of grasping evolved before the specialized, parallel myoseptal organization seen in seahorses. However, as the grasping system in pipehorses is a totally different one, this cannot be concluded from this study.
Keywords: musculoskeletal morphology, prehensile tail, Syngnathidae
Introduction
Syngnathid fishes, comprising pipefishes, pipehorses, seahorses and seadragons, rely on fast oscillations of the dorsal – up to 35 times per second (Breder & Edgerton, 1942) – and pectoral fins for propulsion (Consi et al. 2001). Unlike in pipefishes and other teleost fishes, the tail of seahorses and pipehorses has become modified into a prehensile apparatus, a function unique among fishes. Seahorses and pipehorses no longer possess a caudal fin (which is also characteristic of seadragons) and are able to grasp and hold on substrates during feeding and hiding, by using their tail (Weber, 1926; Blake, 1976; Hale, 1996; Teske & Beheregaray, 2009). In seahorses, the grasping occurs with a vertical posture in the water column, recently suggested to be adaptive for their specific way of feeding, i.e. pivot feeding (Van Wassenbergh et al. 2011), as well as having originated in association with an Indo-West Pacific expansion of sea grass habitats (Teske & Beheregaray, 2009). Despite the fact that they are slowly moving organisms, they can escape predation by crypsis, adopting colors and camouflage specific to their environment, by their fast dorsal fin movement, which exceeds the flicker fusion threshold of their predators (Breder & Edgerton, 1942), or by being nocturnal (Kuiter, 2009; Teske & Beheregaray, 2009).
The syngnathid tail comprises a series of articulating, and frequently ornamented (Kuiter, 2009; Lees et al. 2012), bony plates (four plates per body segment) surrounding the caudal muscle bundles, which are anchored on the central vertebral column (Hale, 1996). A recent study (Porter et al. 2013) showed that this array of bony plates in seahorses functions as a flexible, subdermal armor and protects the tail segments and vertebrae from fracture. The ancestral syngnathid condition, as seen in ghost pipefishes (genus Solenostomus, sister taxon to Syngnathidae) and true pipefishes (Jungersen, 1910), is characterized by a relatively stiff tail. This explains its limited use in swimming, which is mainly done with dorsal and pectoral fins (Ashley-Ross, 2002). Keeping their body horizontal, pipefishes forage in the open water (especially the flagtail pipefishes – Doryrhamphinae; Kuiter, 2009) or close to the sea floor. Two morphotypes, seemingly intermediate between seahorses and pipefishes, also exist and are characterized by a horizontal body posture, the absence of a tail fin and the presence of a prehensile tail. These intermediate types comprise pipehorses and Haliichthys taeniophorus. The first type (further referred to as ‘type I pipehorse’) comprises the very rare pygmy pipehorses (genera Idiotropiscis, Acentronura, Kyonemichthys and Amphelikturus), which superficially look like seahorses (to which they are a sister group), but with a horizontal body posture (Gomon, 2007; Kuiter, 2009; Teske & Beheregaray, 2009). The second type (further referred to as ‘type II pipehorse’) shows a similar morphology to that of pipefishes but possesses a prehensile tail. These pipehorses do not form a monophyletic group and are phylogenetically nested within the pipefishes (Fig. 1). The name pipehorse thus refers rather to a morphological characteristic (the presence of a prehensile tail) than to a phylogenetically accepted name. There is still some debate about the common name of H. taeniophorus. Like seahorse and pipehorse, it possesses a prehensile tail and is referred to as the ribboned seadragon (Kuiter, 2009), the ribboned pipehorse (Munro, 1958) or the ribboned pipefish (Dawson, 1985). There is also no consensus on the phylogenetic position of H. taeniophorus. According to some studies, this species is nested within the pipefishes (Wilson & Rouse, 2010; Wilson & Orr, 2011), whereas others (Hamilton et al. 2009) suggest a close relation to the pygmy pipehorses and a sister-relation to the seahorses. To avoid confusion, we will refer to this species by its Latin name only and not assign it to a main syngnathid group (pipefish, pipehorse, seahorse or seadragon). Seadragons – comprising only two species, Phyllopteryx taeniolatus and Phycodurus eques – are characterized by a horizontal body posture and the absence of both a caudal fin and a prehensile tail.
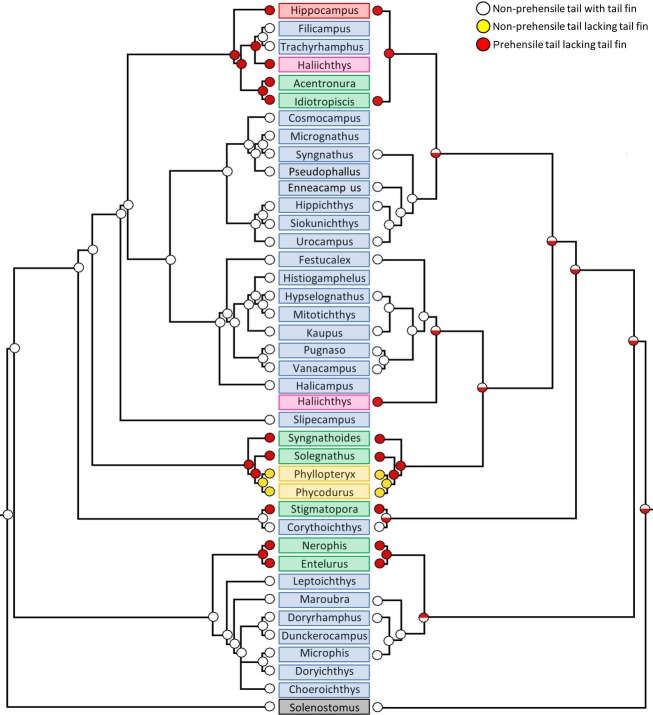
Figure 1.
Polyphyletic origin of grasping tails in syngnathids. Phylogenetic pattern towards the independent evolution from an ancestral tail possessing a tail fin (blue), towards a tail lacking a tail fin without grasping capacities in the seadragons Phycodurus eques and Phyllopteryx taeniolatus (yellow) and with grasping capacities in pipehorses (green) and seahorses (red). Solenostomus (black) belongs to a separate family of Solenostomidae, sister to Syngnathidae and is used as an outgroup. Cladogram based on Hamilton et al. (2009).
Tail prehension is not unique for vertebrates, having arisen in sauropsids (Zippel et al. 1999; Bergmann et al. 2003) and mammals (German, 1982; Organ, 2010) as well. Although it might seem quite unlikely that during evolution, a complex system such as the musculoskeletal caudal system in fishes would have independently evolved into a prehensile tail more than once within a single clade at the family level, recent phylogenetic studies (Hamilton et al. 2009; Teske & Beheregaray, 2009; Wilson & Orr, 2011) suggest this to be a likely scenario (Fig. 1). Prehensile tails seem to have originated several times independently in syngnathid fishes, although it is not yet clear how many times exactly (Teske & Beheregaray, 2009; Wilson & Rouse, 2010; Wilson & Orr, 2011). Little is known about the modifications of the caudal musculoskeletal system associated with such a drastic functional shift (from a non-prehensile to a prehensile tail). Previous studies (Chevroton, 1912; Hale, 1996) showed that seahorses have plates interconnected by sliding joints that are created by elongated spines. In addition, hypaxial muscle fibers lie in between parallel sheets of connective tissue (myosepta), rather than within a conical organization more typically found in teleost fishes (Gemballa & Roder, 2004). Unlike in these teleosts, the myosepta in seahorses span several vertebral segments between their medial and lateral insertion (Hale, 1996). Median ventral muscles, interconnecting hemal spines on the vertebrae in seahorses, are considered to be modified ventral fin muscles (infracarinalis posterior muscles) (Hale, 1996). It is hypothesized that these median ventral muscles are responsible for sustained holding, whereas hypaxials may power fast grasping (Chevroton, 1912; Hale, 1996). However, the structural basis for the apparent complex motor control of which seahorses seem to be capable, is so far not properly understood. As no comparison with the ancestral condition, as found in pipefishes, or the convergent condition in pipehorses has been performed, it is not possible to draw any conclusions about how this unique trajectory towards tail grasping within syngnathid fishes (and fishes in general) could have evolved.
In this study, we test several hypotheses that may help to elucidate this, by comparing the musculoskeletal anatomy and bending capacity in pipefish, seahorse, pipehorse (both morphotypes) and H. taeniophorus. As such, this study may provide a solid base for a more comprehensive survey of the structural modifications of the tail in the different lineages within the Syngnathidae, and their implications for function with respect to grasping. The overall hypothesis of this study is that prehensile function only became possible after substantial modifications of the musculoskeletal system (as observed in seahorses; Hale, 1996) and that the configuration in the studied pipehorse representatives and H. taeniophorus would be similar to that in seahorses, thus differing from that in the pipefish representatives. The maximal bending angle of the tail (in lateral, ventral and dorsal direction) is quantified to determine the passive prehensile capacities of the tail of pipefish (reflecting the ancestral syngnathid condition with limited ventral and dorsal bending), seahorse, pipehorse (type II) and H. taeniophorus. For the latter three, we expect ventral bending capacities to be more extensive (as in seahorses; Weber, 1926; Peters, 1951). We expect that the passive bending capacity is mainly influenced by the bony plate morphology and that the vertebral column shows the same flexibility in all species studied. Concerning the skeletal morphology, we expect that species with a prehensile tail share the condition where plates are interconnected by elongated, sliding joints (rather than fully abutting edges, as seen in non-prehensile species). As gradual reduction in plate size is present towards the distal tip in seahorses (Hale, 1996), with a consequent increasing flexibility, we expected to find a similar reduction in plate size in those species possessing a prehensile tail. The vertebral morphology of Hippocampus kuda and Hippocampus hippocampus, both seahorses, has already been described (Hale, 1996; Bruner & Bartolino, 2008). To our knowledge, no detailed information on that in other seahorses, pipehorses, pipefishes and seadragons is available. As it is generally assumed that the number of vertebrae is related to body flexibility (Breder, 1926; Brainerd & Patek, 1998), we expect species with a prehensile tail to have a higher vertebral count than pipefishes. Concerning the muscular organization, we expect to find parallel myoseptal sheet spanning multiple vertebrae in species with a prehensile tail (compared with a conical organization in pipefishes). We also expect the presence of median ventral muscles (MVM), interconnecting subsequent hemal spines, hypothesized to be responsible for sustained grasping (Hale, 1996). Also, as extensive ventral bending is presumed to be facilitated by a substantial shortening of muscles involved (based on the assumption that a maximal shortening of 50% of the muscle fibers is achieved; Gordon et al. 1966; Hall, 1999), we expect to find longer muscle fibers in the hypaxial muscles of species possessing a prehensile tail. To further test the hypothesis on the convergent evolution of the prehensile tail in syngnathid fishes, a character state reconstruction was performed based on two different phylogenies (Hamilton et al. 2009; Wilson & Orr, 2011).
As shown below, the predictability of the morphology based on functional performance is not as straightforward as might be suggested. Even more striking than the fact that grasping tails evolved more than once within syngnathids, is that at least two evolutionary strategies have resulted in different solutions to the same problem. Although no generalizing predictions can yet be made about evolutionary modifications that characterize the multiple lineages of pipehorses, these different evolutionary strategies do seem to corroborate the hypothesis that seahorses, pipehorses (both morphotypes) and H. taeniophorus became adapted within a different ecological context where grasping proved to be beneficial: hanging vertically while grasping on sea grasses vs. horizontally while attached to macroalgae (Teske & Beheregaray, 2009). Taking into account estimated time divergences of the different lineages (Teske et al. 2004), these apparently extreme functional shifts towards tail grasping in syngnathids did occur multiple times during evolution, within a short time frame (i.e. somewhere between 50 and 25 mya).
Material and methods
Specimens studied
Several specimens of four seahorse species (Hippocampus reidi, Hippocampus capensis, Hippocampus abdominalis and Hippocampus breviceps), four pipehorse species (Syngnathoides biaculeatus, Solegnathus hardwickii, Idiotropiscis australe and Acentronura gracilissima), one seadragon (Haliichthys taeniophorus) and four pipefish species (Corythoichthys intestinalis, Dunckerocampus pessuliferus, Doryrhamphus melanopleura and Syngnathus acus) were studied. Species were selected based on their phylogenetic position within syngnathids, so that the major clade-related morphotypes, from primitive to derived, were included. All pipefishes, H. reidi, H. capensis, H. abdominalis and S. biaculeatus were commercially obtained through the aquarium trade. These specimens were euthanized with an overdose of MS222 (Sigma Aldrich), prior to fixation in 4% formaldehyde. Specimens of H. breviceps, S. hardwickii and A. gracilissima were obtained from the Muséum National d'Histoire Naturelle (MNHN – France, resp. MNHN 1890-0371, MNHN 0000-6042 and MNHN 1904-0298) and two H. taeniophorus specimens from the Commonwealth Scientific and Industrial Research Organization (CSIRO – Australia, unregistered specimens). The I. australe specimen belongs to the collections of the South Australian Museum (SAMA F720).
Musculoskeletal morphology
One specimen of D. pessuliferus, D. melanopleura, H. breviceps, S. hardwickii and A. gracilissima were μCT-scanned to study the skeletal organization. To visualize 3D soft tissue anatomy, one specimen each of S. acus, C. intestinalis and H. abdominalis was treated with phosphomolybdic acid (submerged in 2.5% solutions for 36 h) (Metscher, 2009) and submitted to μCT-scanning. All the above specimens were scanned at the Centre for X-ray Tomography at Ghent University (UGCT), using the following setup: 7 kV tube voltage and 1000 projections over 360°. The pixel pitch of the detector varied between 127 μm and 400 μm and the resulting voxel sizes were respectively 3.52, 35.17, 33.12, 32.99 and 8.91 μm for the non-stained specimens and 23.56, 26.54 and 32.14 μm for the specimens treated with phosphomolybdic acid.
Two adult specimens of H. reidi and one specimen each of S. biaculeatus and H. taeniophorus were scanned at the European Synchrotron Radiation Facility in Grenoble, using phase contrast synchrotron x-ray radiation. A total of respectively 1677, 1844 and 1844 radiographic images with a resolution of 2048 × 2048 pixels and a voxel size of 7.46 μm were acquired using a FReLoN CCD camera. This allowed a detailed visualization of muscle and connective tissue organization. One specimen of I. australe was scanned at the Australian Museum (with a pixel pitch of the detector of 8.60 μm and a resulting voxel size of 6.46 μm) and the raw data was reconstructed at the UGCT. All μCT-data were processed to generate graphical 3D-reconstructions of bony plates and myosepta using amira 5.2.2 (Visage Imaging, San Diego, CA, USA). Based on these detailed reconstructions, schematic overviews of muscles and tendons were made using rhinoceros (Version 5, R. McNeel & associates).
Gross plate morphology was studied on in toto cleared and stained specimens of S. biaculeatus, C. intestinalis, H. reidi, H. capensis and H. taeniophorus (protocol following Taylor & Van Dyke, 1985). The organization of myosepta and the attachment of tendons on the bony plates and vertebrae were also examined based on histological sections of S. rostellatus, H. reidi and S. biaculeatus. To generate these sections, the tail was embedded in Technovit 7100 and 5-μm sections were cut off with a Prosan Leica SM2500 microtome and stained with toluidine. Images of the sections were obtained using an Olympus BX41 microscope and Colorview camera.
A parsimony ancestral state reconstruction was performed on cladograms resulting from different phylogenetic studies (Hamilton et al. 2009; Teske & Beheregaray, 2009; Wilson & Orr, 2011) using mesquite 2.75 (http://mesquiteproject.org).
Muscle fiber length
The hypaxial muscles of the distal part of the tail of an S. acus and H. abdominalis specimen were dissected and treated with nitric acid to separate the individual muscle fibers. Fibers were photographed using a Colorview 8CCD camera mounted on an Olympus SZX9 stereoscope. The length of 30 muscle fibers was measured using analysis 5.0. The hypaxial muscle fiber length of S. biaculeatus and H. reidi was determined using serial histological sections of the tail (embedded in Technovit 7100, 5-μm sections cut with a Leica SM2500 microtome and stained with toluidine). Images were obtained using an Olympus BX41 microscope and Colorview camera, and aligned in amira 5.2.2 after which x, y and z coordinates of the origin and insertion of 30 muscle fibers were determined to calculate the average muscle fiber length. As specimens differed in total body size and hence segment length, differences in muscle fiber length between species were tested using the ratio of the muscle fiber length vs. vertebral length in four species, referred to as relative muscle fiber length, with a Bonferroni-corrected Kruskal–Wallis test using past (Hammer et al. 2001).
Bending performance
Passive joint flexibility was determined by measuring the maximum angle of bending in both a ventral, dorsal and lateral direction. One cleared and stained specimen (Taylor & Van Dyke, 1985) of a seahorse (H. reidi), a pipehorse (S. biaculeatus), a pipefish (C. intestinalis) and H. taeniophorus was secured with pins in a maximally bent position and photographs were taken with a canon EOS550 camera and a Sigma 150-mm macro lens. The line at the level of the vertebral column was traced three times for each species and each view using tpsDIG (Rohlf, 2008), which was subsequently subdivided into 100 equally sized segments. The angle between all segments was calculated, and the average angles (of the three replicated digitizations) were plotted.
Results
Musculoskeletal morphology
Plotting the characteristic ‘prehensile tail’ on the existing phylogenies, one can conclude that the prehensile tail evolved multiple times independently. If the phylogenetic study of Hamilton et al. (2009) is considered, the prehensile tail evolved four times independently within the family of syngnathid fishes (Fig. 2). However, based on the second ancestral state reconstruction (phylogenetic study of Wilson & Orr, 2011, combined with the one of Teske & Beherahay, 2009 for the position of the pygmy pipehorses), the prehensile tail evolved five times, but the independent evolution of it is not confirmed.
Figure 2.
Ancestral state reconstruction of grasping tails in syngnathids. Phylogenetic pattern towards the independent evolution of the prehensile tail in syngnathid fishes. Three different conditions can be discerned: (white circle) species with a non-prehensile tail possessing a tail fin, as seen in pipefish; (yellow circle) species with a non-prehensile tail lacking a tail fin, as seen in the seadragon species Phyllopteryx taeniolatus and Phycodurus eques; (red circle) species with a prehensile tail lacking a tail fin, as seen in pipe- and seahorses. The left cladrogram is based on the phylogenetic study of Hamilton et al. (2009), the right on the study of Wilson & Orr (2011), with inclusion of the pygmy pipehorse (genus Idiotropiscis) based on Teske & Beheregaray (2009). Solenostomus belongs to a separate family of Solenostomidae, sister to Syngnathidae, and is used as an outgroup. The background colour behind each genus name represents the common group to which the genus belongs (pipefish in blue, seadragon in yellow, pipehorse in green, seahorse in red, Solenostomus in black and Haliichthys in pink).
In pipefish, each vertebra is surrounded by four bony plates (further referred to as one segment), being a bilateral set of a dorsal and a ventral plate. Plates within a single segment interconnect through scarf joints [joints with overlapping flat surfaces (Hildebrand, 1995)], whereas subsequent segments interlock through a distinct caudal spine, which slides into a corresponding furrow on the plate posterior to it (further called the anterior furrow). Plates in pipefish form a tight set of articulating, skeletal segments, where any space in between segments is covered by intercalating plates (Fig. 3A). Plates are connected through connective tissue to the vertebrae at the level of the neural arch (dorsal) and the transverse (lateral) and hemal (ventral) spines. In Hippocampus, plates are substantially reduced to a tetrahedral-like structure, still bearing a caudal spine that glides into a distinctive anterior furrow in the corresponding plate of the consecutive segment. The caudal spine is relatively longer than in pipefish, due to the observed plate reduction. The intrasegmental connection is a reduced scarf joint, with only three ridges and corresponding furrows (Fig. 4A). In the four pipehorse species studied, three different skeletal conditions were observed. In S. biaculeatus (a type II pipehorse), a similar condition as in the pipefish is found in the proximal part of the tail (first 70% of the tail), but without the presence of intercalating plates. However, towards the distal tip (last 30%), plates become smaller until completely absent, ending in a series of approximately 20 vertebrae that are no longer surrounded by any plates (Fig. 5A). This reduction of the plates starts more anteriorly at the ventral side, at a point where the dorsal plates still interlock through a caudal spine. In seahorse, the plates also become reduced in size, but each vertebra is covered by four bony plates up to the distal-most vertebrae. The organization of the proximal part of the tail (first 70%) in S. hardwickii (also a type II pipehorse and closely related to S. biaculeatus) is also similar to the one in pipefish (but with the presence of intercalating plates). In the prehensile part of the tail (last 30%), however, the ventral and dorsal plates of one segment are no longer directly opposite to each other, but show an alternating pattern of ventral and dorsal plates in the lateral view (Fig. 5B1). There is also a reduction of the medial side of the ventral plates, which leaves out a gap in between the contralateral ventral plates (Fig. 5B2). Thus, in this last 30% of the tail of S. hardwickii, there is, within one segment, no longer a connection through scarf joints between the contralateral ventral plates and between the dorsal and ventral plates. In addition, the last four vertebrae are no longer covered by any plates at the ventral side (Fig. 5B). Acentronura gracilissima and I. australe (pygmy pipehorses, type I), both belonging to the sister group of Hippocampus, are characterized by an intermediate plate morphology. The dorsal bony plates are very similar to the plates of pipefish (forming a tight set of articulating, skeletal elements), but without the presence of intercalating plates, whereas the ventral plates are more similar to those observed in seahorses, with a strong reduction in plate size (Fig. 5C). The plate morphology of H. taeniophorus strongly resembles the intermediate plate morphology seen in A. gracilissima and I. australe (Fig. 6A).
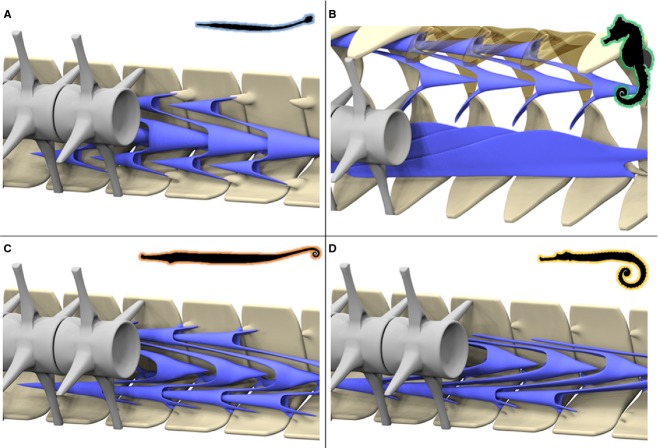
Figure 3.
Musculoskeletal organisation in pipefish. High resolution 3D reconstructions of CT-data on the caudal part of the tail in pipefish (horizontal body posture, non-prehensile tail with tail fin), represented by Corytoichthys intestinalis. (A) Reconstruction of the caudal skeletal part of the tail (dermal and intercalating plates in grey), detail shows a lateral view of the caudal spine on the dorsal plates fitting into an anterior groove on the subsequent plate. (B) Reconstruction of the conical myoseptal organization (dermal plates in grey, myosepta in blue), with a main anterior cone (MAC), ventral posterior cone (VPC) and a secondary hypaxial cone (sHAC).
Figure 4.
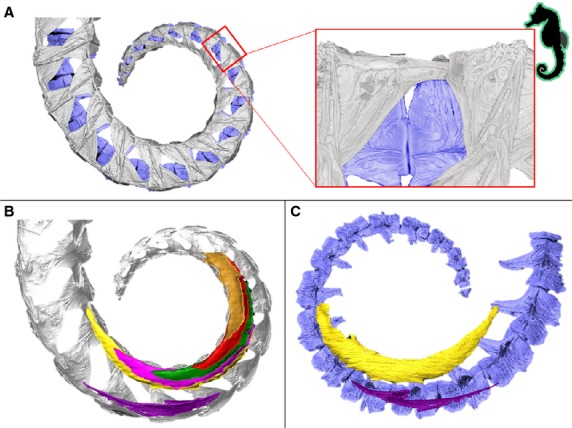
Musculoskeletal organization in seahorse. High resolution 3D reconstructions of CT-data on the caudal part of the tail in seahorses (vertical body posture, prehensile tail lacking a tail fin), represented by Hippocampus reidi. (A) Reconstruction of the caudal skeletal part of the tail (dermal plates in grey, vertebrae in blue); detail shows a lateral view of the caudal spine on the dorsal plates fitting into an anterior groove on the subsequent plate, (B-C) Reconstruction of the parallel myoseptal organization (dermal plates in grey, vertebrae in blue, myosepta in different bright colours), from (B) a medial viewpoint with vertebrae removed and (C) a lateral viewpoint with dermal plates removed.
Figure 5.
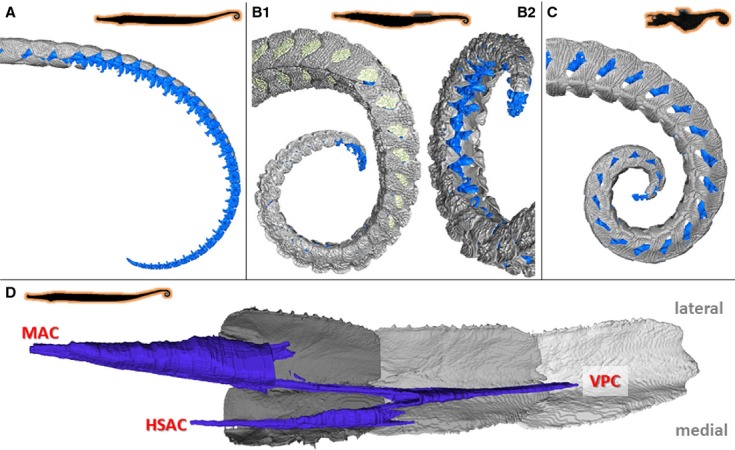
Musculoskeletal organization in pipehorse. High resolution 3D reconstructions of CT-data on the caudal part of the tail in pipehorses (horizontal body posture, prehensile tail lacking a tail fin), represented by (A,D) Syngnathoides biaculeatus (type II pipehorse), (B) Solegnathus hardwickii (type II pipehorse) and (C) Acentronura gracilissima (type I/pygmy pipehorse). (A-C) Reconstruction of the caudal skeletal part of the tail (dermal plates in grey, vertebrae in blue). (D) Reconstruction of the conical myoseptal organization (dermal plates in grey, myosepta in blue), with a main anterior cone (MAC), ventral posterior cone (VPC) and a secondary hypaxial cone (sHAC).
Figure 6.
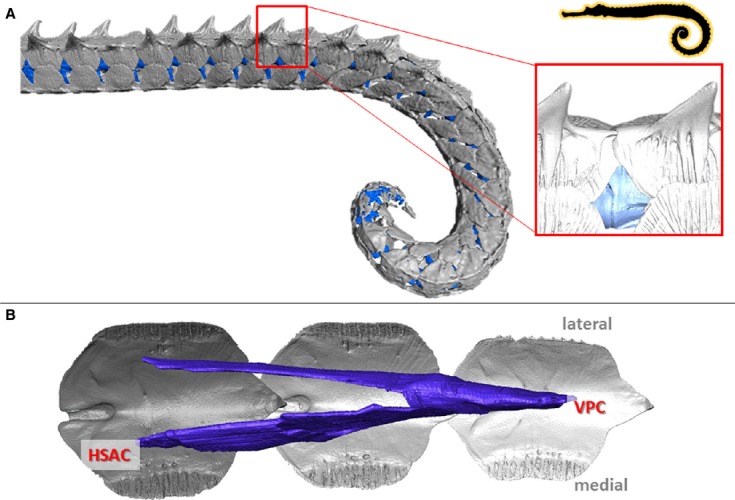
Musculoskeletal organization in Haliichthys taeniophorus. High resolution 3D reconstructions of CT-data on the caudal part of the tail in H. taeniophorys (horizontal body posture, prehensile tail lacking a tail fin). (A) Reconstruction of the caudal skeletal part of the tail (dermal plates in grey, vertebrae in blue); detail shows a lateral view of the caudal spine on the dorsal plates fitting into an anterior groove on the subsequent plate. (B) Reconstruction of the conical myoseptal organization (dermal plates in grey, myosepta in blue), with ventral posterior cone (VPC) and a secondary hypaxial cone (sHAC).
The vertebral morphology in pipefish and pipehorse is similar to that of seahorse, i.e. amphicoelous vertebrae with a well-developed neural arch and anteroposteriorly thickened lateral processes (Hale, 1996). In a lateral view, the ventral side of the body of the vertebrae in the prehensile part of the tail of seahorse, pipehorse and H. taeniophorus is shorter than its dorsal side, whereas the vertebrae in the non-prehensile part and those of pipefish are rectangular, as seen in most teleost fishes. As for the vertebral count, there is no significant difference in the total number of vertebrae between seahorse, pipehorse, seadragon and pipefish (P > 0.05) but the ratio of the number of vertebrae in the tail relative to the total number of vertebrae was significantly higher in species with a prehensile tail (F = 11.216; P = 0.0092).
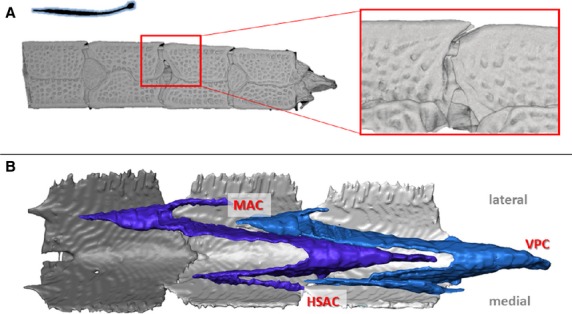
In Corythoichthys and Syngnathus pipefish, myosepta in the hypaxial muscles are organized as conical structures, where three cones per segment lie between the transverse process and the hemal spine. This three-cone organization consists of two anterior pointing cones – one main (hMAC) and one secondary (hSAC) anterior cone – and one ventral posterior pointing cone (VPC). In pipefish, this VPC is the largest cone, the hMAC and hSAC being of equal size, but smaller than the VPC (Gemballa et al. 2006). All three cones are connected to the ventral plates through a distinctive tendon (Fig. 7). The hMAC connects through a lateral tendon (LT) on the plate anterior to the one bearing the connection with the hSAC through a secondary myorhabdoid tendon (SMT). These two tendons attach to two small spines at the anterior side of the plate, respectively at the lateral and ventral side of the anterior furrow. The VPC attaches through a myorhabdoid tendon (MT), which anteriorly splits into two slips that attach on both sides of the distinctive furrow at the rostral side of each plate. The tip of the caudal spine is embedded in this tendon (Fig. 7). The epineural tendon (ENT) in these pipefish species is fused with the horizontal septum and thus is no longer visible as a thick, distinct tendon (terminology based on Gemballa et al., 2003, 2006). In seahorse, a completely different hypaxial myoseptal pattern is present, as was suggested in the literature (Hale, 1996), where parallel sheets of connective tissue interconnect the vertebrae with the plates. However, it is still not clear whether this connection is continuous along the length of the whole myoseptum or occurs by means of one attachment point (e.g. through a tendon) at both the anterior and the posterior side. In this study we found that these myosepta span up to eight segments, resulting in an increased number of septa enclosed in a single segment (Fig. 5B–C). At their ventral face, the myosepta bear a distinct MT, inserting on a small elevation on the anterior side of the plate, at the ventral side of the anterior furrow (Fig. 8B). In seahorse, the tip of the caudal spine is not embedded in the MT, which is the only tendon that can be distinguished in the hypaxial muscles of seahorses. The epaxial muscles show a modified conical pattern, in which the epaxial main anterior cone (eMAC) is reduced. At the dorsal side, only the epaxial secondary anterior cone (eSAC) interconnects to the small anterior spine on the plate through a tendon, whereas the epaxial sloping part (ESP – the part between the dorsal posterior cone (DPC) and the eMAC] is connected to the parapophysis of the vertebrae (Figs 5B,C and 8B). In all seahorse species studied, the hemal spines are interconnected with median ventral muscles (MVM) (Fig. 9B), which are absent in pipefish (Fig. 9A). In H. abdominalis (one of the largest seahorse species), a similar muscular organization is also present on the dorsal side (median dorsal muscles – MDM), interconnecting two consecutive neural arches. In the Syngnathoides pipehorse, a pipefish-like three-cone organization of the hypaxial myosepta is present, but is distinct at two levels. First, the cones are extended, hence spanning about three segments instead of two. Secondly, the VPC is equal in size to the hSAC and the hMAC is the largest in size (Fig. 6D). The myosepta are attached to the bony elements through three distinct tendons, similar to the pipefish. In the tip of the tail, where the bony plates are lacking, the myosepta fuse with the connective tissue of the skin. This pipehorse shares the presence of MVM with seahorses, interconnecting two consecutive hemal spines (Fig. 9C). In the seadragon (H. taeniophorus), a conical organization of the myosepta was observed, with a reduced hMAC, similar to the epaxials in seahorses (Fig. 7B). However, as no attachment site of the myosepta to the bony plates could be unambiguously visualized, it cannot be excluded that the reduction of the hMAC may be an artefact due to limited quality of the synchrotron scans. For the same reason, it was also not possible to visualize the MVM. Due to the rarity of the S. hardwickii, A. gracilissima and I. australe specimens, they could not be stained with contrast agents for soft tissue visualization or histology, and thus only information on the skeletal elements could be obtained.
Figure 7.
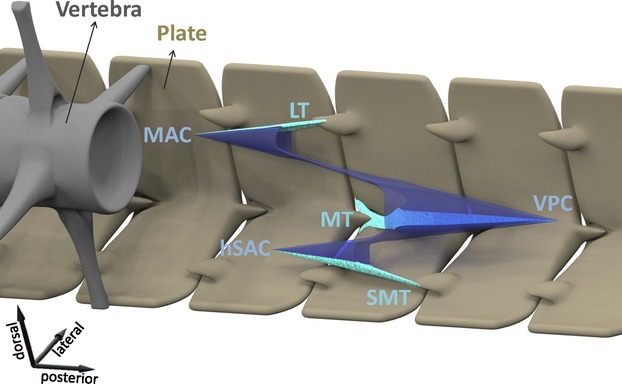
Schematic overview of the conical organization of myosepta and tendons as found in the ancestral condition. Plates in brown, vertebra in grey, myosepta in blue (MAC, main anterior cone; VPC, ventral posterior cone; hSAC, hypaxial secondary anterior cone) and tendons in light green (LT, lateral tendon; MT, myorhabdoid tendon; SMT, secondary myorhabdoid tendon).
Figure 8.
Schematic overview of the myoseptal organization in (A) pipefish, (B) seahorse, (C) pipehorse and (D) seadragon.
Figure 9.
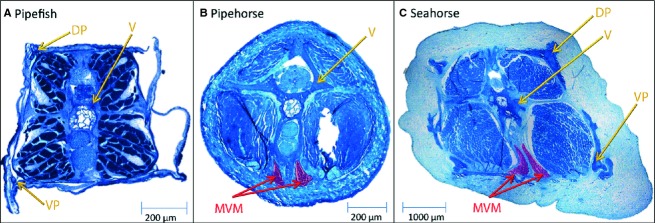
Median ventral muscle illustrated on histological sections. The median ventral muscle (MVM) is absent in (A) pipefish and present in both (B) pipehorse and (C) seahorse. DP, dorsal plate;VP, ventral plate; V, vertebra.
Bending capacity
The hypothesis on relative muscle fiber length of the hypaxials (with respect to the vertebral length) seems to be supported, as in both seahorse species studied (H. abdominalis and H. reidi, 0.2412 ± 0.0206 and 0.2295 ± 0.0557 SD, respectively) the relative muscle fiber length was significantly longer than in a pipefish (Syngnathus acus, 0.1465 ± 0.0236 SD; P < 0.05). However, this was not the case for S. biaculeatus (a pipehorse, 0.1541 ± 0.0262), where the relative muscle fiber length was not significantly different from that in pipefish (and hence significantly shorter than in seahorses; P = 0.3327). Among the two seahorse species studied, the fibers had similar relative length values, although with a larger range in the largest specimen (H. abdominalis). However, this could be a methodological artifact (as fiber length was measured on histological sections with a thickness of 5 μm in H. reidi and those of H. abdominalis on dissected fibers).
As expected, plate interconnectivity proved to constrain bending capacities substantially in pipefish (Corythoichthys): maximal passive bending in all three directions (dorsal, ventral and lateral) was similar along the tail, gradually increasing to a total angle of about 300°. In a seahorse (H. capensis), the bending angle also increases gradually, but at a steeper slope, giving an increased flexibility in the caudal 30% of the tail length. A maximal cumulative bending of around 800° was achieved in a ventral direction, but also in a lateral one, in contrast to what we expected. As expected, dorsal bending was most constrained, but still reached a total of around 600°. We expected a similar pattern for S. biaculeatus (a pipehorse); however, aquarium observations already showed that they grasp with the distal part of the tail only, keeping the proximal part rather rigid. The data obtained confirm this, the anterior 70% of the tail following a pattern very similar to that in pipefish, whereas the caudal 30% is highly flexible, even more than in seahorses. As such, they also reach a cumulative total curvature of around 800° for ventral bending and only slightly lower values for lateral and dorsal bending. In the seadragon (H. taeniophorus), extensive ventral and lateral bending was observed (respectively ca. 900° and 750°). The bending range in the dorsal direction, however, is restricted (ca. 350°) and only slightly higher than the dorsal bending capacity of pipefishes, which is ca. 300° (Fig. 10). To our knowledge, there are no morphological indications that there should be marked differences in the way the vertebrae influence the passive bending capacities of the above syngnathid fishes.
Figure 10.
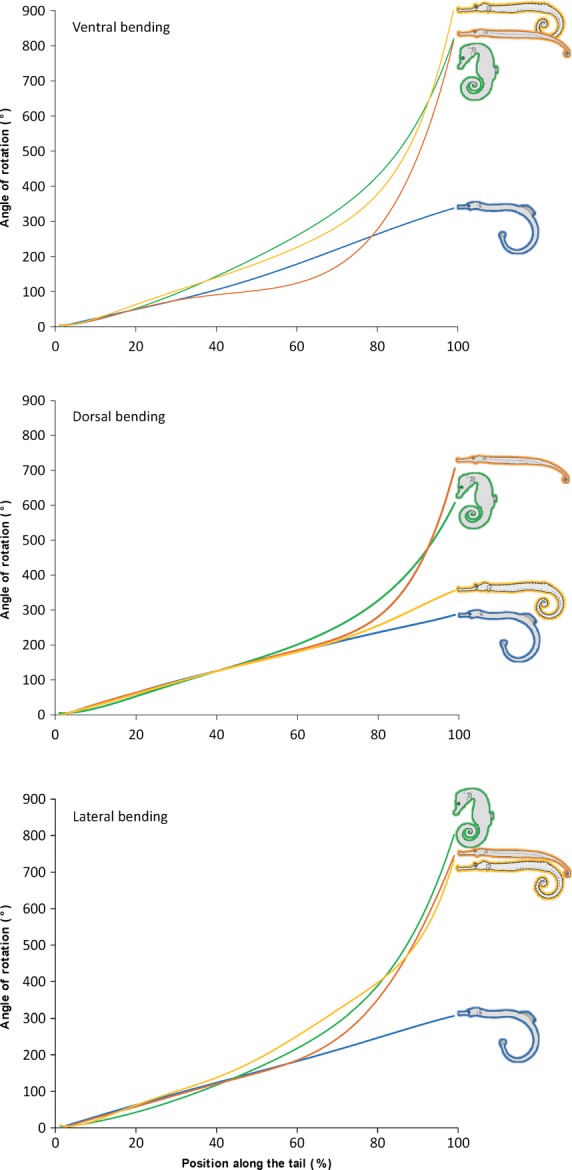
Passive bending capacities. Bending pattern of pipefish (blue), seahorse (green), pipehorse (orange) and seadragon (yellow). The upper graph shows the passive bending pattern in the ventral plane, the middle one in the dorsal plane and the lower one in the lateral plane.
Discussion
As expected, seahorse, pipehorse and H. taeniophorus are capable of bending the caudal part of their tail more ventrally, compared with pipefish. However, our hypothesis that bending capacities would be highest in a ventral direction is refuted, as the tail in seahorse, pipehorse and H. taeniophorus bends almost equally well in a lateral direction. Our observations from manipulating the tail confirm life observations of tail grasping on vertical objects, such as sea grass leaves (Weber, 1926; Peters, 1951), where extensive lateral bending is functionally important for a proper hold while keeping the body both vertical (seahorse) or horizontal (pipehorse and H. taeniophorus). Still, extensive bending in a vertical plane is structurally and functionally not inherently linked to extensive lateral bending, as seen in chameleons, where tail mobility is extensive in the vertical plane, but only a limited amount of lateral undulation occurs during locomotion (Bergmann et al. 2003); nor is the presence of a dorsoventral symmetry in the tail musculature a prerequisite for multidirectional bending (as suggested for prehensile skinks; Zippel et al. 1999). As it was not possible to clear and stain a specimen of S. hardwickii and A. gracilissima (due to their rarity and value), the same bending experiment could not be performed on these species. However, based on the skeletal morphology, we would expect a similar bending pattern in S. hardwickii as seen in the Syngnathoides pipehorse (with the anterior 70% following a pattern similar to that in pipefish and a flexible caudal 30% of the tail). The dermal plates in the first 70% of the tail of S. hardwickii form a tight set of articulating segments, where any space in between plates is covered by intercalating plates. Based on the similarities in plate morphology between S. hardwickii and pipefish, we expect that this part of the tail is rather stiff, just like the tail of pipefish. However, the last 30% of the tail is characterized by a loss of connection between dermal plates (either by reduction or by a shift) at the ventral and lateral side. Although there is a difference in plate morphology between S. harwickii and S. biaculeatus in the last 30% of the tail (only a loss of connection and respectively a complete reduction until absent in the tip), we still would expect this last part of the tail of Solegnathus to be highly flexible compared with the first 70%. Acentronura, on the other hand, is characterized by a plate morphology similar to pipefish at the dorsal side and one similar to seahorse at the ventral side, just as in Haliichthys. Based on this plate morphology, we would expect a similar bending pattern for A. gracilissima as observed in H. taeniophorus, with extensive bending in a ventral and lateral direction but a limited bending range in the dorsal direction.
Plates in pipefish form a tight set of articulating, skeletal segments, where any space in between segments is covered by intercalating plates (Fig. 3A).
The ancestral state reconstruction based on the phylogenetic hypothesis of Hamilton et al. (2009) (Fig. 2) suggests that two different evolutionary strategies enabling the capacity for tail bending (both at the structural level and at subsequent functional one) evolved independently. In all syngnathid fishes possessing a prehensile tail, plates become reduced distally, being present but small in seahorse and H. taeniophorus, only present at the dorsal side in S. hardwickii and completely absent in S. biaculeatus (both at the ventral and dorsal side). In S. hardwickii, plates are no longer contralaterally and medially interconnected. Also, an intermediate skeletal morphology between pipefish and seahorse was found, as both H. taeniophorus (sister relationship to Hippocampus according to Hamilton et al., 2009) and the two studied pygmy pipehorses (type I) share similar body plating with seahorse at their ventral side and the rigid plate structure of pipefish at their dorsal side. These differences at the level of plate modifications could explain the passive bending capacities, where the tail in seahorse, H. taeniophorus and, based on the morphology, probably also in pygmy pipehorse (type I), bends in a more gradual manner, whereas in S. biaculeatus (and based on the external habitus, possibly also in S. hardwickii) the tail combines an extremely flexible distal tip with a rigid proximal part. This rigidity in type II pipehorse is explained by the large plates that lack sliding joints around a large posterior process (as found in seahorse), and this, in contrast to our expectations, reflects the ancestral condition as seen in pipefish. Although there is still some debate about the effect of dermal ossification on bending capacities (Long et al. 1996; Gemballa & Bartsch, 2002), we believe that they are, in this case, related to each other. In the studied type II pipehorse species, the change in dermal plate morphology [from reduced to distally absent (S. biaculeatus) or absence of a connection between the contralateral ventral plates and between the dorsal and ventral plates within one segment (S. hardwickii)] starts at the same position along the tail as the sudden increase in bending angle. Another example that supports this conclusion is the restricted passive bending capacity in dorsal direction (ca. 350°) in H. taeniophorus (and, given the similarities in body plating, pygmy pipehorses probably share this restricted dorsal bending), combined with an extensive passive bending capacity in both a ventral and lateral direction, as the skeletal morphology of the dorsal plates strongly resembles that of pipefish and the ventral plates show the similar characteristics as in seahorse. This also shows, as would be expected, that compression is more constrained by the plates than is extension. The prehensile tail of all of the above described species is also characterized by a substantial reduction in size of the distal vertebrae, a feature already observed in H. hippocampus (Bruner & Bartolino, 2008), as well as in other vertebrates with a prehensile tail (Boistel et al. 2010).
The modifications on the myoseptal level in seahorse suggest a structural link with the ventral bending capacities. However, these parallel myosepta were not observed in pipehorse or H. taeniophorus. Based on the muscular organization, it seems that it is the presence of the modified median ventral muscles that is important for extensive coiling (ventral muscle differentiations are also present in tail grasping chameleons; Boistel et al., 2010), whereas the apparent drastic myoseptal reorganization found in seahorse (longitudinal sheets instead of conical septa) seems to be an autapomorphy for the genus Hippocampus and not a necessity for extensive tail bending. However, the different skeletal configuration in S. biaculeatus and H. taeniophorus may dictate a different musculoskeletal mechanism for tail bending. The fact that we only observe longer muscle fibers (relative to vertebral length) in hypaxial muscles in seahorse and not in pipehorse may also indicate that this is not crucial for prehensility.
Although there is no consensus about the extensiveness of the convergent lineages leading to a prehensile tail in syngnathid fishes based on the two phylogenetic hypotheses, it seems well supported, considering the morphological data from this study, that prehensile tails in syngnathids are the result of convergent evolution. The modifications at the skeletal level allowing bending in S. biaculeatus and S. hardwickii are far from similar compared with the ones in seahorse, pygmy pipehorse and H. taeniophorus. Looking at the bending patterns, there is a clear distinction between the studied type II pipehorses (having a rigid first 70% of the tail and a highly flexible last 30%) and all other studies species possessing a prehensile tail (showing a gradual bending pattern). The skeletal morphology of H. taeniophorus is very similar to the one of I. australe and A. gracilissima and supports their sister relationship as indicated in the phylogenetic study of Hamilton et al. (2009). It has been proposed that, based on recent phylogenetic research, this sister relationship to Hippocampus is no longer supported and that Idiotropiscis, Acentronura and Haliichthys are nested within the clade of the pacific pipefishes (G. Short, personal communication). Based on this phylogeny, the intermediate body plating as seen in both Idiotropiscis, Acentronura and Haliichthys may be due to convergent evolution and similar requirements, and does not represent an intermediate stage between pipefish and seahorse. The phylogeny still supports the independent evolution of tail grasping within the family of syngnathid fishes.
With the current information, the differences in ecological affinities of seahorses, seadragons and pipehorses cannot be related to the observed structural and functional strategies for tail bending. Although we now have an idea about the musculoskeletal organization of the tail of syngnathid fishes possessing a prehensile tail, we still don't know how the kinematics across species work (although some data are available on seahorse kinematics during grasping; A. Maia and D. Adriaens, in preparation). Having a plated (body and) tail is considered to be a multifunctional device that provides structural support and protection (Porter et al. 2013) and that can guide bending in a certain plane, but also impede it in another one (Gemballa & Bartsch, 2002). The reduced plate morphology in seahorses (and in type I pipehorses and H. taeniophorus) thus may improve sustained grasping performance, whereas this may not be an issue for the less stationary pipehorses. Syngnathiform fishes (comprising Syngnathidae, Aulostomidae, Centriscidae, Fistulariidae and Solenostomidae) all possess a body covered with bony plates, including its closely related group of Pegasidae (seamoths). This is unlike fishes belonging to the sister groups Mullidae and Scombriformes, all of which lack any form of dermal plating (Near et al. 2013). Other families characterized by dermal bony plates, such as the Gasterosteidae or Loricariidae, are only distantly related to these Syngnathiformes. As it has been suggested that only a small number of genes control major alterations in body armor, a parallel evolution towards non-plated but highly flexible tail tips in the independent pipehorse lineages might not be so unlikely and may be an explanation for the multiple origin of tail prehensility. However, with feeding performances also being linked to body posture, it has already been suggested that differences in feeding kinematics (especially related to the distance to the prey at a feeding strike) may also explain the evolutionary transitions from pipefish morphotypes to seahorse morphotypes, through intermediate pigmy pipehorse (type I) morphotypes (Van Wassenbergh et al. 2011). A comparative developmental study of the caudal musculoskeletal system in syngnathids may also unravel the mechanism behind the extensive reorganization of myosepta, which seems to have originated in Hippocampus (or in the clade leading to seahorses and pygmy pipehorses, as there are indications of a reduction of the hMAC in H. taeniophorus and in the epaxial muscles of Hippocampus), a hypothesis that remains to be tested.
The more complex evolutionary problems are, the less frequent one would expect solutions to have occurred. Still, in this study we showed that in syngnathids it seems that at least two solutions arose towards improved prehensile capacities, a unique key innovation in fishes. However, further work on additional pipehorse species from different lineages (certainly on the muscular morphology) is required to unravel the detailed pattern and mechanisms that gave rise to these highly modified caudal systems.
Conclusions
This study confirms that at least two quite different strategies led to a grasping tail within the family of syngnathid fishes. Also, intermediate morphotypes showing characteristics of both seahorses and pipefishes could be found (i.e. the pygmy pipehorses and Haliichthys taeniophorus). Our hypothesis that the typical parallel myoseptal organization in seahorses is a necessity for grasping has been refuted, as this configuration was not found in other species possessing a prehensile tail.
Acknowledgments
This research was supported by a research grand of the Research Foundation – Flanders (FWO – project 3G013709) and the agency for Innovation by Science and Technology (IWT – SBO scholarship 111249). We would also like to thank the MNHN for the loan of the specimens used in this research and CSIRO for the Haliichthys taeniophorus specimens.
References
- Ashley-Ross MA. Mechanical properties of the dorsal fin muscle of seahorse (Hippocampus) and pipefish (Syngnathus. J Exp Zool. 2002;293:561–577. doi: 10.1002/jez.10183. [DOI] [PubMed] [Google Scholar]
- Bergmann PJ, Lessard S, Russell AP. Tail growth in Chamaeleo dilepis (Sauria: Chamaeleonidae): functional implications of segmental patterns. J Zool. 2003;261:417–425. [Google Scholar]
- Blake RW. On seahorse locomotion. J Mar Biol Assoc UK. 1976;56:939–949. [Google Scholar]
- Boistel R, Herrel A, Daghfous G, et al. Assisted walking in Malagasy dwarf chamaeleons. Biol Lett. 2010;6:740–743. doi: 10.1098/rsbl.2010.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd EL, Patek SN. Vertebral column morphology, C-start curvature, and the evolution of mechanical defences in tetraodontiform fishes. Copeia. 1998;4:971–984. [Google Scholar]
- Breder CM. The locomotion of fishes. Zoologica. 1926;4:159–297. [Google Scholar]
- Breder CM, Edgerton HE. An analysis of the locomotion of the seahorse, Hippocampus, by means of high speed cinematography. Ann New York Acad Sci. 1942;XLIII:145–172. [Google Scholar]
- Bruner E, Bartolino V. Morphological variation in the seahorse vertebral system. Int J Morphol. 2008;26:247–262. [Google Scholar]
- Chevroton A. Considérations sur les attitudes et la locomotion de l'Hippocampus (étude chronophotographique) Archives de Zool. 1912;51:11–22. [Google Scholar]
- Consi TR, Seifert PA, Triantafyllou MS, et al. The dorsal fin engine of the seahorse (Hippocampus sp.) J Morphol. 2001;248:80–97. doi: 10.1002/jmor.1022. [DOI] [PubMed] [Google Scholar]
- Dawson CE. Indo-Pacific Pipefishes. Ocean Springs, MS: Gulf Coast Research Laboratory; 1985. [Google Scholar]
- Gemballa S, Bartsch P. Architecture of the integument in lower teleostomes: functional morphology and evolutionary implications. J Morphol. 2002;253:290–309. doi: 10.1002/jmor.10007. [DOI] [PubMed] [Google Scholar]
- Gemballa S, Roder K. From head to tail: the myoseptal system in basal actinopterygians. J Morphol. 2004;259:155–171. doi: 10.1002/jmor.10175. [DOI] [PubMed] [Google Scholar]
- Gemballa S, Ebmeyer L, Hagen K, et al. Evolutionary transformations of myoseptal tendons in gnathostomes. Proc R Soc B Biol Sci. 2003;270:1229–1235. doi: 10.1098/rspb.2003.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemballa S, Konstantinidis P, Donley JM, et al. Evolution of high-performance swimming in sharks: transformations of the musculotendinous system from subcarangiform to thunniform swimmers. J Morphol. 2006;267:477–493. doi: 10.1002/jmor.10412. [DOI] [PubMed] [Google Scholar]
- German RZ. The functional morphology of the caudal vertebrae in New World monkeys. Am J Phys Anthropol. 1982;58:453–459. doi: 10.1002/ajpa.1330580414. [DOI] [PubMed] [Google Scholar]
- Gomon MF. A new genus and miniature species of pipehorse (Syngnathidae) from Indonesia. Int J Ichthyol. 2007;13:25–30. [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale ME. Functional morphology of ventral tail bending and prehensile abilities of the seahorse, Hippocampus kuda. J Morphol. 1996;227:51–65. [Google Scholar]
- Hall SJ. Basic biomechanics. Boston: McGraw-Hill; 1999. [Google Scholar]
- Hamilton H, Saarman NP, Moore E, et al. A molecular phylogeny of syngnathid fishes. 8th Annual Indo-Pacific fish conference; Fremantle, WA. 2009. [Google Scholar]
- Hammer O, Harper DAT, Ryan PD. Past: paleontological statistic software package for education and data analysis. Paleontol Electron. 2001;4:1–9. [Google Scholar]
- Hildebrand CM. Analysis of Vertebrate Structure. New York: John Wiley and Sons Inc; 1995. [Google Scholar]
- Jungersen HFE. 1910. The Structure of the Aulostomidae Syngnathidae and Solenostomidae, Høst.
- Kuiter RH. Seahorses and their relatives. Seaford, Australia: Aquatic Photographics; 2009. [Google Scholar]
- Lees J, Märss T, Wilson MVH, et al. The sculpture and morphology of postcranial dermal armor plates and associated bones in gasterosteiforms and syngnathiforms inhabiting Estonian coastal waters. Acta Zool. 2012;93:422–435. [Google Scholar]
- Long JH, Hale ME, McHenry MJ, et al. Functions of fish skin: flexural stiffness and steady swimming of longnose gar Lepisosteus osseus. J Exp Biol. 1996;199:2139–2151. doi: 10.1242/jeb.199.10.2139. [DOI] [PubMed] [Google Scholar]
- Metscher BD. MicroCT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Dev Dyn. 2009;238:632–640. doi: 10.1002/dvdy.21857. [DOI] [PubMed] [Google Scholar]
- Munro ISR. Handbook of Australian Fishes, no. 24. Aust Fish Newsl. 1958;17:97–100. [Google Scholar]
- Near TJ, Dornburg A, Eytan RI, et al. Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. Proc Natl Acad Sci U S A. 2013;110:12738–12743. doi: 10.1073/pnas.1304661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organ JM. Structure and function of platyrrhine caudal vertebrae. Anat Rec (Hoboken) 2010;293:730–745. doi: 10.1002/ar.21129. [DOI] [PubMed] [Google Scholar]
- Peters HM. Beiträge zur ökologischen Physiologie des Seepherdes (Hippocampus brevirostris. Z Vergl Physiol. 1951;33:207–265. [PubMed] [Google Scholar]
- Porter MM, Novitskaya E, Castro-Ceseña AB, et al. Highly deformable bones: unusual deformation mechanisms of seahorse armor. Acta Biomater. 2013;9:6763–6770. doi: 10.1016/j.actbio.2013.02.045. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ. tpsDig: Thin Plate Spline Digitize. Stony Brook: State University of New York at Stony Brook; 2008. [Google Scholar]
- Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985;9:107–119. [Google Scholar]
- Teske PR, Beheregaray LB. Evolution of seahorses' upright posture was linked to Oligocene expansion of seagrass habitats. Biol Lett. 2009;5:521–523. doi: 10.1098/rsbl.2009.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske PR, Cherry MI, Matthee CA. The evolutionary history of seahorses (Syngnathidae: Hippocampus): molecular data suggest a West Pacific origin and two invasions of the Atlantic Ocean. Mol Phylogenet Evol. 2004;30:273–286. doi: 10.1016/s1055-7903(03)00214-8. [DOI] [PubMed] [Google Scholar]
- Van Wassenbergh S, Roos G, Ferry L. An adaptive explanation for the horse-like shape of seahorses. Nat Commun. 2011;2:5. doi: 10.1038/ncomms1168. [DOI] [PubMed] [Google Scholar]
- Weber H. Beiträge zur Bewegungsphysiologie der Hippocampus-Arten. Z Vergl Physiol. 1926;5:1–36. [Google Scholar]
- Wilson AB, Orr JW. The evolutionary origins of Syngnathidae: pipefishes and seahorses. J Fish Biol. 2011;78:1603–1623. doi: 10.1111/j.1095-8649.2011.02988.x. [DOI] [PubMed] [Google Scholar]
- Wilson NG, Rouse GW. Convergent camouflage and the non-monophyly of ‘seadragons’ (Syngnathidae: Teleostei): suggestions for a revised taxonomy of syngnathids. Zoolog Scr. 2010;39:551–558. [Google Scholar]
- Zippel KC, Glor RE, Bertram JEA. On caudal prehensility and phylogenetic constraint in lizards: the influence of ancestral anatomy on function in Corucia and Furcifer. J Morphol. 1999;239:143–155. doi: 10.1002/(SICI)1097-4687(199902)239:2<143::AID-JMOR3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]