Abstract
Background & Aims
Obese patients rarely achieve long-term weight loss with only lifestyle interventions. We evaluated the use of endoscopic aspiration therapy for obesity. Aspiration therapy involves endoscopic placement of a gastrostomy tube (A-Tube) and the AspireAssist siphon assembly (Aspire Bariatrics, King of Prussia, PA) to aspirate gastric contents 20 minutes after meal consumption.
Methods
We performed a pilot study of 18 obese subjects who were randomly assigned (2:1) to groups that underwent aspiration therapy for 1 year plus lifestyle therapy (n = 11; mean body mass index, 42.6 ± 1.4 kg/m2) or lifestyle therapy only (n = 7; mean body mass index, 43.4 ± 2.0 kg/m). Lifestyle intervention comprised a 15-session diet and behavioral education program.
Results
Ten of the 11 subjects who underwent aspiration therapy and 4 of the 7 subjects who underwent lifestyle therapy completed the first year of the study. After 1 year, subjects in the aspiration therapy group lost 18.6% ± 2.3% of their body weight (49.0% ± 7.7% of excess weight loss [EWL]) and those in the lifestyle therapy group lost 5.9% ± 5.0% (14.9% ± 12.2% of EWL) (P < .04). Seven of the 10 subjects in the aspiration therapy group completed an additional year of therapy and maintained a 20.1% ± 3.5% body weight loss (54.6% ± 12.0% of EWL). There were no adverse effects of aspiration therapy on eating behavior and no evidence of compensation for aspirated calories with increased food intake. No episodes of binge eating in the aspiration therapy group or serious adverse were reported.
Conclusions
In a pilot study, aspiration therapy appears to be a safe and effective long-term weight loss therapy for obesity. ClinicalTrials.gov, Number: NCT00773903.
Keywords: Obesity, Endoscopic Bariatric Therapy, Overweight, Percutaneous Endoscopic Gastrostomy
Obesity is a major global health problem because of its high prevalence, causal relationship with a large number of medical comorbidities, adverse effect on quality of life, and considerable economic consequences.1,2 In all persons, obesity is caused by ingesting more energy than is expended over a long period. Accordingly, the principle of obesity therapy is to have patients consume less energy than expended, which mobilizes endogenous adipose tissue triglyceride stores for use as fuel. The current therapeutic approaches for obesity include lifestyle therapy to change eating and physical activity behaviors, pharmacotherapy to reduce food intake or energy absorption, and bariatric surgery to reduce food intake and, in some procedures, also cause malabsorption.3
Although many patients lose 5% to 10% of their body weight with intensive lifestyle therapy,4–6 long-term weight loss maintenance is rarely achieved, with most people regaining lost weight over time.7–9 Pharmacotherapy can provide additional weight loss when used as an adjunct to lifestyle intervention.10,11 Bariatric surgery is the most effective available therapy for obesity, but it is expensive, is associated with serious complications, and can only be performed on a small number of patients per year relative to the number of eligible patients.12–14 The limitations of current obesity treatment options have led to an increased interest in developing endoscopic therapies for obesity. Endoscopic therapy could have several advantages over existing therapies by being more effective than pharmacotherapy and less expensive, safer, and potentially more available than bariatric surgery.
The purpose of this study was to conduct a 1-year clinical randomized controlled trial (RCT) with an additional 1 year of follow-up to evaluate the safety and efficacy of a novel endoscopic therapy for obesity. This approach takes advantage of percutaneous endoscopic gastrostomy (PEG) tube technology to induce weight loss by aspirating a portion of ingested meals from the stomach.
Patients and Methods
Trial Design
This was a 12-month RCT performed at a single center with 2:1 randomization (aspiration therapy plus lifestyle therapy [AT]/lifestyle therapy only [LT]) conducted at the Washington University School of Medicine (St Louis, MO). After completion of the 12-month RCT, subjects in the AT group were allowed to continue participating in the study for an additional 12 months if they lost at least 25% of their excess body weight. Excess body weight was determined as current body weight (in kilograms) minus calculated body weight (in kilograms) at a body mass index (BMI) of 25 kg/m2. The primary study end point was percent absolute weight loss. Secondary study end points were (1) percentage of excess weight loss (EWL) and (2) percentage of subjects achieving >25% EWL. All authors had access to the study data and reviewed and approved the final manuscript.
Participants
Eighteen obese adults (BMI between 40.0 and 50.0 kg/m2 or between 35.0 and 39.9 kg/m2 with comorbidities) recruited between February and October 2009 participated in this study (Table 1). The flow of study participants is shown in Supplementary Figure 1. All subjects completed a comprehensive medical examination, which included a history and physical examination, blood tests, and a 12-lead electrocardiogram. All subjects also completed a careful psychological assessment, including the Eating Disorder Examination (EDE),15,16 Stunkard Eating Inventory,17 and Beck Depression Inventory (BDI-II).18,19 The EDE is a structured interview-based assessment of disordered attitudes and behaviors related to eating, body shape, and weight that has items designed to diagnose eating disorders based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria.15 The Stunkard Eating Inventory is a self-administered questionnaire that assesses 3 behavioral traits that can affect control of body weight: cognitive dietary restraint (deliberate control of intake), disinhibition (loss of control over food intake), and perceived hunger (awareness of and susceptibility to hunger). The BDI-II is a self-administered 21-item questionnaire that assesses the existence and severity of symptoms of depression. Potential subjects were excluded if they had evidence of an eating disorder or major depression, history of gastrointestinal disease or previous gastric surgery that would increase the risk of A-Tube placement, uncontrolled hypertension, sleep apnea, fasting serum glucose level ≥105 mg/dL, diabetes, or serum triglyceride level >400 mg/dL or were pregnant/lactating. In addition, women of childbearing potential were required to be on at least one form of birth control. All subjects were weight stable (<3% change in self-reported weight for at least 3 months before the study). All subjects provided written informed consent before participating in this study, which was approved by Washington University's Institutional Review Board (protocol no. 201111076). This study was registered at ClinicalTrials.gov (NCT00773903).
Table 1. Baseline Subject Characteristics.
| LT group | AT group | P value | |
|---|---|---|---|
| No. (male/female) | 4 (1/3) | 10 (0/10) | |
| Age (y) | 45.3 ± 2.8 | 38.7 ± 2.3 | .129 |
| Weight (kg) | 105.3 ± 2.5 | 112.2 ± 4.6 | .384 |
| BMI (kg/m2) | 39.3 ± 1.1 | 42.0 ± 1.4 | .155 |
| Total cholesterol (mg/dL) | 192.3 ± 13.1 | 189.2 ± 6.1 | .813 |
| Low-density lipoprotein cholesterol (mg/dL) | 116.0 ± 13.0 | 112.8 ± 6.9 | .818 |
| High-density lipoprotein cholesterol (mg/dL) | 48.5 ± 4.1 | 53.6 ± 2.9 | .354 |
| Total triglyceride (mg/dL) | 139.3 ± 12.8 | 113.4 ± 18.8 | .425 |
| Glucose (mg/dL) | 86.8 ± 3.4 | 83.9 ± 1.9 | .448 |
| ALT (IU/L) | 26.8 ± 7.3 | 20.6 ± 2.6 | .325 |
| Magnesium (mEq/L) | 1.6 ± 0.03 | 1.6 ± 0.03 | .395 |
| Calcium (mg/dL) | 9.2 ± 0.17 | 9.2 ± 0.08 | .749 |
| Iron (μm/dL) | 83.8 ± 8.9 | 68.9 ± 8.1 | .308 |
| 25-Hydroxyvitamin D (nmol/L) | 45.2 ± 11.4 | 65.3 ± 3.6 | .128 |
| Vitamin B12 (pg/mL) | 465.3 ± 110.6 | 395.6 ± 60.8 | .567 |
NOTE. Values are expressed as means ± SEM.
Randomization
Eligible subjects were randomized to the AT group or LT group using a 2:1 computer-generated randomization scheme developed by an independent statistician who did not participate in subject enrollment. Randomization allocations were sealed in envelopes, which were opened sequentially by study coordinators as subjects were enrolled in the study.
AspireAssist Components
The device used to perform AT, AspireAssist (Aspire Bariatrics, King of Prussia, PA), consists of the following (Figure 1):
The A-Tube, which has holes in the intragastric portion to allow aspiration of gastric contents.
The Skin-Port, which is a flange 3.5 cm in diameter and 0.9 mm in height that connects to the external end of the A-Tube, contains a valve that is normally closed to prevent gastric leakage and is opened by engaging the connector.
The connector, which mates with the Skin-Port and opens the Skin-Port valve to allow aspiration of gastric contents. In addition, the connector contains a “counter” that tracks the number of times the connector is attached to the Skin-Port. When the count reaches 115 aspiration cycles (approximately 5-6 weeks of therapy), the connector locks and the Skin-Port can no longer be accessed for aspiration. The connector provides an additional safety measure against long-term unsupervised use, and the subject must return to the clinic to obtain a new connector to continue aspiration therapy.
The companion, which is a siphon that allows 2-way flow of fluids (draining stomach contents and infusing water into the stomach).
The reservoir, which is a 600-mL soft water bottle that allows subjects to flush tap water into the stomach to facilitate aspiration.
The drain tube, which provides a clean exit of aspirated gastric contents into the toilet (Figure 1A and B).
Figure 1.
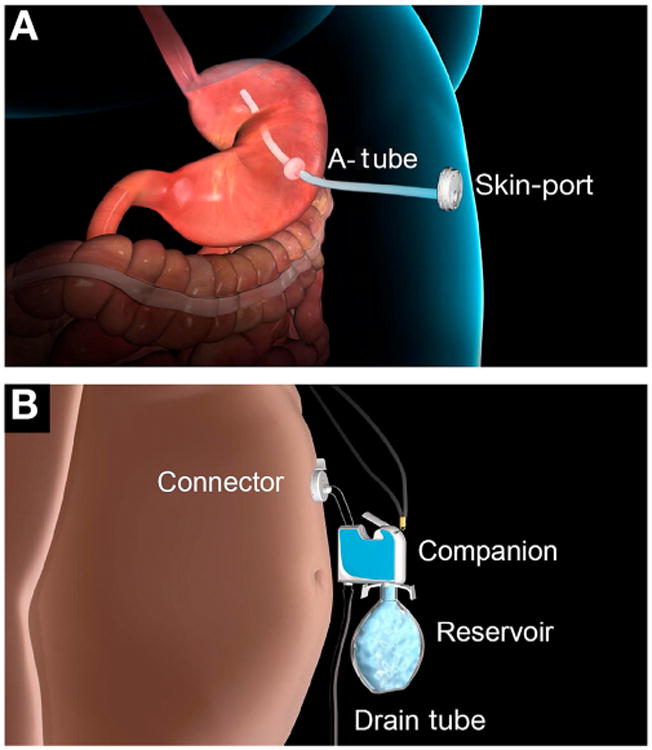
Components of AT. (A) Internal components and Skin-Port and (B) external components.
The original A-Tube contained an extragastric portion made of an expanded polytetrafluoroethylene tube reinforced with a helical expanded polytetrafluoroethylene wire and an intragastric portion composed of silicone. The A-Tube was changed during the study to an all-silicone tube without a helical wrap because subjects reported abdominal discomfort at the fistula site, presumably caused by irritation from the helical wire, and deterioration of the external portion of the tube affected the integrity of the connection to the Skin-Port. The all-silicone A-Tube was placed through the existing fistula tract in all subjects between September and October 2011.
A-Tube Placement and Aspiration Therapy
Subjects reported to the outpatient endoscopy unit after they fasted for ∼ 12 hours overnight. Cefazolin (1 g intravenous bolus) was given 30 minutes before placement of the A-Tube. After completing a full diagnostic upper gastrointestinal endoscopy, the A-Tube placement site was identified by both transillumination of the light from the endoscope and finger indentation in the left upper quadrant of the abdomen. The site was prepped and draped in sterile fashion, and standard pull technique was used to place the A-Tube using a Wilson-Cook 20F or 24F kit (Cook Medical, Bloomington, IN). The endoscope was reintroduced to verify correct placement of the A-Tube. All subjects continued antibiotic prophylaxis (cephalexin 500 mg every 12 hours) for 7 days after the procedure.
Ten to 14 days after placement of the A-Tube, the proximal end of the A-Tube was cut to within 1 cm of the abdominal wall and then attached to the Skin-Port. Subjects were given instructions on how to aspirate after meals and proper care and cleaning of the device. Subjects were instructed to aspirate 20 minutes after breakfast, lunch, and dinner whenever the meal contained more than 200 kcal. To aspirate, subjects flush food particles out through the A-Tube by infusing water into the stomach from the reservoir in 150- to 200-mL increments and then reversing the flow by lowering the lever on the companion to allow contents to drain out of the stomach (Figure 2). This process is repeated as many times as necessary (typically 3-8 infusions) until food particles are no longer seen in the aspirate. This process takes 5 to 15 minutes to perform, depending on the size of the meal consumed. Subjects in the AT group also started treatment with omeprazole (20 mg orally twice daily) and potassium chloride (20 mEq by mouth twice daily) to reduce acid loss and potential potassium depletion. After completion of the 12-month RCT, subjects in the AT group were invited to continue participation in the study if they met the goal of ≥25% EWL.
Figure 2.

Subject performing aspiration.
Blood Tests
A fasting lipid panel, comprehensive metabolic panel, vitamin D level, vitamin B12 level, complete blood count, iron panel, uric acid level, urine analysis, and plasma protein level (albumin and prealbumin) were obtained at weeks 0, 24, and 52 in the LT group and weeks 0, 12, 24, 52, 76, and 104 in the AT group. A basic metabolic panel was obtained at weeks 4, 12, 24, 36, and 52 in the LT group and every 4 weeks in the AT group to monitor plasma electrolyte concentrations. All blood tests were analyzed by an independent laboratory (Quest Diagnostics, St Louis, MO).
Psychological and Eating Behavior Assessments
The following evaluations were performed at baseline and weeks 12, 24, and 52: (1) the EDE,15 (2) Stunkard Eating Inventory, 17 (3) BDI-II,18 and (4) Hunger Visual Analog Scale, which is a self-administered questionnaire to assess hunger, satiety, and motivation to eat.20 The EDE was also performed at weeks 76 and 104 in subjects in the AT group who completed 104 weeks of therapy.
See Supplementary Methods for information on lifestyle therapy intervention and assessment of aspiration efficiency.
Statistical Analyses
Power calculation
The primary end point of the study was the percent weight loss at 12 months. Assuming an average (±SD) weight loss of 2.5% ± 6.4% in the LT group4 and 15.9% ± 8.3% in the AT group (based on unpublished data from a pilot study conducted by the sponsor in Mexico), it was estimated that with 12 subjects in the AT group and 6 subjects in the LT group, we would be able to detect a 13% absolute difference in weight loss between groups at 12 months, with a power of 0.94 and an α value of <0.05. With a total of 10 subjects in the AT group and 4 subjects in the LT group, we would be able to detect a 13% absolute difference in weight loss between groups at 12 months, with a power of 0.8 and an α value of <0.05. Power calculations were performed using G*Power 3.1.2 software (Franz Faul, Universität Kiel, Kiel, Germany).
Data analysis
All data were normally distributed according to the Kolmogorov-Smirnov test. The Student t test for independent samples was used to evaluate the statistical significance of differences in outcome measures between the AT and LT groups. Analysis of variance was used to determine the group × time interaction for serum lipid concentrations, glucose concentrations, and psychological assessment variables. Paired t test was used to determine within-group changes from baseline to week 52 in all subjects and week 104 in those subjects in the AT group who completed 104 weeks of therapy. Independent t tests were used to determine differences in aspiration efficiency due to an unequal number of meal tests. All results are expressed as mean ± SEM unless otherwise stated. A 2-sided P value of ≤.05 was considered statistically significant. Statistical analyses were performed using SPSS version 20.0 for Windows (IBM, Armonk, New York).
Results
Subject Disposition
A total of 14 subjects completed the 12-month RCT (4 subjects in the LT group and 10 subjects in the AT group) and were included in the analysis (Supplementary Figure 1). Transillumination for placement of the A-Tube was successful in all placements. Three subjects in the LT group dropped out of the study. These subjects were called at least 5 times by the study team and a certified letter was sent to their homes; however, further details on why they dropped out of the study could not be obtained. All 10 subjects in the AT group who completed the 12-month RCT met the weight loss criterion needed to continue aspiration therapy for an additional 12 months. Seven of these subjects completed 24 months of aspiration therapy, and 3 subjects discontinued therapy during the second year because of a job change and out-of-state relocation (n = 1), pain at the A-Tube site (n = 1), and personal life issues (n = 1). All 7 subjects who completed 2 years of therapy requested to continue aspiration therapy beyond 2 years.
Weight Loss
The percent weight loss in the AT group was greater than that in the LT group at 52 weeks (18.6% ± 2.3% and 5.9% ± 5.0%; P = .021; Figure 3A [see Supplementary Figure 2 for data on individual weight loss]). No significant change in weight loss occurred from week 52 to week 104 in the 7 subjects who continued aspiration therapy (21.2% ± 2.8% and 20.1% ± 3.5%, respectively; P = .547). The percentage of EWL was also greater in the AT group than in the LT group at 52 weeks (49.0% ± 7.7% and 14.9% ± 12.2%; P = .036; Figure 3B). No significant change in the percentage of EWL occurred from week 52 to week 104 in the 7 subjects who continued aspiration therapy (57.0% ± 9.6% and 54.6% ± 12.0%, respectively; P = .611).
Figure 3.
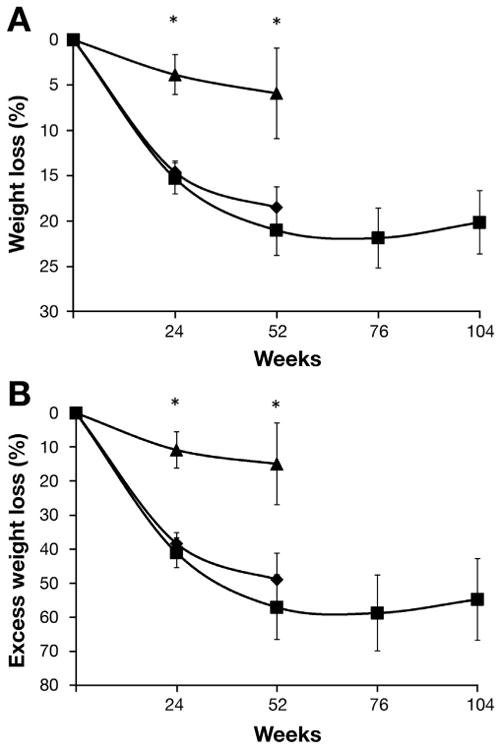
(A) Percentage of absolute weight loss and (B) percentage of EWL in subjects in the LT group (n = 4, black triangles), in subjects in the AT group who completed 52 weeks of therapy (n = 10, black diamonds), and in subjects in the AT group who completed 104 weeks of therapy (n = 7, black squares). *Value significantly different from the corresponding value in the LT group, P < .05. Data are expressed as means ± SEM.
Aspiration Efficiency
The amount of time required for aspiration (∼ 10 minutes) was not different when subjects aspirated at 20 or 60 minutes after either a 450- or 800-kcal meal (Table 2). Approximately 30% of ingested calories were removed by aspiration 20 minutes after consuming a 450- or 800-kcal meal. Aspirating 20 or 60 minutes after consuming the 800-kcal meal did not significantly affect the percentage of calories removed by aspiration. However, the percentage of calories aspirated was greater at 20 minutes than 60 minutes after consuming the 450-kcal meal (P = .023).
Table 2. Aspiration Characteristics.
| 450 kcal | 800 kcal | |||
|---|---|---|---|---|
|
|
|
|||
| 20 min (n = 6) | 60 min (n = 7) | 20 min (n = 7) | 60 min (n = 7) | |
| Amount aspirated (g) | 1747 ± 186 | 1761 ± 197 | 2081 ± 240 | 2034 ± 200 |
| Calories aspirated (% of ingested kcal) | 29.3 ± 4.0 | 17.3 ± 2.5a | 28.3 ± 4.3 | 26.9 ± 5.1 |
| Aspiration time (min) | 10.2 ± 1.1 | 8.5 ± 1.2 | 9.9 ± 1.1 | 8.4 ± 1.1 |
NOTE. Data are expressed as mean ± SEM.
Value significantly different from the corresponding 20-minute value (P = .023).
Plasma Chemistry and Lipid, Iron, 25-Hydroxyvitamin D, and Vitamin B12 Concentrations
Weight loss did not result in any significant changes in plasma glucose, magnesium, calcium, total cholesterol, low-density lipoprotein cholesterol, triglyceride, or high-density lipoprotein cholesterol concentrations in either group. However, baseline values were already within the normal range. There was a trend toward a decrease in plasma alanine transaminase (ALT) concentration in the AT group (20.6 ± 2.6 to 12.8 ± 1.9 IU/L at baseline to week 52, respectively) compared with the LT group (26.8 ± 7.3 to 34.0 ± 18.2 IU/L at baseline to week 52, respectively) (group × time interaction P = .076). Further analysis with paired t tests for within-group changes from baseline to week 52 showed a significant decrease in serum ALT concentration in the AT group only (P = .014). In the AT group, 4 subjects required iron supplementation, 3 subjects required vitamin D supplementation, and one subject required vitamin B12 supplementation. With supplementation, aspiration therapy did not result in a difference in plasma iron, 25-hydroxyvitamin D, or vitamin B12 concentrations at week 52 in the AT group compared with the LT group.
Psychological Assessments
EDE
The use of aspiration therapy did not induce any adverse eating behaviors (Supplementary Table 1). One subject in the LT group reported one binge-eating episode during the 52-week study, defined as consuming a large amount of food accompanied by a feeling of loss of control over eating. No binge-eating episodes occurred in the AT group. Body weight and shape did not become significantly more important, and participants' desired weight did not significantly change (indicating that they did not develop unrealistically low or extreme weight goals) in either the AT or LT group. Discomfort exposing body shape to others decreased in the AT but not the LT group (P = .005), and discomfort seeing one's own body decreased in the AT but not the LT group (P = .012). No changes in EDE scores occurred from week 52 to week 104 in the 7 subjects in the AT group who continued study participation to week 104.
BDI-II
The BDI-II values in the AT group were low at baseline and remained low throughout the study (Supplementary Table 2). One subject in the control group had a baseline value of 14, suggesting the presence of mild depression. The BDI-II score in this subject increased to 28 at week 24 (moderate depression) and decreased to a score of 18 (back to mild depression) at week 52 without psychiatric or antidepressant therapy. No subject in either group had evidence of suicidal ideation at any time point.
Stunkard Eating Inventory
All 3 behavioral traits improved in the AT group; subjects showed improved restraint, less disinhibition (more control over food intake), and decreased hunger (Supplementary Table 3). The control group showed improved restraint but no change in disinhibition or hunger.
Hunger Visual Analogue Scale
No significant changes in any measures of the visual analogue scale were detected in either the AT or LT groups, and there were no differences between groups (Supplementary Table 4). There was no evidence of increased hunger, increased thoughts about food, increased cravings, or decreased feeling of fullness in the AT group despite regular meal aspiration.
Adverse Events
No serious adverse events occurred in either the LT or AT group. The most common adverse events included peristomal pain within the first 4 weeks after placement of the A-Tube, peristomal pain more than 4 weeks after placement of the A-Tube, peristomal irritation, and constipation (Table 3). Pain within the first 4 weeks after placement of the A-Tube was related to placement of the tube through the abdominal wall and resolved within 4 weeks of placement. Pain more than 4 weeks after placement was different in character and believed to be related to the original tube design. This resolved in all but 2 subjects after the original A-Tubes were replaced with the redesigned A-Tubes. Three infections occurred; 2 were peristomal cutaneous candidal infections treated with improved hygiene and nystatin cream, and one was a presumed peristomal soft tissue infection diagnosed by computed tomography and treated with oral cephalexin. Nausea with or without emesis occurred in 7 of 11 subjects after placement of the A-Tube; this resolved within 4 weeks and could have been related to the procedure or the A-Tube. All adverse events resolved with observation or conservative medical treatment. One episode of hypokalemia occurred (serum potassium concentration of 3.4 mEq/L) due to patient noncompliance with potassium supplementation. This resolved with reinforcement of the importance of compliance with study-related medications. The average serum potassium concentration in the AT group at baseline, week 24, and week 52 was 4.2 ± 0.2, 4.1 ± 0.3, and 4.1 ± 0.3, respectively. Although A-Tube blockages were not considered adverse events, 5 episodes of A-Tube blockage occurred during the 2-year trial. These were treated conservatively with an endoscopy brush in the outpatient setting.
Table 3. Adverse Events in Subjects Randomized to the AT Group.
| Adverse event | No. of subjects affected | No. of reports | Severity |
|---|---|---|---|
| Abdominal discomfort/pain within 4 wk after A-tube placement | 11 | 13 | Minimal to moderate |
| Abdominal discomfort/pain >4 wk after A-tube placement | 10 | 34 | Minimal to moderate |
| Pain (other) | 4 | 4 | Minimal to mild |
| Peristomal skin irritation | 6 | 9 | Minimal to moderate |
| Peristomal bleeding | 5 | 9 | Minimal to mild |
| Peristomal infection | 3 | 3 | Minimal to mild |
| Bloating, gas, burping, cramps | 5 | 13 | Minimal to moderate |
| Constipation | 8 | 14 | Minimal to moderate |
| Diarrhea | 3 | 3 | Minimal |
| Nausea and/or vomiting | 7 | 11 | Minimal to mild |
| Anemia | 4 | 4 | Minimal |
| Sore throat after A-Tube placement procedure | 5 | 7 | Minimal |
| Decreased appetite | 2 | 2 | Minimal |
| Shortness of breath | 1 | 1 | Minimal |
| Insomnia | 1 | 1 | Moderate |
| Thirst | 1 | 2 | Minimal |
| Pruritus | 1 | 1 | Mild |
| Persistent fistula after A-Tube removal | 1 | 1 | Moderate |
Minimal, awareness of the symptom but easily tolerated; mild, tolerated with some difficulty; moderate, interference with some normal daily activities; significant, inability to perform normal daily activities; severe, requires hospitalization.
Discussion
The purpose of this study was to evaluate the safety and efficacy of aspiration therapy as a novel endoscopic weight loss approach for obese people. The results of this pilot study show that subjects in the AT group had greater weight loss than those in the LT group at 1 year and that the weight loss achieved in the AT group at 1 year was maintained at 2 years. The amount of weight loss achieved with AT (∼ 19% of initial body weight) was much greater than that usually observed with intensive lifestyle therapy alone8,9 or obesity pharmacotherapy10 and nearly the same as that observed after laparoscopic adjustable gastric banding.21 Weight regain, which is typically seen after 1 year of intensive weight loss therapy8,9 and at 1 to 10 years after bariatric surgery,22-24 was not seen in this study. In addition, although serious complications and death occur in 2.5% to 4.2% and 0.04% to 0.3% of patients who undergo bariatric surgery, respectively,25-27 these events did not occur in any subject in the AT group. Furthermore, there was no evidence of adverse effects on eating patterns, eating disorder psychopathology, or hunger in the AT group. These data show that aspiration therapy may be a safe and effective long-term treatment option for people with obesity.
Although aspiration after meals resulted in removal of ∼30% of ingested energy, this reduction in calories would account for only about 80% of the total amount of weight loss, even if subjects were completely compliant by aspirating after all main meals within 20 minutes of meal completion.28,29 Therefore, it is likely that subjects in the AT group also decreased their daily energy intake. The precise mechanisms responsible for the effect of aspiration therapy on food intake are not known, but several possibilities were reported by the study subjects. First, the dietary changes needed for effective aspiration therapy also reinforce the basic behavioral principles of weight management, such as increased chewing of food and drinking more water with each meal to facilitate meal aspiration, limiting between-meal snacks to avoid the need for additional aspirations, and developing a structured meal plan to accommodate aspiration inside and outside the home. Second, the fistula formed between the stomach and abdominal wall because of the A-Tube implant could affect gastric distention needed to accommodate large amounts of food and result in earlier satiation during meals. Finally, the use of aspiration therapy lowers the threshold needed to achieve successful weight loss because it reduces the stress around meals and empowers subjects to feel in control of their food choices. Additional studies are needed to further investigate the potential mechanisms responsible for the decrease in energy intake induced by aspiration therapy along with carful dietary analysis to determine the percentage of weight loss derived from calorie reduction.
An important finding of this study is that there was no evidence of an increase in food intake during main meals or between main meals to compensate for aspirated calories in the AT group, despite removing up to 30% of the calories consumed in a meal. This observation has important implications in understanding the drive to eat in obese people. In the past 15 years, sophisticated studies conducted in rodent models have led to a better understanding of the complex adipose tissue-gut-brain interactions involved in regulating food intake.30 However, the psychological factors, such as liking and wanting food, stress eating, and social influences, are poorly understood.31,32 The results of the present study suggest that psychosocial factors and the reward of smelling, chewing, and tasting food are important drivers of food intake in obese people rather than a physiological signal for more calories to meet a specific set point. Accordingly, removal of up to one-third of the calories consumed did not result in subsequent compensation with increased food intake. The central mechanisms that explain satiation (level of fullness during a meal) and satiety (level of hunger after a meal is consumed) presumably functioned normally in our subjects despite the decrease in absorbed calories; however, this study was not designed to investigate these mechanisms. Further studies are required to understand the effects of aspiration therapy on the regulation of food intake.
Although no serious adverse events occurred, multiple mild to moderate adverse events were identified that resolved with observation or conservative medical management. The most common adverse event was abdominal pain at the A-Tube site. This symptom prompted a redesign of the A-Tube to a smooth, all-silicone tube from the original ridged, expanded polytetrafluoroethylene tube, which resulted in a marked decrease in symptoms of pain. Therefore, it is likely that the pain experienced by subjects at the beginning of the trial was related to the design of the tube. Infection was another important adverse event; however, 2 of the infections were local candida infections that were treated with topical medications and improved hygiene of the A-Tube site. One presumed soft tissue infection occurred that did resolve with oral antibiotics but was not verified with bacterial culture. One of the 4 subjects who had the A-Tube removed had a persistent fistula. Three endoscopies were performed to treat the persistent fistula; however, the third endoscopy revealed that the tract had closed spontaneously. No other additional procedures were needed for the fistulas to close after the other A-Tube removals. In addition, despite aspirating gastric contents after meals, plasma potassium concentrations remained within the normal range throughout the study. Although therapy with a proton pump inhibitor was used to reduce acid losses and daily oral potassium supplementation was instituted to help ensure plasma potassium concentrations remained normal, further studies are needed to determine whether this prophylactic treatment is essential. It is notable that despite the removal of ingested calories after meal consumption in the AT group, careful psychological and eating assessments did not find any evidence that aspiration therapy had adverse effects on mood, eating behavior, eating disorder psychopathology, attitudes toward eating, or perceived hunger or satiety.
This study has several important limitations. First, this study contained a small number of relatively healthy obese subjects. Although the number of subjects was adequate to demonstrate the clinical weight loss efficacy of aspiration therapy, this study was not powered to detect all of the possible adverse events that could occur with this therapy. However, it is likely that the complications of an implanted A-Tube are similar to those of standard PEG tubes, which have been carefully characterized from experience in thousands of patients in the past 30 years.33–35 Moreover, PEG tubes have been placed successfully in obese patients with few complications in the hands of experienced endoscopists.36,37 Second, the subjects enrolled in this study did not have any serious obesity-related comorbidities, which limited our ability to detect a therapeutic effect of aspiration therapy on the medical complications of obesity or cardiovascular disease risk factors. Nonetheless, we were able to detect a decrease in plasma ALT concentrations in the AT group at 1 year, which suggests subjects may have had excess intrahepatic triglyceride levels at baseline that decreased with weight loss38 and may decrease participants' risk of developing diabetes.39 Third, this study was not blinded, and this may have introduced bias in the AT group because subjects knew they were getting additional therapy and may have biased the interviewers administering the EDE. Furthermore, subjects in the AT group had additional contact with the study team. Although this additional contact was solely to manage the A-tube site and any adverse events related to the tube, this may also have influenced the subjects in the AT group and led to increased weight loss. Fourth, careful dietary analysis was not performed, and therefore the percentage of weight loss related to aspiration is only a rough estimate.
In summary, the use of the AspireAssist system provides a novel approach for weight loss and has the potential to bridge an important gap in efficacy between current medical and surgical therapies for obesity. The results of the present study show that aspiration therapy results in considerable weight loss for up to 2 years in obese people, with minimal adverse events and no evidence of harmful effects on eating behaviors. In fact, based on the bomb calorimetry of aspirated gastric fluid, food intake likely decreased with therapy and accounted for at least 20% of the weight loss seen in this pilot study. The AspireAssist system has multiple advantages compared with the currently available bariatric surgical procedures. The A-Tube is easily placed without the need for general anesthesia, and it can be easily removed if it is decided to discontinue therapy. The aspiration technique is easily mastered by the patient, and placement and care of the device cost less than current bariatric surgeries. The techniques used for placing and retrieving the A-Tube are the same as those for PEG tubes and are part of the standard training provided in gastroenterology fellowship programs. Therefore, most gastroenterologists already have the skills needed to place and manage this device and would require only minimal additional instruction. Moreover, subjects who completed 2 years of therapy wanted to continue therapy beyond the 2-year study, indicating that the long-term acceptability of this therapy may be high. Although this study was limited to patients with a BMI up to 50 kg/m2, there were no difficulties placing the A-Tube, which suggests that obese people with higher BMIs could be candidates for this procedure. This may allow many obese people to avoid bariatric surgery but would not prevent a patient from undergoing bariatric surgery if needed in the future. The results from this pilot study support further research of aspiration therapy as an endoscopic approach for treating people with obesity.
Supplementary Material
Supplementary Figure 1. Flow of study participants.
Supplementary Figure 2. Individual percent weight loss for the LT group (dashed lines) and the AT group (solid lines).
Supplementary Table 1. EDE at Baseline and Change at Week 52
Supplementary Table 2. BDI-II at Baseline, Week 24, and Week 52
Supplementary Table 3. Stunkard Eating Inventory
Supplementary Table 4. Hunger Visual Analogue Scale
Supplementary Table 5. Recommended Energy Intake for Study Subjects Based on Initial Body Weight and Group Assignment
Acknowledgments
The authors would like to thank Samuel Klein for the design of the study. The authors also would like to thank Melisa Moore, RN, and Courtney Lueking, RD, who were dedicated to the completion of the study and to our study subjects.
Funding: Supported by Aspire Bariatrics and grant UL1 TR000448.
Abbreviations used in this paper
- ALT
alanine aminotransferase
- AT
aspiration therapy plus lifestyle therapy
- BDI-II
Beck Depression Inventory
- BMI
body mass index
- EDE
Eating Disorder Examination
- EWL
excess weight loss
- LT
lifestyle therapy only
- PEG
percutaneous endoscopic gastrostomy
- RCT
randomized controlled trial
Footnotes
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2013.08.056.
Conflicts of interest: The authors disclose the following: Dr Sullivan has performed contracted research for Aspire Bariatrics, ReShape Medical, and GI Dynamics. Dr Stein has performed contracted research for Aspire Bariatrics, EnteroMedics Inc, and Orexigen Therapeutics, Inc, and has served as a consultant for Aspire Bariatrics on a pivotal trial. Dr Jonnalagadda has performed contracted research for Aspire Bariatrics. Dr Mullady has performed contracted research for Aspire Bariatrics. Dr Edmundowicz has performed contracted research for Aspire Bariatrics, ReShape Medical, and GI Dynamics and has served as a consultant for GI Dynamics.
References
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 3.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- 4.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 5.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the look AHEAD Study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadden TA, Frey DL. A multicenter evaluation of a proprietary weight loss program for the treatment of marked obesity: a five-year follow-up. Int J Eat Disord. 1997;22:203–212. doi: 10.1002/(sici)1098-108x(199709)22:2<203::aid-eat13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 11.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 12.Sugerman HJ, Kellum JM, Engle KM, et al. Gastric bypass for treating severe obesity. Am J Clin Nutr. 1992;55:560S–566S. doi: 10.1093/ajcn/55.2.560s. [DOI] [PubMed] [Google Scholar]
- 13.Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93:s89–s96. doi: 10.1210/jc.2008-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumon KR, Murayama KM. Bariatric surgery outcomes. Surg Clin North Am. 2011;91:1313–1338. doi: 10.1016/j.suc.2011.08.014. x. [DOI] [PubMed] [Google Scholar]
- 15.Cooper Z, Cooper PJ, Fairburn CG. The validity of the eating disorder examination and its subscales. Br J Psychiatr. 1989;154:807–812. doi: 10.1192/bjp.154.6.807. [DOI] [PubMed] [Google Scholar]
- 16.Fairburn CG, Cooper Z. Binge eating: nature, assessment and treatment. In: Fairburn CG, Wilson G, editors. The eating disorder examination. 12th. New York, NY: Guilford Press; 1993. pp. 317–360. [Google Scholar]
- 17.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosini PJ, Metz C, Bianchi MD, et al. Concurrent validity and psychometric properties of the Beck Depression Inventory in outpatient adolescents. J Am Acad Child Adolesc Psychiatry. 1991;30:51–57. doi: 10.1097/00004583-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 20.Flint A, Raben A, Blundell JE, et al. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 21.Cunneen SA. Review of meta-analytic comparisons of bariatric surgery with a focus on laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2008;4:S47–S55. doi: 10.1016/j.soard.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Himpens J, Verbrugghe A, Cadiere GB, et al. Long-term results of laparoscopic Roux-en-Y Gastric bypass: evaluation after 9 years. Obes Surg. 2012;22:1586–1593. doi: 10.1007/s11695-012-0707-z. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson J, Taft C, Ryden A, et al. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond) 2007;31:1248–1261. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 24.Langer FB, Prager G, Poglitsch M, et al. Weight loss and weight regain-5-year follow-up for circular- vs. linear-stapled gastrojejunostomy in laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2013;23:776–781. doi: 10.1007/s11695-013-0892-4. [DOI] [PubMed] [Google Scholar]
- 25.Finks JF, Kole KL, Yenumula PR, et al. Predicting risk for serious complications with bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Ann Surg. 2011;254:633–640. doi: 10.1097/SLA.0b013e318230058c. [DOI] [PubMed] [Google Scholar]
- 26.Wahed AS, Berk P, Chapman W, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta PK, Franck C, Miller WJ, et al. Development and validation of a bariatric surgery morbidity risk calculator using the prospective, multicenter NSQIP dataset. J Am Coll Surg. 2011;212:301–309. doi: 10.1016/j.jamcollsurg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Thomas DM, Schoeller LA, Redman LA, et al. Pennington Biomedical Research Center. Weight loss predictor. [Accessed September 20, 2012]; Available at: http://www.pbrc.edu/research-and-faculty/calculators/weight-loss-predictor/
- 29.Thomas DM, Schoeller DA, Redman LA, et al. A computational model to determine energy intake during weight loss. Am J Clin Nutr. 2010;92:1326–1331. doi: 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 31.French SA, Epstein LH, Jeffery RW, et al. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite. 2012;59:541–549. doi: 10.1016/j.appet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadden TA, Butryn ML. Behavioral treatment of obesity. Endocrinol Metab Clin North Am. 2003;32:981–1003. doi: 10.1016/s0889-8529(03)00072-0. x. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson FB, Korman MG, Richardson MA. Percutaneous endoscopic gastrostomy: a review of indications, complications and outcome. J Gastroenterol Hepatol. 2000;15:21–25. doi: 10.1046/j.1440-1746.2000.02004.x. [DOI] [PubMed] [Google Scholar]
- 34.Ljungdahl M, Sundbom M. Complication rate lower after percutaneous endoscopic gastrostomy than after surgical gastrostomy: a prospective, randomized trial. Surg Endosc. 2006;20:1248–1251. doi: 10.1007/s00464-005-0757-6. [DOI] [PubMed] [Google Scholar]
- 35.Finocchiaro C, Galletti R, Rovera G, et al. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13:520–523. doi: 10.1016/s0899-9007(97)00030-0. [DOI] [PubMed] [Google Scholar]
- 36.Wiggins TF, Garrow DA, DeLegge MH. Evaluation of percutaneous endoscopic feeding tube placement in obese patients. Nutr Clin Pract. 2009;24:723–727. doi: 10.1177/0884533609349250. [DOI] [PubMed] [Google Scholar]
- 37.Bochicchio GV, Guzzo JL, Scalea TM. Percutaneous endoscopic gastrostomy in the supermorbidly obese patient. JSLS. 2006;10:409–413. [PMC free article] [PubMed] [Google Scholar]
- 38.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 39.Fraser A, Harris R, Sattar N, et al. Alanine aminotransferase, γ-glutamyltransferase, and incident diabetes. Diabetes Care. 2009;32:741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Flow of study participants.
Supplementary Figure 2. Individual percent weight loss for the LT group (dashed lines) and the AT group (solid lines).
Supplementary Table 1. EDE at Baseline and Change at Week 52
Supplementary Table 2. BDI-II at Baseline, Week 24, and Week 52
Supplementary Table 3. Stunkard Eating Inventory
Supplementary Table 4. Hunger Visual Analogue Scale
Supplementary Table 5. Recommended Energy Intake for Study Subjects Based on Initial Body Weight and Group Assignment


