Abstract
Drug-induced phospholipidosis (PLD) is a common adverse effect which has led to the termination of clinical trials for many candidate pharmaceuticals. However, this lipid-inducing effect may be beneficial in the treatment of meibomian gland dysfunction (MGD). MGD is the major cause of dry eye disease (DED), which affects 40 million people in the USA and has no cure. Azithromycin (AZM) is a PLD-inducing antibiotic that is used off-label to treat MGD, and is presumably effective because it suppresses the MGD-associated conjunctival inflammation (i.e. posterior blepharitis) and growth of lid bacteria. We hypothesize that AZM can act directly to promote the function of human meibomian gland epithelial cells by inducing PLD in these cells, characterized by the accumulation of lipids and lysosomes. We cultured immortalized human meibomian gland epithelial cells (HMGEC) were cultured with or without azithromycin for 5 days. Cells were evaluated for cholesterol (Filipin) and neutral lipid (LipidTox) staining, as well as the appearance of lysosomes (LysoTracker) and lamellar bodies (transmission electron microscopy, TEM). The lipid composition of cellular lysates was analyzed by high performance thin-layer chromatography. Our findings demonstrate that AZM stimulates the accumulation of free cholesterol, neutral lipids and lysosomes in HMGEC. This AZM-induced increase of neutral lipid content occurred predominantly within lysosomes. Many of these vesicles appeared to be lamellar bodies by TEM, which is the characteristic of PLD. Our findings also show that AZM promotes an accumulation of free and esterified cholesterol, as well as phospholipids in HMGEC. Our results support our hypothesis and confirm the beneficial effect of PLD induced by AZM on HMGEC. Our discovery reveals a new potential use of PLD-inducing drugs, and makes this adverse effect a beneficial effect.
Keywords: Azithromycin, Phospholipidosis, Cationic amphiphilic drugs, Meibomian gland dysfunction, Dry eye disease
1. Introduction
Drug-induced phospholipidosis (PLD) is an excessive intracellular accumulation of phospholipid, characterized by the formation of distinct, onion-shaped secretory lysosomes, termed lamellar bodies (Anderson and Borlak 2006). Drug-induced PLD can be caused by many drugs, especially cationic amphiphilic drugs (CADs) (Anderson and Borlak 2006). It is considered to be a major problem for the pharmaceutical industry because of potential toxicity and the huge expense to screen out PLD-inducing drugs each year (Shayman and Abe 2013).The development of some lead compounds has been terminated when PLD was seen in certain organs in clinic trials (Shayman and Abe 2013).The mechanism of PLD has been linked to enhanced cholesterol synthesis (Lowe et al. 2012), but its significance to humans is unclear. Although it is generally considered as a “poisonous” effect that the pharmaceutical industry wants to eliminate, we think this lipid accumulation effect may be beneficial in the treatment of meibomian gland dysfunction (MGD).
MGD is the most common cause of dry eye disease (DED), which afflicts tens of millions of people in the United States, and is one of the leading reasons for patient visits to eye care practitioners (2007), Meibomian glands normally produce abundant lipids (e.g. cholesterol and phospholipids), that accumulate in lysosomes, are secreted in a holocrine manner into lateral ducts, and ultimately released onto the ocular surface (Green-Church et al. 2011; Knop et al. 2011) (Unpublished results). These secretions enhance the stability and prevent the evaporation of the tear film, thereby playing a critical role in the wellbeing of the eye (Green-Church et al. 2011; Knop et al. 2011). However, MGD, and the associated lipid deficiency, disrupts this process, destabilizes the tear film, increases its evaporation and promotes DED (Ding and Sullivan 2012; Knop et al. 2011). MGD also facilitates bacterial growth on the lid margin and inflammation in the adjacent conjunctiva (e.g. posterior blepharitis) (Knop et al. 2011). There is no global cure for MGD.
We hypothesize that azithromycin (AZM), a potent PLD-inducing CAD (Ribeiro et al. 2009; Tyteca et al. 2001), can elicit a PLD-like effect in human meibomian gland epithelial cells and serve as a treatment for MGD. More specifically, we hypothesize that AZM will increase cholesterol and phospholipid levels, stimulate the formation of the lamellar bodies, and promote lipid accumulation in the lamellar lysosomes of these cells. Our goal was to test this hypothesis.
2. Material and Methods
2.1 Cell cultures
Immortalized human meibomian gland epithelial cells (Liu et al. 2010) were cultured in the presence or absence of 10% fetal bovine serum, according to published techniques (Liu et al. 2013). After reaching 80 to 90% confluence (∼ 5 × 10/6well), cells were exposed to the vehicle (0.02% ethanol) or azithromycin (10 μg/ml; Santa Cruz Biotechnology, TX) for 5 days and then processed for histological and biochemical procedures.
2.2 Cell analyses
Cell numbers were enumerated with a hemocytometer. Cellular lipid and lysosome accumulation were examined by staining cells with: [a] Filipin III (Sigma-Aldrich, Saint Louis, MO), a fluorometric probe for identifying unesterified cholesterol; [b] LipidTOX green neutral lipid stain (Invitrogen, Grand Island, NY); and [c] LysoTracker® Red DND-99 (Invitrogen), a fluorescent technique designed for labeling acidic organelles (e.g. lysosomes). Lysosomal lamellar bodies were identified by transmission electron microscopy. In brief, after culture and treatment on 24 mm polycarbonate membrane transwell inserts (0.4 μm pore size; Corning Incorporated, Corning, NY), cells were fixed in Karnovsky solution (2% formaldehyde + 2.5% glutaraldehyde, in 0.08 M sodium cacodylate buffer, pH 7.4; Electron Microscopy Sciences, Hatfield, Pennsylvania), post-fixed with 2% osmium tetroxide in 0.1M sodium cacodylate buffer, and staineden bloc with 2% aqueous uranyl acetate. Samples were then dehydrated in graded ethyl alcohol solutions and embedded by using the tEPON-812 epoxy resin kit (Tousimis, Rockville, Maryland). Cross sections (1 μM) were stained with 1% toluidine blue in 1% sodium tetraborate solution. Ultrathin sections (60-90 nm) were imaged with a FEI Tecnai G2 Spirit transmission electron microscope (Hillsboro, Oregon) interfaced with an AMT XR41 digital CCD camera (Advanced Microscopy Techniques, Woburn, Massachusetts). For biochemical assessments, cellular lipids were extracted with chloroform and methanol from samples with the same amount of protein (Suzuki et al. 2012). Lipids were then evaluated with high-performance thin-layer chromatography (HPTLC, Silica Gel 60, Merck, Darmstadt, Germany) (Bligh and Dyer 1959; Miyazaki et al. 2001; Persson et al. 2006; Ponec et al. 1988).
With regard to the staining results, three Filipin, five LipidTox and four LysoTracker studies were conducted, and each experiment was performed in duplicate. Four random pictures were taken of each group, and four random areas were selected for the measurement of fluorescent intensities. The HPTLC experiments were performed in duplicate more than three times. Image data were quantified with ImageJ (http://rsbweb.nih.gov/ij/index.html). Electron microscopic analyses were performed in triplicate. Statistical analyses were performed with Student's t-test (two-tailed, unpaired).
3. Results
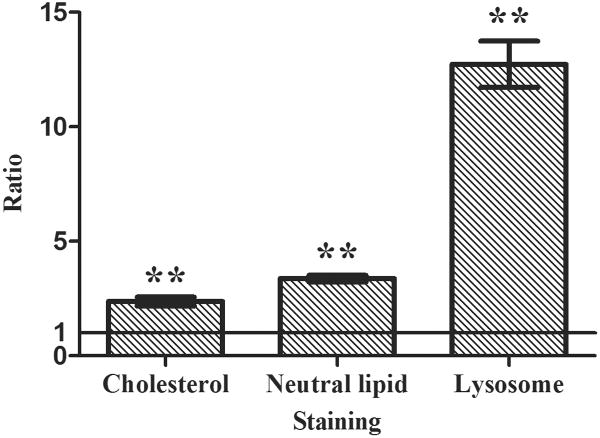
Our results demonstrate that AZM significantly stimulates the accumulation of free cholesterol, neutral lipids and lysosomes in human meibomian gland epithelial cells (Figure 1a and 1c). As shown in Figure 1b, this AZM-induced increase of neutral lipid content occurred predominantly within lysosomes. Furthermore, our electron microscopic analyses reveal that many of these lysosomes appear to be onion-shaped lamellar bodies (Figure 2).
Figure 1. Effect of AZM on intracellular accumulation of lipids and lysosomes.
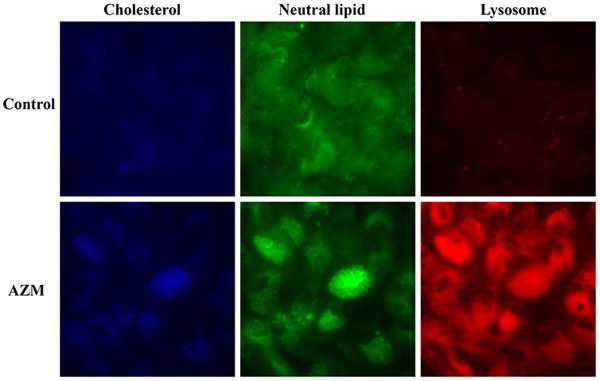
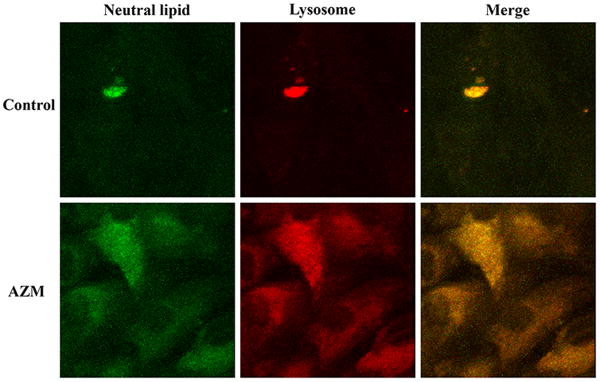
Cells were treated with vehicle or 10μg/ml AZM for 5 days. a. The blue color is Filipin stain indicating free cholesterol, green color is LipidTOX green neutral lipid stain, and red color is LysoTracker red stain indicating lysosomes. Images were obtained with a fluorescent microscope. b. Cells were stained as in (a), and images were obtained with a confocal microscope in order to show colocalization of neutral lipids and lysosomes. c. The fluorescence intensity was measured by using ImageJ; control image intensity was set to 1 and data (mean ± SE) are reported as fold-change compared to control values. **p < 0.005 versus control. Free choleserol staining was repeated 3 times, neutral lipid staining 5 times and lysosome staining 4 times. All results shown are from a single experiment.
Figure 2. Influence of AZM on the accumulation of lysosomal lamellar bodies in human meibomian gland epithelial cells.
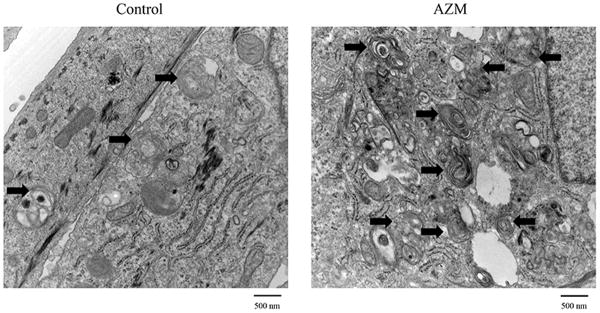
TEM images were obtained after cellular exposure to vehicle or 10μg/ml AZM for 5 days. Arrows indicate the lamellar bodies. Bar=500 nm
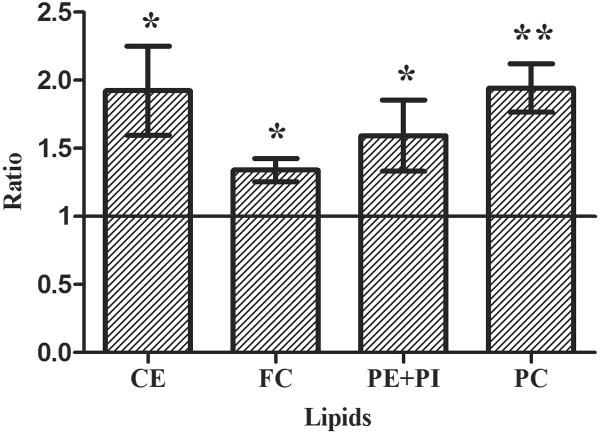
Our biochemical studies indicate that AZM significantly promotes an accumulation of free and esterified cholesterol, as well as phosphatidylethanolamine, phosphatidylcholine and phosphatidylinositol in human meibomian gland epithelial cells (Figure 3).
Figure 3. The effect of AZM on the expression of cholesterol ester (CE), free cholesterol (FC), phosphatidylethanolamine (PE) phosphatidylcholine (PC) and phosphatidylinositol (PI) in human meibomian gland epithelial cells.
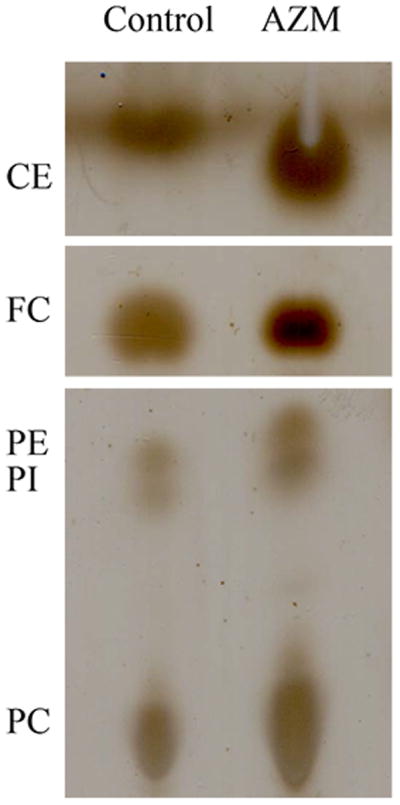
a. Cells were treated with vehicle or 10μg/ml AZM for 5 days before performing chromatographic analyses of total lipid extracts. b. Band intensity was measured by using ImageJ; control band instensity was set to 1, and data (mean ± SE) are reported as fold-change compared to control values. *p < 0.05, **p < 0.005, versus control. Band intensity analysis includes data from at least three independent expreiments.
4. Discussion
Our study supports our hypothesis that AZM induces a PLD-like effect in human meibomian gland epithelial cells. This macrolide antibiotic significantly stimulates the cellular accumulation of free and esterified cholesterol, neutral lipids, phospholipids and lysosomes. The increase of neutral lipid content occurred predominantly within lysosomes, many of which appeared to be lamellar bodies. Given our findings, it is possible that topical AZM could be beneficial in the treatment of human MGD.
We hypothesized that AZM would induce such a PLD-like effect in human meibomian gland epithelial cells for several reasons. First, AZM is a CAD and more than 50 marketed and experimental pharmaceuticals containing a CAD structure have been reported to elicit PLD (Sadrieh 2010). Second, AZM has been shown to promote the accumulation of phospholipids, cholesterol and lysosomal lamellar bodies in other cells (Gerbaux et al. 1996; Munic et al. 2011; Ribeiro et al. 2009; Tyteca et al. 2001; Van Bambeke et al. 1998; Van Bambeke et al. 1996). The hallmark feature, and indeed the gold standard, for identifying PLD is the demonstration of an intracellular accumulation of phospholipids and the concurrent development of lamellar bodies (Reasor and Kacew 2001; Sadrieh 2010). A lamellar body is a type of lysosome specialized for lipid storage and secretion (Baronas et al. 2007; Dietl et al. 2001; Rabionet et al. 2013; Schmitz and Muller 1991; Weaver et al. 2002), and concentric vesicles like lamellar bodies appear to accumulate lipids in the meibomian gland (Jester et al. 1981; Sirigu et al. 1992). Third, we have recently discovered that AZM can act directly on human meibomian gland epithelial cells to seemingly stimulate their maturation and holocrine-like secretion (Liu et al. 2014). The effective concentration (i.e. 10μg/ml) of AZM in vitro in that and the present study is clinically relevant. Following the topical application of 0.5, 1.0 and 1.5% AZM eye drops, the tear concentration of AZM remained above 7 μg/ml for 24 hours (Chiambaretta et al. 2008).
Of particular note, the AZM-induced generation of lysosomes and the accumulation of lipids within these vesicles are analogous to events that typically occur during the differentiation of human meibomian gland epithelial cells. This cellular process is characterized by a pronounced increase in lysosome number and lipid production, and culminates with a profusion of lipid-filled vesicles and nuclear pyknosis. Following this terminal differentiation cells undergo holocrine secretion, which entails autophagy, apoptosis, disintegration and release of lipid-laden contents into glandular ductules (Brandes and Bertini 1965; Knop et al. 2011; Mesquita-Guimarãtes and Coimbra 1976; Mesquita-Guimarãtes et al. 1979; Sirigu et al. 1992; Thody and Shuster 1989).
The ability of AZM to stimulate the differentiation, and apparently secretion, of human meibomian gland epithelial cells is clinically very significant. This action could explain why the off-label use of topical AZM is the most commonly used pharmaceutical treatment for MGD in the United States (Lemp and Nichols 2009). This antibiotic's efficacy has been presumed to be due to its anti-inflammatory and anti-bacterial actions, which may suppress the MGD-associated posterior blepharitis and growth of lid bacteria (Foulks et al. 2013; Foulks et al. 2010; Geerling et al. 2011). However, our data indicate that AZM has the capability to enhance directly the function of human meibomian gland epithelial cells, and thereby possibly ameliorate the pathophysiology of MGD.
In summary, our studies demonstrate that the PLD induced by AZM, although often considered an adverse, “poisonous” side effect in many cell types, may be beneficial in the treatment of MGD. This is not the first time the “side effect” of a drug was found to be helpful in another tissue. For example, sildenafil citrate, or Viagra, was originally created to treat high blood pressure and angina pectoris, but its “side effect” was beneficial for patients with erectile dysfunction. As the old saying goes, one man's poison is another man's meat. Our discovery opens a new potential use for PLD-inducing drugs, such as CADs. Instead of a “poisonous” problem to the pharmaceutical industry, they could be “meat” for the treatment of MGD.
Acknowledgments
We thank Donald Pottle and Philip Seifert, MS, (Boston, MA) for providing technical support.
Funding information: This research was supported by NIH grants EY05612 and EY003790, the Margaret S. Sinon Scholar in Ocular Surface Research Fund, and the Guoxing Yao Research Fund.
Abbreviation
- PLD
phospholipidosis
- CADs
cationic amphiphilic drugs
- MGD
meibomian gland dysfunction
- DED
dry eye disease
- AZM
azithromycin
- HMGEC
Immortalized human meibomian gland epithelial cells
- TEM
transmission electron microscopy
- CE
cholesterol ester
- FC
free cholesterol
- PE
phosphatidylethanolamine
- PC
phosphatidylcholine
- PI
phosphatidylinositol
Footnotes
Conflict of Interest: A provisional patent has been filed around this technology. The intellectual property for this application is owned by the Schepens Eye Research Institute/Massachusetts Eye and Ear.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) The ocular surface. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 2.Anderson N, Borlak J. Drug-induced phospholipidosis. FEBS letters. 2006;580:5533–5540. doi: 10.1016/j.febslet.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 3.Baronas ET, Lee JW, Alden C, Hsieh FY. Biomarkers to monitor drug-induced phospholipidosis. Toxicology and applied pharmacology. 2007;218:72–78. doi: 10.1016/j.taap.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 5.Brandes D, Bertini F. Role of Lysosomes in Cellular Lytic Processes. Ii. Cell Death during Holocrine Secretion in Sebaceous Glands. Experimental and molecular pathology. 1965;11:245–265. doi: 10.1016/0014-4800(65)90001-8. [DOI] [PubMed] [Google Scholar]
- 6.Chiambaretta F, Garraffo R, Elena PP, Pouliquen P, Delval L, Rigal D, Dubray C, Goldschmidt P, Tabbara K, Cochereau I. Tear concentrations of azithromycin following topical administration of a single dose of azithromycin 0.5%, 1.0%, and 1.5% eyedrops (T1225) in healthy volunteers. European journal of ophthalmology. 2008;18:13–20. doi: 10.1177/112067210801800103. [DOI] [PubMed] [Google Scholar]
- 7.Dietl P, Haller T, Mair N, Frick M. Mechanisms of surfactant exocytosis in alveolar type II cells in vitro and in vivo. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2001;16:239–243. doi: 10.1152/physiologyonline.2001.16.5.239. [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Sullivan DA. Aging and dry eye disease. Experimental gerontology. 2012;47:483–490. doi: 10.1016/j.exger.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foulks GN, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2013;32:44–53. doi: 10.1097/ICO.0b013e318254205f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foulks GN, Borchman D, Yappert M, Kim SH, McKay JW. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29:781–788. doi: 10.1097/ICO.0b013e3181cda38f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O'Brien T, Rolando M, Tsubota K, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Investigative ophthalmology & visual science. 2011;52:2050–2064. doi: 10.1167/iovs.10-6997g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerbaux C, Van Bambeke F, Montenez JP, Piret J, Morlighem G, Tulkens PM. Hyperactivity of cathepsin B and other lysosomal enzymes in fibroblasts exposed to azithromycin, a dicationic macrolide antibiotic with exceptional tissue accumulation. FEBS letters. 1996;394:307–310. doi: 10.1016/0014-5793(96)00975-1. [DOI] [PubMed] [Google Scholar]
- 13.Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, Glasgow BJ. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Investigative ophthalmology & visual science. 2011;52:1979–1993. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jester JV, Nicolaides N, Smith RE. Meibomian gland studies: histologic and ultrastructural investigations. Investigative ophthalmology & visual science. 1981;20:537–547. [PubMed] [Google Scholar]
- 15.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigative ophthalmology & visual science. 2011;52:1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. The ocular surface. 2009;7:S1–S14. doi: 10.1016/s1542-0124(12)70620-1. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Hatton MP, Khandelwal P, Sullivan DA. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2010;51:3993–4005. doi: 10.1167/iovs.09-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Kam WR, Ding J, Hatton MP, Sullivan DA. Effect of growth factors on the proliferation and gene expression of human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2013;54:2541–2550. doi: 10.1167/iovs.12-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Kam WR, Ding J, Sullivan DA. Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA ophthalmology. 2014;132:226–228. doi: 10.1001/jamaophthalmol.2013.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe R, Mussa HY, Nigsch F, Glen RC, Mitchell JB. Predicting the mechanism of phospholipidosis. Journal of cheminformatics. 2012;4:2. doi: 10.1186/1758-2946-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesquita-Guimarãtes J, Coimbra A. Holocrine cell Lysis in the rat preputial sebaceous gland. Evidence of autophagocytosis during cell involution. The Anatomical Record. 1976;186:49–67. [Google Scholar]
- 22.Mesquita-Guimarãtes J, Pignatelli D, Coimbra A. Autophagy during holocrine cell lysis in skin sebaceous glands. J Submicrosc Cytol. 1979;11:435–447. [Google Scholar]
- 23.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. The Journal of nutrition. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 24.Munic V, Banjanac M, Kostrun S, Nujic K, Bosnar M, Marjanovic N, Ralic J, Matijasic M, Hlevnjak M, Erakovic Haber V. Intensity of macrolide anti-inflammatory activity in J774A.1 cells positively correlates with cellular accumulation and phospholipidosis. Pharmacological research : the official journal of the Italian Pharmacological Society. 2011;64:298–307. doi: 10.1016/j.phrs.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Persson J, Nilsson J, Lindholm MW. Cytokine response to lipoprotein lipid loading in human monocyte-derived macrophages. Lipids in health and disease. 2006;5:17. doi: 10.1186/1476-511X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponec M, Weerheim A, Kempenaar J, Mommaas AM, Nugteren DH. Lipid composition of cultured human keratinocytes in relation to their differentiation. Journal of lipid research. 1988;29:949–961. [PubMed] [Google Scholar]
- 27.Rabionet M, Gorgas K, Sandhoff R. Ceramide synthesis in the epidermis. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbalip.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Reasor MJ, Kacew S. Drug-induced phospholipidosis: are there functional consequences? Experimental biology and medicine. 2001;226:825–830. doi: 10.1177/153537020122600903. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro CM, Hurd H, Wu Y, Martino ME, Jones L, Brighton B, Boucher RC, O'Neal WK. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PloS one. 2009;4:e5806. doi: 10.1371/journal.pone.0005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadrieh N. The Regulatory Challenges of Drug-induced Phospholipidosis. 2010 FDA website http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/advisorycommitteeforpharmaceuticalscienceandclinicalpharmacology/ucm210798.pdf.
- 31.Schmitz G, Muller G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. Journal of lipid research. 1991;32:1539–1570. [PubMed] [Google Scholar]
- 32.Shayman JA, Abe A. Drug induced phospholipidosis: an acquired lysosomal storage disorder. Biochimica et biophysica acta. 2013;1831:602–611. doi: 10.1016/j.bbalip.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirigu P, Shen RL, Pinto da Silva P. Human meibomian glands: the ultrastructure of acinar cells as viewed by thin section and freeze-fracture transmission electron microscopies. Investigative ophthalmology & visual science. 1992;33:2284–2292. [PubMed] [Google Scholar]
- 34.Suzuki M, Ohsaki Y, Tatematsu T, Shinohara Y, Maeda T, Cheng J, Fujimoto T. Translation inhibitors induce formation of cholesterol ester-rich lipid droplets. PloS one. 2012;7:e42379. doi: 10.1371/journal.pone.0042379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thody AJ, Shuster S. Control and function of sebaceous glands. Physiological reviews. 1989;69:383–416. doi: 10.1152/physrev.1989.69.2.383. [DOI] [PubMed] [Google Scholar]
- 36.Tyteca D, Van Der Smissen P, Van Bambeke F, Leys K, Tulkens PM, Courtoy PJ, Mingeot-Leclercq MP. Azithromycin, a lysosomotropic antibiotic, impairs fluid-phase pinocytosis in cultured fibroblasts. European journal of cell biology. 2001;80:466–478. doi: 10.1078/0171-9335-00180. [DOI] [PubMed] [Google Scholar]
- 37.Van Bambeke F, Gerbaux C, Michot JM, d'Yvoire MB, Montenez JP, Tulkens PM. Lysosomal alterations induced in cultured rat fibroblasts by long-term exposure to low concentrations of azithromycin. The Journal of antimicrobial chemotherapy. 1998;42:761–767. doi: 10.1093/jac/42.6.761. [DOI] [PubMed] [Google Scholar]
- 38.Van Bambeke F, Montenez JP, Piret J, Tulkens PM, Courtoy PJ, Mingeot-Leclercq MP. Interaction of the macrolide azithromycin with phospholipids. I. Inhibition of lysosomal phospholipase A1 activity. European journal of pharmacology. 1996;314:203–214. doi: 10.1016/s0014-2999(96)00552-3. [DOI] [PubMed] [Google Scholar]
- 39.Weaver TE, Na CL, Stahlman M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Seminars in cell & developmental biology. 2002;13:263–270. doi: 10.1016/s1084952102000551. [DOI] [PubMed] [Google Scholar]