Abstract
BACKGROUND
It has been speculated that the Bethesda Classification System for thyroid fine needle aspirate (FNA) may be used to predict aggressive features among histologically proven malignancies. We sought to evaluate whether malignancies that were characterized as Bethesda category V or VI have more aggressive features than malignancies that were category III or IV.
METHODS
A prospectively maintained database was reviewed to identify thyroid malignancies treated at a single center from 2004–2009. Only cancers that could be definitively matched to a preoperative FNA were included. Associations between Bethesda category, patient demographics, histopathologic findings, and outcomes were examined.
RESULTS
Three hundred and sixty cancers were analyzed: 73(20%) were Bethesda category III or IV and 287 (80%) were category V or VI. The majority of Bethesda III and IV cancers were follicular variants of PTC (fvPTC) whereas the majority of Bethesda V and VI cancers were classic PTC (52% and 67%, respectively, p<0.01). Extrathyroidal extension (30% vs. 16%, p=0.02), lymph node metastases (50% vs. 31%, p=0.05), and multifocality (51% vs. 37%, p=0.03) were more common among Bethesda V and VI nodules. However, when Bethesda III or IV classic PTC and fvPTC were compared to Bethesda V or VI cancers of the same histologic subtype, there were no differences in any features. Recurrence and overall survival were the same in all groups.
CONCLUSION
Bethesda category may be helpful in predicting the most likely histologic subtype of thyroid cancer, but it does not carry any prognostic significance once the histologic diagnosis is known.
INTRODUCTION
Thyroid cancer is the most common endocrine malignancy, with an age-adjusted incidence rate of 12.2 per 100,000 men and women per year between 2006–2010, and the incidence has been increasing by an estimated 6.4% per year between 1997 and 20101. After an initial ultrasound, fine needle aspiration (FNA) is the next step in assessing the risk of malignancy of a thyroid nodule2–5. Biopsy results can fall within one of six categories as defined by the Bethesda system for reporting FNA cytopathology results: I (non-diagnostic), II (benign), III (atypia of undetermined significance/follicular lesion of undetermined significance), IV (follicular neoplasm), V (suspicious for malignancy), and VI (malignant)6.
Thyroid nodules that fall within Bethesda categories III and IV have an overall risk of malignancy of between 15–40%6, 7. If Bethesda III or IV nodules are found to be malignant, the most common histologic subtype is the follicular variant of papillary thyroid carcinoma (fvPTC)4, 8. This subtype is generally less aggressive than classic PTC and has been shown to have a lower risk of lymph node metastases, extrathyroidal extension, and recurrence, especially if the tumor is encapsulated9–11.
A major focus of thyroid research in recent years has been to identify markers that can be screened for on FNA to predict the presence of aggressive features. Recently, some have suggested that the Bethesda classification system itself may be used as a prognostic marker for a more aggressive phenotype12. These authors hypothesized that thyroid malignancies that were classified as Bethesda category VI on preoperative FNA have more aggressive features than malignancies that were classified as category III or IV. However, it remains unclear whether these differences simply represent the ability to identify classic papillary thyroid cancers as opposed to fvPTC. Whether this always translates to identification of a more aggressive tumor remains uncertain.
We hypothesized that although thyroid malignancies that are classified as Bethesda category V or VI on preoperative FNA may appear more aggressive than malignancies that are classified as Bethesda category III or IV, these differences are primarily due to a preponderance of classic papillary thyroid carcinomas in those categories rather than the less aggressive fvPTC that predominate among Bethesda category III or IV nodules.
METHODS
Patient Selection and Clinical Data Collection
A prospectively maintained database was retrospectively reviewed to identify all histopathologically diagnosed thyroid malignancies treated by two endocrine surgeons at a single academic tertiary care center between January 2004 and December 2011. To be included in this study, all patients had to have a preoperative fine needle aspiration (FNA) of a nodule that could be definitively matched by size and location with the malignancy identified on histopathology. Patients whose FNA results were either non-diagnostic (Bethesda category I), benign (Bethesda category II), or who had biopsy-proven lymph node metastases preoperatively were excluded, since the Bethesda category of these nodules did not factor into their management.
The following information was collected for each patient: age, sex, Bethesda category of the preoperative FNA, size of the nodule, extent of surgical resection, histologic subtype, extrathyroidal extension, lymphovascular invasion, multifocality, margin status, number of lymph nodes with metastases, and total number of lymph nodes examined. Follow-up data including whether the patient had postoperative radioactive iodine (RAI), dose of RAI, local or distant recurrence, reoperation for cancer recurrence, length of follow-up, disease-free survival, and overall survival were also reviewed. In general, our institution routinely performs a total thyroidectomy with a prophylactic central compartment neck dissections (CND) for all clinically node-negative Bethesda category V and VI nodules. A CND is typically only performed for Bethesda III and IV nodules if there are grossly suspicious lymph nodes identified on preoperative imaging or intraoperatively, or if there were particularly worrisome features of cytologic atypia (i.e. nuclear grooves or pseudo inclusions) on the preoperative FNA, which impart a significantly greater risk of malignancy13. Cancer recurrence was defined as a rise in serum markers (thyroglobulin and anti-thyroglobulin antibodies) and/or appearance of suspicious findings on imaging studies, which warrant clinical intervention. Due to inter-assay variability of thyroglobulin levels, no strict cutoffs are used, but rather the trend in results over time is considered.
If the Bethesda category of the preoperative FNA was not explicitly stated in the biopsy report (i.e. biopsies performed prior to 2009 and biopsies performed at institutions that do not utilize the Bethesda system in their reports), the appropriate Bethesda category was assigned based on the findings noted in the report. For this reason, Bethesda categories III and IV and categories V and VI were grouped together, as it was often difficult to distinguish these groups based on the terminology of those cytology reports.
The Institutional Review Board of Weill Cornell Medical College approved this study.
Statistical Analysis
Thyroid malignancies that were classified as Bethesda categories III and IV preoperatively were compared to malignancies that were classified as categories V and VI. Next, classic papillary thyroid carcinomas (PTC) that were Bethesda categories III or IV on preoperative FNA were compared to classic PTC that were Bethesda categories V or VI. A similar comparison was performed for fvPTC. P-values were calculated using Fisher’s exact test, Pearson’s chi-squared, student’s T-test (normally distributed continuous variables), or Mann-Whitney U-test (non-normally distributed continuous variables) as appropriate. Continuous variables that followed a normal distribution are presented as mean ± standard deviation (SD), while those that were not normally distributed are presented as median (range). A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using STATA 12.0 (College Station, TX).
RESULTS
Study Population
Five hundred and sixty-two thyroid malignancies were reviewed. Of these, 164 were excluded either because a preoperative FNA was not performed or the nodule that was biopsied could not be definitively matched to the histopathologically-confirmed malignancy. Thirty-eight patients were excluded because they were Bethesda category I, category II, or had biopsy-proven lateral neck lymph node metastases. Therefore, 360 patients remained in the final study population. Seventy-three (20%) malignancies were characterized as Bethesda category III or IV on preoperative FNA and 287 (80%) were Bethesda categories V or VI (60 category V, 227 category VI).
Bethesda III/IV Malignancies vs. Bethesda V/VI Malignancies
Patient demographics, extent of surgical resection, and a complete listing of histologic subtypes are shown in Table 1. There were no differences in age or gender between malignancies that were Bethesda category III or IV compared to malignancies that were Bethesda category V or VI. Only 15% of the category III/IV group underwent a total thyroidectomy with central compartment lymph node dissection compared to85% of the category III/IV group (p<0.001). The most common histologic subtype among Bethesda III/IV malignancies was fvPTC (52%) whereas the majority of Bethesda V/VI malignancies were classic PTC (67%) (Figure 1).
Table 1.
Demographics, Procedure, and Histologic Subtypes of Bethesda Categories III/IV vs. V/VI
| Bethesda Category | |||
|---|---|---|---|
|
| |||
| N=360 | III and IV (n=73) | V and VI (n=287) | p-value |
| Age (mean ± SD) | 48.3 ± 14.8 | 47.0 ± 14.5 | 0.499 |
|
| |||
| Females | 60 (82%) | 227 (79%) | 0.557 |
|
| |||
| Procedure | |||
| Total thyroidectomy | 33 (45%) | 29 (10%) | <0.001 |
| Total thyroidectomy w/CND | 11 (15%) | 244 (85%) | |
| Hemithyroidectomy | 28 (38%) | 10 (4%) | |
| Isthmusectomy | 1 (2%) | 4 (1%) | |
| Completion* | 13 (45%) | 10 (71%) | 0.101 |
|
| |||
| HISTOLOGIC SUBTYPE | |||
| PTC, classic | 14 (19%) | 191 (67%) | <0.001 |
| PTC, follicular variant | 38 (52%) | 34 (12%) | |
| PTC, tall cell features/variant | 0 (0%) | 14 (5%) | |
| PTC w/Hurthle cell features | 1 (1%) | 4 (1%) | |
| PTC, cribriform-morular variant | 0 (0%) | 2 (1%) | |
| PTC, mixed type | 4 (6%) | 32 (11%) | |
| Follicular carcinoma | 7 (10%) | 0 (0%) | |
| Poorly differentiated | 4 (6%) | 1 (<1%) | |
| Anaplastic | 1 (1%) | 2 (1%) | |
| Medullary | 0 (0%) | 7 (2%) | |
| Hurthle cell carcinoma | 3 (4%) | 0 (0%) | |
Percentage of patients who had a hemithyroidectomy or isthmusectomy.
FIGURE 1.
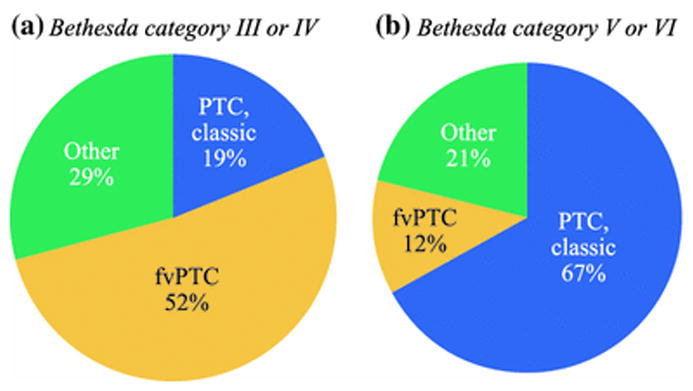
Histologic subtypes of thyroid malignancies by preoperative Bethesda category. PTC=papillary thyroid carcinoma, fvPTC=follicular variant of PTC.
Bethesda category V and VI tumors were slightly smaller than Bethesda category III and IV tumors, but they were more likely to have extrathyroidal extension (30% vs. 16%, p=0.018), multifocality (51% vs. 37%, p=0.030), lymph node metastases (50% vs. 31%, p=0.053), and a greater percentage of their sampled lymph nodes were positive for metastatic disease (23% vs. 14%, p=0.035) (Table 2). However, if a central compartment neck dissection was performed, patients with Bethesda III and IV tumors were more likely than Bethesda V and VI tumors to have suspicious or enlarged lymph nodes noticed at the time of surgery (36% vs. 14%, respectively, p=0.037).
Table 2.
Histopathologic Findings of Bethesda Categories III/IV vs. V/VI
| Bethesda Category | |||
|---|---|---|---|
| N=360 | III or IV (n=73) | V or VI (n=287) | p-value |
| Tumor size (cm)1 | 1.5 (0.5–8.0) | 1.3 (0.2–11.0) | 0.025 |
| Extrathyroidal extension | 12 (16%) | 87 (30%) | 0.018 |
| Lymphovascular invasion | 14 (19%) | 39 (14%) | 0.229 |
| Multifocal | 27 (37%) | 147 (51%) | 0.030 |
| Positive margins | 4 (6%) | 35 (12%) | 0.105 |
| Suspicious lymph nodes (if CND performed) | 4 (36%) | 34 (14%) | 0.037 |
| Lymph node metastases2 | 9 (31%) | 129 (50%) | 0.053 |
| % Positive lymph nodes | 15/106 (14%) | 520/2269 (23%) | 0.035 |
| # lymph nodes sampled1 | 0 (0–14) | 7 (0–33) | <0.001 |
Median (range).
Percentages reflect only patients who had lymph nodes sampled.
CND = central compartment lymph node dissection.
There were no significant differences in the percentage of patients who received RAI postoperatively or in the dose of RAI that was administered (Table 3). The median length of follow-up for both groups was 20 months. The overall risk of recurrence for the Bethesda III/IV group was 3% compared to 7% for the Bethesda V/VI group (p=0.152). The overall survival of both groups was excellent, as 99% of all patients were alive at the end of this study.
Table 3.
Follow-up Data of Bethesda Categories III/IV vs. V/VI
| Bethesda Category | |||
|---|---|---|---|
| N=360 | III or IV (n=73) | V or VI (n=287) | p-value |
| Length of follow-up (months)1 | 20 (0.5–97) | 20 (0.5–105) | 0.973 |
| % Radioactive Iodine | 43 (59%) | 199 (69%) | 0.082 |
| 131I dose (mCi)1 | 51 (0–446) | 75 (0–353) | 0.092 |
| Recurrence | 2 (3%) | 21 (7%) | 0.152 |
| Time to recurrence (months)1 | 12 (0–24) | 10 (0–60) | 0.912 |
| Overall survival | 99% | 99% | 1.000 |
Median (range).
Subgroup Analysis by Histologic Subtype
Classic PTC and fvPTC accounted for 77% of the total study population, and therefore those two histologic subtypes were isolated for subgroup analysis. Classic and fvPTC that were Bethesda categories III or IV were compared to tumors of the same histologic subtype that were Bethesda categories V or VI to see whether preceding cytologic diagnosis is associated with more aggressive clinical features within the same histologic subtypes. When compared to tumors of the same subtype, there were no differences in any histopathologic findings, except that Bethesda V/VI classic PTC and fvPTC had a greater number of lymph nodes sampled (Table 4). Patient outcomes were also similar between the groups, as 7% of both Bethesda III/IV and Bethesda V/VI classic PTC recurred, while 0% and 3% of Bethesda III/IV and V/VI fvPTC recurred, respectively (p=NS). The only deaths in this study were in patients with anaplastic thyroid carcinoma, which were excluded from the subgroup analysis. Therefore, the overall survival of all patients with classic PTC and fvPTC was 100%, regardless of Bethesda category.
Table 4.
Histopathologic Findings of Only Classic Papillary Thyroid Carcinomas and Follicular Variant of Papillary Thyroid Carcinomas
| Classic PTC | fvPTC | |||||
|---|---|---|---|---|---|---|
| III and IV (n=14) | V and VI (n=191) | p-value | III and IV (n=38) | V and VI (n=34) | p-value | |
| Tumor size (cm)1 | 0.9 (0.5–3.0) | 1.2 (0.2–11.0) | 0.144 | 1.5 (0.7–7.5) | 1.3 (0.4–10.0) | 0.523 |
| Extrathyroidal extension | 4 (29%) | 57 (30%) | 0.920 | 5 (13%) | 8 (24%) | 0.253 |
| Lymphovascular invasion | 2 (14%) | 23 (12%) | 0.804 | 3 (8%) | 5 (15%) | 0.359 |
| Multifocal | 5 (36%) | 92 (48%) | 0.368 | 15 (40%) | 20 (59%) | 0.101 |
| Positive margins | 1 (7%) | 19 (10%) | 0.733 | 2 (5%) | 2 (6%) | 0.909 |
| Suspicious lymph nodes (only if CND performed) | 1 (33%) | 22 (13%) | 0.350 | 3 (50%) | 5 (21%) | 0.300 |
| Lymph node metastases2 | 3 (50%) | 88 (50%) | 0.989 | 6 (46%) | 11 (41%) | 0.746 |
| % Positive Lymph nodes | 6/33 (18%) | 378/1515 (25%) | 0.568 | 9/48 (19%) | 37/234 (16%) | 0.737 |
| # lymph nodes sampled1 | 0 (0–14) | 7 (0–33) | <0.001 | 0 (0–13) | 6 (0–18) | <0.001 |
| Extranodal ext.3 | 1 (33%) | 9 (10%) | 0.208 | 2 (33%) | 2 (18%) | 0.584 |
| % Radioactive Iodine | 8 (57%) | 131 (69%) | 0.376 | 21 (55%) | 23 (68%) | 0.282 |
| Radioactive iodine dose | 49 (0–152) | 53 (0–353) | 0.181 | 51 (0–446) | 75 (0–293) | 0.091 |
| Length of followup | 13.5 (2–78) | 21 (0–105) | 0.334 | 20 (0.5–97) | 13 (0.3–104 | 0.446 |
| Recurrence | 1 (7%) | 14 (7%) | 0.979 | 0 (0%) | 1 (3%) | 0.465 |
| Time to recurrence | 24 (24–24) | 11.5 (0–60) | 0.352 | n/a | 23 (23–23) | n/a |
| Overall survival | 100% | 100% | 1.000 | 100% | 100% | 1.000 |
Median (range).
Percentages reflect only patients who had lymph nodes sampled.
Percentages restricted to only tumors with positive lymph nodes.
PTC=papillary thyroid carcinoma, fvPTC=follicular variant of papillary thyroid carcinoma. CND = central compartment lymph node dissection.
DISCUSSION
Risk stratification of patients with newly diagnosed thyroid cancer plays a critical role in guiding their post-operative care. Decisions regarding adjuvant radioactive iodine (RAI) administration, the need for a completion thyroidectomy (if less than a total thyroidectomy was performed initially), and how to advise the patient regarding their risk of recurrence is multifactorial. Several risk stratification systems have been proposed that use a combination of demographic(i.e. male gender and advanced age) and histopathologic information(i.e. extrathyroidal extension, lymph node metastases, and positive margins) to estimate the risk of recurrence and/or mortality14–16. However, a better understanding of cytologic and molecular markers of more aggressive thyroid tumors is needed.
Recently, VanderLaan and colleagues suggested that a preceding malignant cytologic diagnosis (i.e. Bethesda category VI) is associated with aggressive features of PTC12. They found that PTC that fell within Bethesda category VI were more likely to have a higher American Joint Committee on Cancer T and N stage, angiolymphatic invasion, and extrathyroidal extension. The authors concluded that Bethesda category VI classification identifies higher risk PTC whereas category III and IV classifications identify low-risk PTC. However, it was unclear from this study whether the more aggressive features that were noted among category VI malignancies were merely reflective of more aggressive histologic subtypes stratifying to the Bethesda VI category. We sought to examine this in greater detail in the current study.
In our cohort of 360 thyroid malignancies, we observed that aggressive histologic features were more common among malignancies with a preceding cytologic diagnosis of Bethesda category V or VI compared to category III or IV. The rates of extrathyroidal extension, multifocality, and percent of positive lymph nodes were all significantly higher among Bethesda category V and VI malignancies. There was also a trend towards an increased risk of recurrence, but this did not reach significance. However, subgroup analysis of the two most common histologic subtypes (classic PTC and fvPTC) revealed that when tumors in different cytologic groups were compared to tumors of the same histologic subtype, there were no differences between the groups. This suggests that the differences that were observed initially were largely reflective of a greater proportion of classic PTC among the Bethesda category V and VI nodules and fvPTC in the category III and IV nodules.
The follicular variant of papillary thyroid carcinoma is generally considered a less aggressive histologic subtype, especially when the tumor is completely encapsulated9–11. When compared to classic PTC, fvPTC has a lower risk of lymph node metastases, extrathyroidal extension, and locoregional recurrence. This has led several authors to advocate for a more conservative approach to the management of these patients(i.e. hemithyroidectomy without radioactive iodine ablation)9, 17. In this study, we found that nodules that were characterized as Bethesda category III or IV were most likely to be fvPTC whereas those that were Bethesda category V or VI were most likely to be classic PTC. Therefore, when discussing the management of a suspicious nodule with a patient preoperatively, the findings of this study can be used to advise the patient not only on their overall risk of malignancy, but also on the most likely histologic subtype and the risk of aggressive features associated with that subtype.
The subgroup analysis of the two most common histologic subtypes (classic PTC and fvPTC) was a critical aspect of this study because it allowed us to determine that the differences that were observed between the two cytologic groups were likely due to a preponderance of fvPTC in the Bethesda III and IV nodules. However, one limitation to this study is that other more aggressive histologic subtypes such as tall cell variant, poorly differentiated, and anaplastic thyroid carcinomas were excluded from this subgroup analysis because there were too few of each to allow for any meaningful conclusions about their associations with Bethesda category. In fact, the only deaths that were observed in this study were among patients with anaplastic carcinoma, so the overall survival became 100% in both groups once those patients were excluded. However, it should be noted that aggressive subtypes were seen in both Bethesda category III/IV nodules as well as category V/VI nodules. Therefore, although malignancies that were characterized as Bethesda III or IV on preoperative FNA are most likely to be the more indolent fvPTC, one should keep in mind that there is still a small chance that the tumor may be a subtype that would require more aggressive management (i.e. total thyroidectomy with radioactive iodine).
It is important to note that at our institution, prophylactic central neck dissections (CND) are performed on all patients who present with Bethesda VI nodules and most patients with Bethesda V nodules. However, a CND is typically only performed on patients with Bethesda III or IV nodules if there is macroscopic suspicion of lymph node metastases (either on preoperative imaging or intraoperatively). In addition, the presence of particularly worrisome features of cytologic atypia on the preoperative FNA of Bethesda III nodules such as nuclear grooves and/or inclusions often compel us to perform a prophylactic CND, since our group has shown that the presence of these two atypical features increases the risk of malignancy to 80%13. This explains why 15% of patients with Bethesda III or IV nodules underwent a CND. Recently, commercially available molecular tests that screen for genetic mutations or gene expression profiles have been introduced to help improve the accuracy of preoperative FNA18, 19. However, these tests were not commercially available when the patients in this study underwent surgery (2004–2009), so they were not used to guide their therapy.
One interesting finding was that despite a lower rate of lymph node metastases and a lower percentage of positive lymph nodes, patients with Bethesda III and IV malignancies were more likely to have enlarged or suspicious lymph nodes encountered at the time of surgery. This is reflective of our institutional practice regarding the selective use of prophylactic CND in patients with Bethesda III and IV nodules, as described above. As such, it follows that patients with Bethesda III and IV nodules who had a CND were more likely to have enlarged or suspicious lymph nodes.
There are several limitations to this study. First, this was a retrospective, single center study and therefore the treatments and follow-up were not standardized and are reflective of our institutional practices. Second, due to the relatively low incidence of locoregional recurrence and the even lower incidence of mortality, the study was underpowered to detect differences in either outcome. Third, evaluation of the risk of lymph node metastases was vulnerable to sampling bias because nodules that were classified as Bethesda category V or VI were more likely to undergo a prophylactic central compartment lymph node dissection and, therefore, had significantly more lymph nodes sampled than Bethesda III or IV nodules. We attempted to control for this bias in two ways. First, the incidence of lymph node metastases was calculated only among patients who had lymph nodes sampled and not among all patients, and second, we also reported the percentage of positive lymph nodes among all lymph nodes sampled as a way of accounting for differences in the extent of lymph node sampling. Fourth, since the FNAs of many patients were performed prior to the widespread use of the Bethesda classification system, the appropriate Bethesda category had to be inferred for many cases. This introduces a risk of erroneously classifying some cases, although we believe that this risk is minimal since Bethesda categories III/IV and V/VI were grouped together and it was always quite clear to which of those two groups the nodule should be assigned. Finally, patients who presented with confirmed lymph node metastases were excluded from this study, regardless of the Bethesda classification of the primary nodule, since the presence of known metastases rendered the Bethesda classification of the nodule irrelevant and those patients were managed as if they were aggressive malignancies regardless of the nodule’s FNA result. This may have introduced selection bias by excluding more aggressive nodules. However, both study groups were prone to the same exclusion criteria, and so it is unknown whether this biased the results towards or away from the null hypothesis.
In conclusion, thyroid malignancies that were classified as Bethesda categories V or VI on preoperative FNA have more aggressive features than malignancies that were classified as Bethesda categories III or IV, however this is likely due to a preponderance of fvPTC in the Bethesda III or IV group. Therefore, the Bethesda classification may be helpful in predicting the most likely histologic subtype of thyroid cancer if the nodule turns out to be malignant, but it does not carry any prognostic significance once the histologic diagnosis is known.
SYNOPSIS.
The Bethesda classification system for reporting results of thyroid fine needle aspirates predicts the most likely histologic subtype if the nodule turns out to be malignant, but it does not carry any prognostic significance once the histologic diagnosis is known.
Acknowledgments
This study was supported in part by grant TL1RR000459of the Clinical and Translational Science Center at Weill Cornell Medical College, and by a donation from the Dancers Care Foundation.
Footnotes
Financial Disclosures: None
References
- 1.Howlader NNA, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. 2013. [Google Scholar]
- 2.Yang GC, Liebeskind D, Messina AV. Ultrasound-guided fine-needle aspiration of the thyroid assessed by Ultrafast Papanicolaou stain: data from 1135 biopsies with a two- to six-year follow-up. Thyroid : official journal of the American Thyroid Association. 2001;11(6):581–9. doi: 10.1089/105072501750302895. [DOI] [PubMed] [Google Scholar]
- 3.Filetti S, Durante C, Torlontano M. Nonsurgical approaches to the management of thyroid nodules. Nature clinical practice Endocrinology & metabolism. 2006;2(7):384–94. doi: 10.1038/ncpendmet0215. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111(5):306–15. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 5.ATA adopts TeleHomecare clinical guidelines. Home healthcare nurse manager. 1999;3(6):24–5. [PubMed] [Google Scholar]
- 6.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid : official journal of the American Thyroid Association. 2009;19(11):1159–65. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2009;19(11):1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 8.Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagnostic cytopathology. 2002;26(1):41–4. doi: 10.1002/dc.10043. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Singh B, Tallini G, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107(6):1255–64. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 10.Jain M, Khan A, Patwardhan N, Reale F, Safran M. Follicular variant of papillary thyroid carcinoma: a comparative study of histopathologic features and cytology results in 141 patients. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2001;7(2):79–84. doi: 10.4158/EP.7.2.79. [DOI] [PubMed] [Google Scholar]
- 11.LiVolsi VA. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survival. Cancer. 2003;98(9):1997. doi: 10.1002/cncr.11750. author reply 97–8. [DOI] [PubMed] [Google Scholar]
- 12.Vanderlaan PA, Marqusee E, Krane JF. Features associated with locoregional spread of papillary carcinoma correlate with diagnostic category in the Bethesda System for reporting thyroid cytopathology. Cancer cytopathology. 2012;120(4):245–53. doi: 10.1002/cncy.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato MA, Buitrago D, Moo TA, et al. Predictive value of cytologic atypia in indeterminate thyroid fine-needle aspirate biopsies. Annals of surgical oncology. 2011;18(10):2893–8. doi: 10.1245/s10434-011-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102(6):1088–95. [PubMed] [Google Scholar]
- 15.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114(6):1050–7. discussion 57–8. [PubMed] [Google Scholar]
- 16.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104(6):947–53. [PubMed] [Google Scholar]
- 17.Rivera M, Tuttle RM, Patel S, Shaha A, Shah JP, Ghossein RA. Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern) Thyroid : official journal of the American Thyroid Association. 2009;19(2):119–27. doi: 10.1089/thy.2008.0303. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. The Journal of clinical endocrinology and metabolism. 2009;94(6):2092–8. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 19.Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. The New England journal of medicine. 2012;367(8):705–15. doi: 10.1056/NEJMoa1203208. [DOI] [PubMed] [Google Scholar]


