Abstract
Implanted vagus nerve stimulation (VNS) has been used to treat seizures and depression. In this study, we explore the mechanism of action of non-invasive vagus nerve stimulation (nVNS) for the treatment of trigeminal allodynia. Rats were repeatedly infused with inflammatory mediators directly onto the dura, which leads to chronic trigeminal allodynia. nVNS for 2min decreases periorbital sensitivity in rats with periorbital trigeminal allodynia for up to 3.5hr after stimulation. Using microdialysis, we quantified levels of extracellular neurotransmitters in the trigeminal nucleus caudalis (TNC). Allodynic rats showed a 7.7±0.9 fold increase in extracellular glutamate in the TNC following i.p. administration of the chemical headache trigger, glyceryl trinitrate (GTN; 0.1mg/kg). Allodynic rats, which received nVNS, had only a 2.3±0.4 fold increase in extracellular glutamate following GTN similar to the response in control naive rats. When nVNS was delayed until 120min after GTN treatment, the high levels of glutamate in the TNC were reversed following nVNS. The nVNS stimulation parameters used in this study did not produce significant changes in blood pressure or heart rate. These data suggest that nVNS may be used to treat trigeminal allodynia.
1. Introduction
The vagus nerve innervates many major organs of the body and relays sensory information about the state of the viscera to the central nervous system [5, 9]. The vagus nerve is composed of 80% afferent and 20% efferent fibers. Vagal afferents convey physiological information from these organs to the central nervous system. Vagus nerve stimulation (VNS) has been approved in the United States for treatment of epilepsy and depression [9, 13]. During clinical trials of V nNS for the treatment of epilepsy, patients reported a reduction in headache frequency and intensity [11, 29], and a prophylactic effect of VNS [14]. These observations suggest that vagus nerve stimulation may be a novel non-pharmacologic treatment for migraine. In this study, we stimulated the vagus nerve non-invasively through the skin on the ventral surface of the neck using a non-invasive vagus nerve stimulator (nVNS). This allowed us to stimulate the vagus nerve without the surgical implantation of stimulation electrodes in the neck of the rat.
VNS reduces tail-flick reflex elicited by noxious heat and formalin-induced nociceptor activation in rodent models [2]. VNS also reduces c-Fos expression in the ipsilateral TNC when applied at the same time as an injection of formalin into the facial vibrissae [2]. Another study found that chronic interruption of the vagal afferent input to the abdominal viscera halted antinociceptive responses to abdominal pain in rats [10].
Our rodent model of trigeminal allodynia relies on repeated activation of trigeminal nociceptive circuits through repeated infusions of prostglandin E2 onto the dura, which leads to chronic trigeminal allodynia similar to the hypersensitivity found in patients with migraine [22, 26-27]. Using this model, this study aims to examine the mechanism of action of acute non-invasive vagus nerve stimulation (nVNS) for the treatment of trigeminal allodynia.
2. Methods
2.1 Ethics Statement
All procedures performed on the animals were reviewed and approved by the Thomas Jefferson University Institutional Animal Care and Use Committee (IACUC) prior to initiating research. All surgeries were performed under isoflurane anesthesia and all efforts were made to reduce animal numbers and suffering.
2.2 Experimental Groups
Male Sprague Dawley rats (350-375g, Charles River) were divided into naive and prostaglandin infused allodynic rats. These groups were further divided by treatment during microdialysis: no treatment, glyceryl trinitrate (GTN), nVNS, and nVNS with GTN.
2.3 Periorbital Von Frey Sensory Testing
Rats were trained to enter a plastic tube restraint uncoaxed for periorbital sensory testing. Sensory testing occurred during the day using von Frey filaments with reproducible calibrated buckling forces varying from 10-0.07g in descending sequential order [26]. Von Frey monofilaments were required to make firm, perpendicular contact with the skin in the periorbital region, causing the filament to bend. The right and left periorbital threshold data were recorded separately. A positive response was characterized by several behavioral criteria: stroking the face with a forepaw, head withdrawal from the stimulus, and head shaking. The threshold was defined as a positive response to 2 of 3 trials. A normal periorbital threshold for rats was defined as 6g and higher; allodynic rats were defined as having periorbital thresholds of 4g or less [26].
2.4 Cannula Implantation and Infusion
After two weeks of habituation and training, the rats were fitted with a stainless steel cannula (26 gauge, Plastics One Inc., Roanoke, VA, USA) under isoflurane anesthesia (3% induction, 1.5% maintenance). First, a ~3mm diameter craniotomy was performed above the junction of the superior sagittal sinus and transverse sinus to expose the dura. The cannula was fixed to the bone with small screws and dental cement. An obdurator that extended just past the end of the cannula was inserted to prevent scar tissue from forming, and thus clogging the cannula. Animals were allowed at least one week of recovery. Periorbital thresholds were monitored during the recovery period to ensure the thresholds returned to presurgery baselines. If animals did not return to baseline, they were excluded from the study.
Rats were permitted free movement within their cages during infusions of sterile saline or 0.1μM prostaglandin E2 in 0.9% sterile saline. Polyethylene tubing (PE20) was connected to a Hamilton syringe, as well as the top of the cannula. 15μL saline or prostaglandin E2 was infused over three minutes while the rats moved freely. Visual inspection of the area around the cap was done to verify there was no leakage of fluid. Obdurators were replaced after the last sensory test of the day, roughly 5 hours after the infusion. Rats were infused three times a week over the course of three to four weeks.
Cannula position was verified post mortem via visual assessment to ensure placement over the superior sagittal and transverse sinuses.
2.5 Trigeminal von Frey thresholds
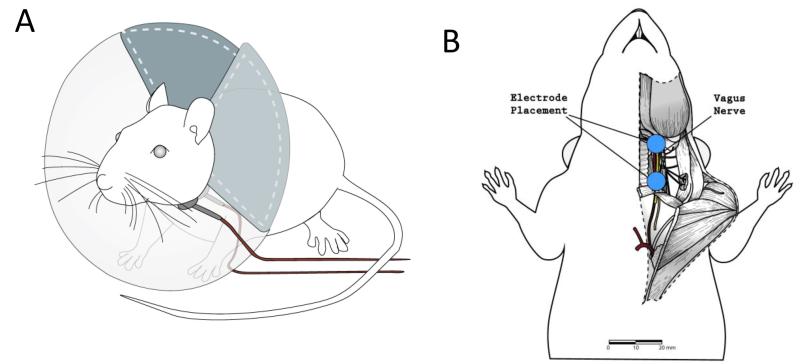
To test the effect of nVNS on periorbital von Frey thresholds, an Elizabethan collar with electrodes and an electrolyte gel (Signa gel, Parker Laboratories, Inc.) was placed on the neck lateral to the trachea (Fig. 1A). The electrodes were placed on the shaved skin of the neck, parallel to and over the vagus nerve (Fig. 1B). nVNS stimulation consisted of a 1ms pulse of 5kHz sine waves, repeated at 25Hz, for two minutes. Periorbital thresholds were taken before stimulation, then 5, 30, 90, 150, 210 min, and 24hr after nVNS stimulation.
Figure 1.
A) An Elizabethan collar with two silver coated electrodes (0.8cm diameter) was placed on conscious naive and allodynic rats. The electrodes were connected to an amplifier that produced 1ms pulse of 5kHz sine waves, repeated at 25Hz. The electrodes were positioned on the skin of the rat, lateral to the rat’s trachea.
Figure 1B) Although in the experiments in this study vagus nerve stimulation was achieved through stimulation of the shaved skin on the neck over the vagus nerve, we have included a diagram of the anatomy of the neck of the rat to illustrate the placement electrodes with respect to the vagus nerve. This diagram is a cutaway of the rat’s neck that shows the trachea (white), carotid artery (red) and vagus nerve (yellow) in relation to the placement of the electrodes (blue).
2.6 Microdialysis of the Trigeminal Nucleus Caudalis
CMA 12MD Elite probes (CMA Microdialysis Inc., Solna, Sweden) with a 1mm PAES membrane length with a 20,000 Dalton cutoff and a 0.5mm outer diameter was placed into the trigeminal nucleus caudalis (TNC) −2.6 to −2.9mm from obex and 1.7 to 1.9mm lateral to the midline. PE10 tubing connected the probe to a 2.5mL glass syringe mounted on a CMA/100 microinjection pump (CMA Microdialysis AB, North Chelmsford, MA, USA). The dialysis system was perfused at 2.0μL/min with sterile, pyrogen-free artificial extracellular spinal fluid (aCSF; composition in mM/L: 135 NaCl; 3 KCl; 1 MgCl2; CaCl2; 2-sodium phosphate mono- and dibasic; pH= 7.4). Probes were inserted 2-3 hours prior to stimulation in order to settle to baseline levels. Samples were collected continuously every 15 minutes and began immediately after probe insertion. Sample collection continued for at least 3.5 hours after stimulation.
At the end of the experiment, animals were injected with 0.5mL of Euthasol via i.p. injection. The probe was then removed and stored in distilled water. The brain and spinal cord were removed and stored in 4% paraformaldehyde for later analysis to check the position of the probe via sectioning and staining.
2.7 HPLC Measurement of Amino Acids
The amino acid content (specifically, glutamate, norepinephrine, glycine, and GABA) of each sample was analyzed via high-performance liquid chromatography (HPLC) using a binary gradient and pre-column derivatization of O-phthal aldehyde (OPA) with fluorescence detection.
Samples were diluted (5μL aCSF + 5μL dialysate) and a 1:2 sample to reagent ratio was used (10μL sample + 20μL OPA). After a 60 second reaction, 20μL of the sample-OPA mixture was auto-injected into an Agilent Zorbax Eclipse AAA column (150x4.6mm; 5μm particle size). A binary gradient of mobile phase A (40mM sodium phosphate monobasic; pH= 7.4) and mobile phase B (45% acetonitrile; 45% methanol; 10% water) with a flow rate of 1.5mL/min was used for separation. The column temperature was maintained at 30°C. EZChrom Elite version 3.1.6 software was used to determine concentrations of extracellular neurotransmitters.
2.8 HPLC Measurement of Monoamines
Measurement of monoamine neurotransmitters, particularly norepinephrine, serotonin (5-HT) and 5-hydroxyindoleacetic acid, was via electrochemical detection utilizing an isocratic flow of mobile phase (75mM sodium phosphate monobasic; 1.7mM 1-Octanesulfonic acid; 100μM triethylamine; 25μM EDTA; 11% acetonitrile; in polished water; pH=3.00) at a constant flow rate of 0.5mL/min. Samples were diluted (5μL aCSF + 5μL dialysate) and manually injected through a 20μL loop into a Thermo 150x3.2mm 3μm particle size column, which was kept at 35°C. Concentrations of extracellular monoamine neurotransmitters were analyzed using EZChrom Elite version 3.1.6 software.
2.9 Statistical Analysis
Unless explicitly stated otherwise, repeated measures ANOVA was used to compare groups and the individual time points were tested using a one-way ANOVA using SPSS version 22. Bonferroni post hoc tests were done where applicable.
2.10 Treatment Groups
Stimulation of the rat included nVNS only, GTN only (0.1mg/kg), or both. Non-invasive electrical vagus nerve stimulation or i.p. injection of GTN was performed after a minimum of one hour of stable baseline extracellular amino acid levels. An electrolyte gel (Signa gel) was smeared onto the nerve stimulator and then placed on the shaved neck of the rat lateral to the trachea and over the vagus nerve, one minute before stimulation occurred. Rats received repeated 25Hz, 1milisecond pulses for 2 minutes. Animals that received both treatments received GTN (0.1mg/kg, i.p.) during electrical stimulation.
2.11 Blood Pressure and Heart Rate
Blood pressure and heart rate were monitored using a femoral artery catheter (UFI instruments, CA). After one hour of stable baseline, rats received nVNS stimulation as described above and were monitored for an additional 45min.
3. Results
3.1 Blood Pressure and Heart Rate
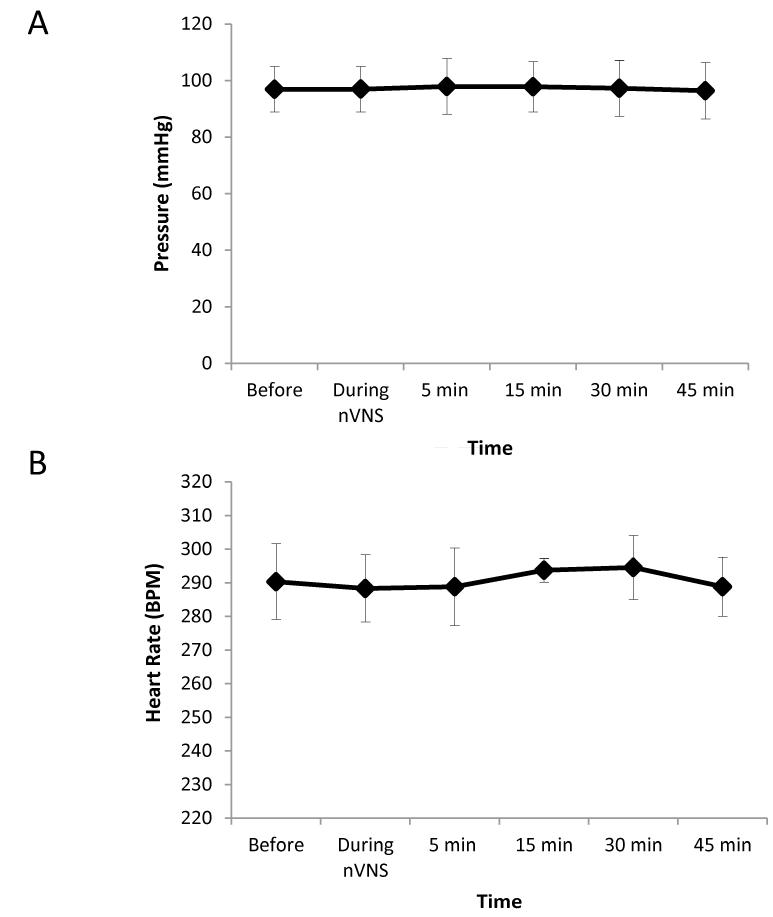
To rule out cardiac effects due to vagus nerve stimulation on the pain behaviors and physiology, we measured blood pressure and heart rate before and after nVNS. Using a femoral artery catheterization, average baseline blood pressure was 97.16±0.57mmHg (Fig. 2A) and average baseline heart rate was 143.32±8.09 beats per minute (Fig. 2B). Following nVNS, there were no significant changes in either blood pressure or heart rate.
Figure 2.
A) Blood pressure was monitored via femoral artery catheterization. Anesthetized rats were monitored for an hour then given non-invasive vagus nerve stimulation for 2 minutes, and monitored for 45min (n=4). We found that nVNS did not cause any significant change in blood pressure (97.17±0.57 mmHg).
Figure 2B) Heart rate was monitored via femoral artery catheterization. Anesthetized rats were monitored for an hour then given non-invasive vagus nerve stimulation for 2 minutes, and monitored for 45min (n=4). We found that nVNS using our parameters did not cause any significant change in heart rate (143.32±8.09 beats/min).
3.2 Periorbital Allodynia Sensory Testing
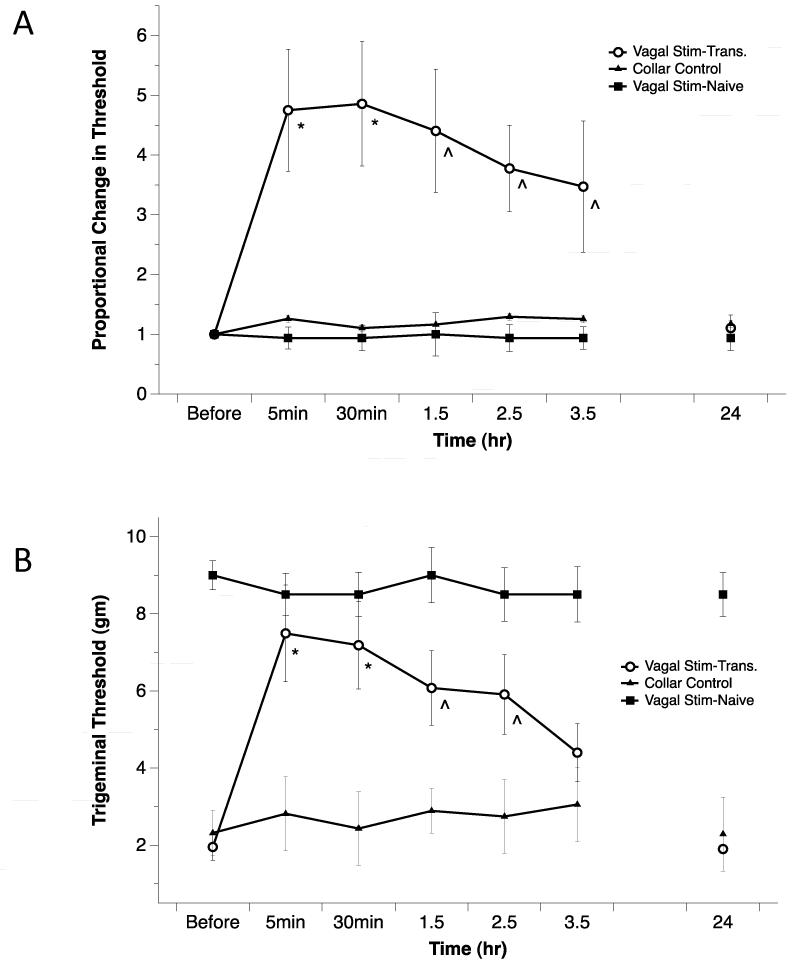
Using silver plated electrodes attached to an Elizabethan collar (Fig. 1A), we quantified the effects of nVNS stimulation in unanaesthetized naive and allodynic rats. Following 2min of nVNS, periorbital thresholds in allodynic rats increased within in the first 5min 4.7±1.0 times compared to their pre-stimulation levels. The analgesic effect of stimulation lasted 3.5 hours (*p<0.01; ^p<0.05), and then returned to baseline by 24 hours. No changes in the periorbital von Frey thresholds were noted in naive animals that received nVNS stimulation or allodynic rats that received the Elizabethan collar placement only without stimulation (Fig. 3A). The collar control group was used to ensure that the person performing the sensory testing was blinded to the treatment. When the data is presented as the absolute values of the von Frey thresholds the thresholds increased 7.5±1.3 fold within the first 5min (Fig. 3B). The increase in threshold was significant for 2.5 hours.
Figure 3.
Using electrodes attached to an Elizabethan collar, conscious naive and allodynic rats received nVNS. Periorbital thresholds were compared to pre-stimulation levels. Following nVNS, allodynic rats significantly increased their thresholds significantly above baseline relative to control animals (Repeated measure ANOVA p<0.05 for both the proportional and gram threshold values, one-way ANOVA of individual time points, *p<0.01; ^p<0.05). A) When the data is presented as a proportional change from baseline, the effect of the stimulation is significant for 3.5 hours, returning to baseline by 24 hours. No threshold change was seen in naive animals that received nVNS stimulation. B) When the data is presented as the absolute values of the von Frey thresholds, the effect is significant for 2.5 hours. (nVNS - transitioned rats n=14, collar control rats n=10, nVNS naive rats n=4).
3.3 Glutamate Levels in the TNC following early and late nVNS treatment
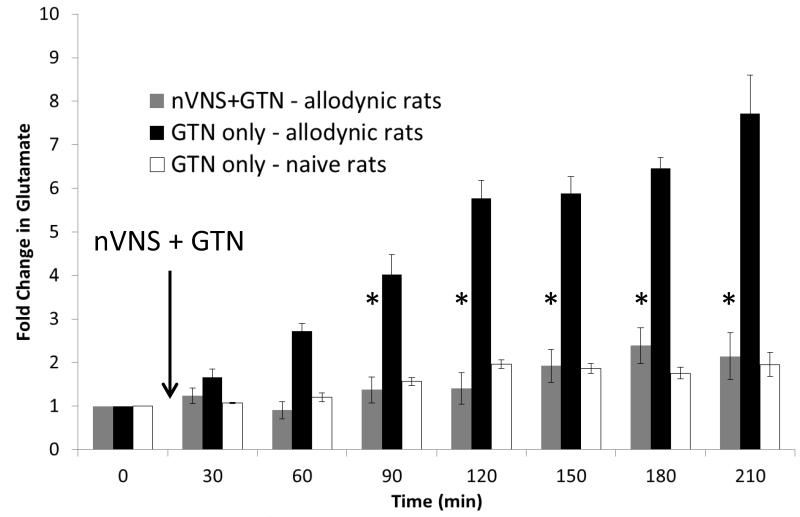
Nitric oxide (NO) donors trigger migraine headaches in migraine patients. Rats with trigeminal allodynia following repeated prostglandin E2 infusions on the dura are also hypersensitive to NO donors [8, 26]. Using microdialysis, we quantified the effect of GTN (0.1mg/kg, i.p.) treatment on extracellular neurotransmitter levels in the TNC of naive and allodynic rats. After 2hr of a stable baseline, microdialysis samples were collected continuously for at least 3.5 hours after GTN treatment. The allodynic rats exhibited 7.7±0.9 fold increase in extracellular glutamate following GTN. When the nVNS stimulation for 2min was coupled with the GTN treatment in the allodynic rats, there was only a 2.3±0.4 fold increase in extracellular glutamate (p<0.01; Fig. 4). The naive rats only had a 1.9±0.1 fold increase in extracellular glutamate; which was not significantly different than the glutamate levels in the nVNS + GTN allodynia rats.
Figure 4.
Allodynic rats which received glyceryl trinitrate (GTN; 0.1mg/kg i.p.; n=5) had a greater than 7 fold increase in extracellular glutamate in the trigeminal nucleus caudalis (TNC). Allodynic rats that were treated with 2 minutes of nVNS at the same time as GTN injection exhibited only a 2.5 fold increase in extracellular glutamate in the TNC (n=5), similar to the GTN response in naive rats (n=5; *p<0.01).
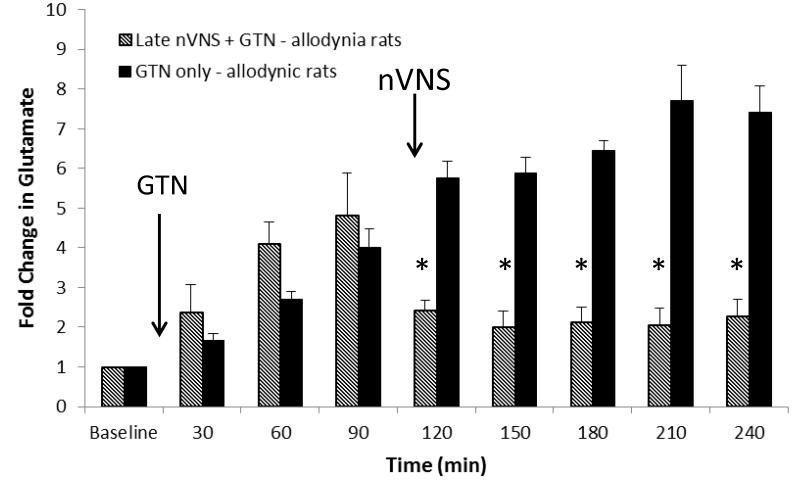
To test if nVNS could reverse the increase in glutamate in the TNC following GTN treatment, we delayed the nVNS for 120min after the injection of GTN (0.1mg/kg, i.p.). In the allodynic rats that received GTN only and the group that was destined to receive nVNS after 120min, there was a comparable increase in glutamate in the TNC during the first 120min. After the 2min nVNS treatment, there was a rapid decrease in the glutamate levels, from 4.8±2.4 fold to 1.9±0.9 fold (Fig. 5; p<0.01).
Figure 5.
Allodynic rats that received i.p. glyceryl trinitrate (GTN; 0.1mg/kg; n=5) had a greater than 7 fold increase in extracellular glutamate in the trigeminal nucleus caudalis (TNC). Allodynic rats that were treated with 2 minutes of nVNS 120 minutes after the GTN injection (n=5) reversed the increase in glutamate noted in the untreated rats. (one-way ANOVA *p<0.01).
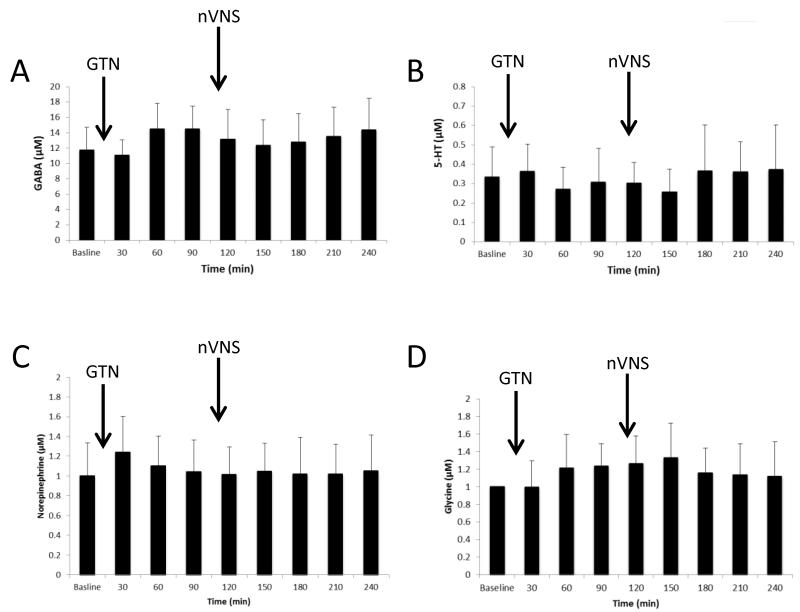
There were no significant differences in the levels of the inhibitory neurotransmitters GABA, serotonin (5-HT), norepinephrine and glycine in allodynic rats that received nVNS 120min after GTN stimulation (Fig. 6). The same samples were used to quantify the glutamate, GABA, 5-HT, and norepinephrine, which act as an internal control for the significant effects on glutamate concentrations that we quantified in Fig. 5.
Figure 6.
Levels of the inhibitor neurotransmitters A) GABA, B) 5-HT C) norepinephrine, and D) glycine in the trigeminal nucleus caudalis (TNC) of allodynic rats that received i.p. glyceryl trinitrate (GTN; 0.1mg/kg) after baseline and 2 min of nVNS 120 minutes later (n=5). There was no significant change in the levels of GABA, 5-HT, norepinephrine and glycine.
4. Discussion
The behavioral experiments of this study demonstrate that non-invasive vagus nerve stimulation alleviates trigeminal allodynia and pain in rats with chronic trigeminal allodynia. Allodynic rats had a significant decrease in periorbital sensitivity for 3.5 hours following acute non-invasive stimulation of the vagus nerve. This effect is achieved after only 2 minutes of stimulation, without the need for surgical implantation of the stimulator directly onto the nerve. These results support the open label clinical studies that suggest that non-invasive vagus nerve stimulation may be beneficial in alleviating migraine and other recurrent headaches [29-31].
Our study identified no changes in blood pressure or heart rate after nVNS. Because there is no change in heart rate and blood pressure, we can rule out the cardiac effects of the vagus nerve as the cause of the behavioral effects noted in Figs. 3A and 3B and the changes in extracellular glutamate levels (Figs. 4 and 5). Following nVNS there was no significant change in the behavior of the rats noted, other than the relief of trigeminal allodynia.
Our microdialysis experiments demonstrate that nVNS stimulation can block or reverse high levels of glutamate in the TNC. High levels of glutamate in the TNC are a marker for increased trigeminal pain. The nVNS suppression of the increase in extracellular glutamate in allodynic rats following GTN treatment matches the time course of the decreases in periorbital sensitivity noted in the behavioral experiments. The suppression of the increase in glutamate in the TNC and the reversal of high levels of glutamate in the TNC following GTN may be the mechanism for nVNS in the treatment of trigeminal allodynia. Other amino acids, such as GABA, glycine, norepinephrine and 5-HT were essentially unchanged in the TNC after nVNS treatment, suggesting that they do not play a role in nVNS suppression of glutamate and the behavioral suppression of allodynia at the level of the TNC. However, these or other inhibitory neurotransmitters in other areas of the brain such as the nucleus of the solitary tract (NTS) may be involved in the suppression of the excessive glutamate levels in the TNC [23].
Several studies have proposed GABA-mediated inhibition as the mechanism for clinical efficacy of VNS [9, 1, 20]. Other nerve stimulating devices, such as occipital nerve and transcutaneous electrical nerve stimulators (ONS and TENS, respectively) work via an increase in GABA in the dorsal horn of the spinal cord [28, 12, 4, 3, 17, 32, 18]. We did not observe an increase in GABA in allodynic rats receiving both nVNS and GTN. This suggests that the mechanism of nVNS stimulation for the treatment of trigeminal allodynia is not through stimulation of the occipital nerve afferents which cause an increase in GABA in the TNC.
In conclusion, non-invasive vagus nerve stimulation decreases trigeminal nociceptive stimulation by suppressing the rise in glutamate after nitric oxide treatment. While the precise mechanism is still unknown, the search for the mechanism of nVNS has the potential to lend new insight into the treatment of trigeminal allodynia and migraine headache.
Acknowledgements
This study was supported by NIH/NINDS R01 NS061571 and Electrocore LLC. We would like to thank Nathan T. Fried for reviewing the manuscript and Elizabeth Cottrell for providing the illustrations in Figures 1A and B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
This study was funded by an unrestricted grant to Michael L. Oshinsky, PhD. from Electrocore LLC. Bruce J. Simon is a full-time employee of Electrocore LLC.
References
- 1.Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, Treig T, Stefan H, Ramsay RE, Wernicke JF, Wilderd BJ. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221–7. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 2.Bohotina C, Scholsema M, Multona S, Martinc D, Bohotinb V, Schoenen J. Vagus nerve stimulation in awake rats reduces formalin-induced nociceptive behaviour and fos immunoreactivity in trigeminal nucleus caudalis. Pain. 2003;101(1-2):3–12. doi: 10.1016/s0304-3959(02)00301-9. [DOI] [PubMed] [Google Scholar]
- 3.DeSantana JM, da Silva LF, De Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience. 2009;163(4):1233–1241. doi: 10.1016/j.neuroscience.2009.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Current Rheumatology Reports. 2008;10(6):492–9. doi: 10.1007/s11926-008-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. Journal of Pharmacology and Experimental Therapeutics. 2006;318(2):890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- 6.Dougherty KJ, Sawchuk MA, Hochman S. Properties of mouse spinal lamina I GABAergic Interneurons. Journal of Neurophysiology. 2005;94(5):3221–3227. doi: 10.1152/jn.00184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goadsby P, Lipton R, Cady R, Mauskop A. Grosberg, Brian. Non-invasive Vagus Nerve Stimulation (nVNS) for Acute Treatment of Migraine: An Open-label Pilot Study. Annual Meeting of the American Academy of Neurology. 2013 Abstract Number: 2571. [Google Scholar]
- 8.Greco R, Mangione AS, Sandrini G, Maccarrone M, Nappi G, Tassorelli C. Effects of anandamide in migraine: data from an animal model. J Headache Pain. 2011 Apr;12(2):177–83. doi: 10.1007/s10194-010-0274-4. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience and Behavioral Reviews. 2005;29(3):493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Gschossmann JM, Mayer EA, Miller JC, Rayboud HE. Subdiaphragmatic vagal afferent innervation in activation of an opioidergic antinociceptive system in response to colorectal distension in rats. Neurogastroenterology and Motility. 2002;14(4):403–408. doi: 10.1046/j.1365-2982.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- 11.Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. Journal of Pain. 2003;4(9):530–534. doi: 10.1016/j.jpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Rev of Neurotherapeutics. 2011 May;11(5):735–753. doi: 10.1586/ern.11.48. [DOI] [PubMed] [Google Scholar]
- 13.Krahl SE, Clark KB, Smith DC, Browning RA. Locus Coeruleus Lesions Suppress the Seizure-Attenuating Effects of Vagus Nerve Stimulation. Epilepsia. 1998;39(7):709–14. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 14.Lenaerts ME, Lenaerts ME, Oommen KJ, Couch JR, Skaggs V. Can vagus nerve stimulation help migraine? Cephalagia. 2008 Apr;28(4):392–5. doi: 10.1111/j.1468-2982.2008.01538.x. [DOI] [PubMed] [Google Scholar]
- 15.Leonardi M, Mathers C. Global burden of migraine in the year 2000: Summary of methods and data Sources. Global Burden of Disease. 2000:1–18. 2000. [Google Scholar]
- 16.Lipton RB, Hamelsky SW, Stewart WF. Epidemiology and impact of headache. In: SD Silberstein, RB Lipton, DJ Dalessio., editors. Wolff’s Headache and Other Head Pain. 7th Edition Oxford University Press; New York, NY: 2001. pp. 85–107. [Google Scholar]
- 17.Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp Brain Res. 2001;137(1):94–102. doi: 10.1007/s002210000629. [DOI] [PubMed] [Google Scholar]
- 18.Maeda Y, Lisi TL, Vance CG, Sluka KA. Release of GABA and activation of GABAA receptors in the spinal cord mediates the effects of TENS in rats. Brain Res. 2007;1136(1):43–50. doi: 10.1016/j.brainres.2006.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manta S, Dong J, Debonnel G, Blier P. Optimization of vagus nerve stimulation parameters using the firing activity of serotonin neurons in the rat dorsal raphe. European Neuropsychopharmacology. 2009;19(4):250–5. doi: 10.1016/j.euroneuro.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Marrosu F, Serra A, Maleci A, Puligheddu M, Biggio G, Piga M. Correlation between GABAA receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy. Epilepsy Research. 2003;55(1-2):59–70. doi: 10.1016/s0920-1211(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 21.Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25(2):82–86. doi: 10.1111/j.1468-2982.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell CR, Spangenberg RJ, Hoek JB, Silberstein SD, Oshinsky ML. Acetate causes alcohol hangover headache in rats. PLoS ONE. 2010;5(12):e15963. doi: 10.1371/journal.pone.0015963. doi: 10.1371/journal.pone.0015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDougal DH, Hermann GE, Rogers RC. Vagal Afferent Stimulation Activates Astrocytes in the Nucleus of the Solitary Tract Via AMPA Receptors: Evidence of an Atypical Neural-Glial Interaction in the Brainstem. Journal of Neuroscience. 2011;31:14037–14045. doi: 10.1523/JNEUROSCI.2855-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesbitt A, Marin J, Goadsby P. Treatment of hemicrania continua by non-invasive vagus nerve stimulation in 2 patients previously treated with occipital nerve stimulation. The Journal of Headache and Pain. 2013;1(Suppl 1):P230. [Google Scholar]
- 25.Nesbitt A, Marin J, Tomkins E, Ruttledge M, Goadsby P. Non-invasive vagus nerve stimulation for the treatment of cluster headache: a case series. The Journal of Headache and Pain. 2013;14:P231. [Google Scholar]
- 26.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007 Jul-Aug;47(7):1026–36. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshinsky ML, Luo J. Neurochemistry of trigeminal activation in an animal model of migraine. Headache. 2006;46(Suppl 1):S39–44. doi: 10.1111/j.1526-4610.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 28.Oshinsky ML, Hirata H, Poletto C, Wacnik P. Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2011. Occipital Nerve Stimulation Suppresses Dural Sensitization, Program No. 701.07/RR2. [Google Scholar]
- 29.Proietti A, Mea E, Tullo V, Curone M, Franzini A, Broggi G, Savino M, Bussone G, Leone M. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurological science. 2009;30(suppl 1):101–104. doi: 10.1007/s10072-009-0073-3. [DOI] [PubMed] [Google Scholar]
- 30.Schoffnegger D, Heinke B, Sommer C, Sandkühler J. Physiological properties of spinal lamina II GABAergic neurons in mice following peripheral nerve injury. Journal of Physiology. 2006;577(Pt 3):869–78. doi: 10.1113/jphysiol.2006.118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silberstein SD, Lipton RB, Dalessio DJ. Overview, diagnosis, and classification of headache. In: Silberstein SD, Lipton RB, Dalessio DJ, editors. Wolff’s Headache and Other Head Pain. 7th Edition Oxford University Press; New York, NY: 2001. pp. 6–26. [Google Scholar]
- 32.Sluka KA, Vance CGT, Lisi TL. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J Neurochem. 2005;95(6):1794–1801. doi: 10.1111/j.1471-4159.2005.03511.x. [DOI] [PubMed] [Google Scholar]