Abstract
Glycolytic ATP synthesis by synaptic vesicles provides an efficient mechanism for fueling vesicular loading of the neurotransmitter glutamate. This is achieved in part by vesicle-bound pyruvate kinase. However, we have found that vesicular glutamate uptake, in the presence of the pyruvate kinase substrates ADP and phosphoenolpyruvate (PEP), substantially exceeds that caused by exogenous ATP. We propose that this much enhanced uptake is in part due to extra ATP produced via a mechanism involving a novel enzyme, PEP-dependent ADP synthase. We discuss implications for this enzyme in energy homeostasis and pathophysiology, as well as in efficient synaptic glutamate transmission.
Keywords: energy metabolism, ADP synthesis, glycolytic intermediate, VGLUT, glutamate transmission
Introduction
Glucose serves as the major substrate for cerebral energy, under normal conditions, and glycolysis plays a vital role in maintaining normal brain function and synaptic transmission [1]. Glucose utilization and cognitive function are directly correlated [2–5]. Reduction of glucose levels results in pathophysiological states and abnormal electrophysiological activity. However, this occurs long before significant decrease in tissue ATP levels is detected [6–10]. Substitution of pyruvate for glucose maintains normal tissue ATP levels but fails to restore normal evoked neuronal activity [11–13]. Abnormal synaptic transmission caused by hypoglycemia occurs in part, if not entirely, by a presynaptic mechanism [10, 14, 15]. Substantial reduction of extracellular glucose results in decreased stimulus-evoked glutamate release, with no detectable changes in global cellular ATP levels [10]. These studies suggest that glycolytic intermediate(s) or local ATP concentration would be critical for normal synaptic transmission independent of global cellular ATP levels.
In a study of these possibilities, Ikemoto et al. [16] have found that a glycolytic ATP-generating complex, consisting of glyceraldehyde phosphate dehydrogenase (GAPDH) and 3-phosphoglycerate kinase (3-PGK), is highly enriched in synaptic vesicles. Activation of this enzyme complex was shown sufficient in order to support loading of synaptic vesicles with the major excitatory neurotransmitter glutamate. Glutamate accumulation into synaptic vesicles in the nerve terminal is an initial crucial step in glutamate transmission [17–22]. The synaptic vesicular glutamate uptake system [17, 23] is comprised of the vesicular glutamate transporter known as VGLUT (24, 25), and v-type proton-pump ATPase [26], and requires ATP to generate an electrochemical gradient, the driving force for glutamate uptake into synaptic vesicles [24, 27–31].
Evidence [16] also suggests that glycolytically produced ATP, rather than ATP made in mitochondria, is a major source of energy for fueling glutamate loading into synaptic vesicles. Compatible with this notion, Ishida et al. [32] have recently shown that ATP produced by synaptic-vesicle bound pyruvate kinase, the other glycolytic ATP-generating enzyme, at the expense of the glycolytic intermediate PEP, can also support vesicular glutamate uptake. Thus, since glycolytic ATP production (which occurs considerably prior to mitochondrial ATP synthesis) can occur at the synaptic vesicle site, local glycolytic ATP synthesis could represent an efficient mechanism for the rapid energy supply needed to sustain normal synaptic transmission. These lines of evidence offer new insight into the molecular mechanism of vital dependence on glycolysis of normal synaptic transmission [33].
In this communication, we report the findings that ATP generated by synaptic vesicles in the presence of PEP and ADP is more effective than exogenous ATP in fueling vesicular glutamate uptake, and that an additional ATP production involving a new ADP-regenerating mechanism (harnessing AMP and PEP) underlies this enhanced glutamate uptake. Wider implications for this ADP synthase are discussed.
Materials and Methods
Reagents and Chemicals
Frozen rat (male) brains used in this study were purchased from Pel-Freez. Thus, this research did not involve handling of live animals. L-[3,4-3H]glutamic acid (50 Ci/mmol), [8-14C]ADP (40–60 Ci/mmol) [α-32P]ATP (3,000 Ci/mmol) and [γ-32P]ATP (6,000 Ci/mmol) were purchased from PerkinElmer. Adenosine 5′-O-thiomonophosphate (AMPS), ATP, ADP, AMP, adenosine, apyrase, and muscle pyruvate kinase were obtained from Sigma-Aldrich.
Assay for Vesicular Glutamate Uptake
A pyruvate kinase-rich synaptic vesicle fraction was prepared from frozen rat cerebrum, and glutamate uptake into these synaptic vesicles measured by a filtration-based assay using Whatman GF/C filters, as described previously [32]. The vesicle fraction (30 or 15 μg) was incubated at 30° C in a solution (final volume, 0.1 ml), which contained 50 μM [3H]glutamate (280 mCi/mmol), 20 mM Hepes-Tris (pH 7.4), 4 mM MgSO4, 4 mM KCl, 2 mM aspartate, 100 mM potassium gluconate, and 50 mM sucrose, in the absence or presence of 0.8 mM ADP alone, a combination of 0.8 mM ADP and 1 mM PEP, or 1 mM ATP alone (Fig. 1), or of 1 mM PEP alone or a combination of 1 mM PEP and 0.5 mM AMP each with various concentrations of ADP (Fig. 5). Glutamate uptake was terminated by the addition of 2.5 ml of ice-cold 150 mM KCl, followed by immediate filtration through Whatman GF/C filters. The incubation tube was washed three times, and the filter was then washed three more times. Radioactivity (3H) retained on the filter was determined in a Beckman LS 6500 scintillation spectrophotometer.
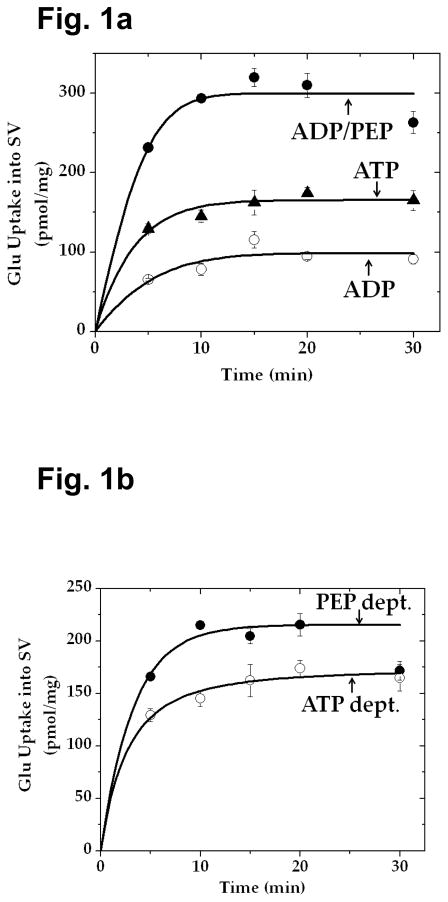
Fig. 1.
Glutamate uptake into synaptic vesicles caused by PEP and ADP is greater than that fueled by exogenous ATP. a Pyruvate kinase-rich synaptic vesicles (SV-A, 30 μg) were incubated with 50 μM [3H]glutamate in the absence or presence of 0.8 mM ADP, 0.8 mM ADP plus 1 mM PEP, or 1 mM ATP alone in the medium described in Materials Methods, and glutamate uptake determined. b PEP-dependent (PEP-dept.) uptake indicates uptake in the presence of a combination of PEP and ADP, minus uptake in the presence of ADP alone; this represents vesicle-bound pyruvate kinase-generated ATP-fueled uptake. The data represent mean ± SEM of three separate vesicle preparations. PEP-dept., PEP-dependent; ATP-dept., ATP-dependent.
Fig. 5.
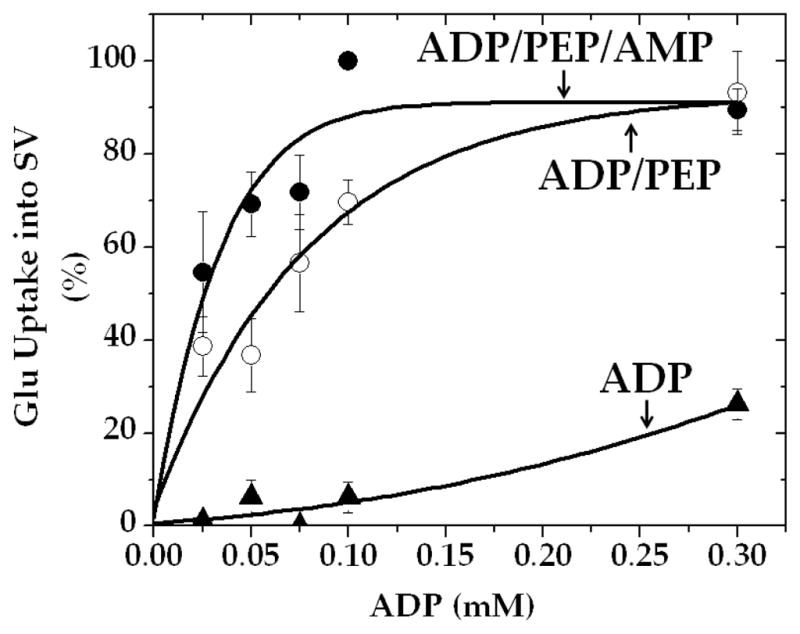
AMP enhances pyruvate kinase-fueled vesicular glutamate uptake in the presence of low, but not high, concentrations of ADP. Synaptic vesicles (15 μg) were incubated for 10 min, with 1 mM PEP or 1 mM PEP plus 0.5 mM AMP, in the presence of various ADP concentrations in the medium described in Materials and Methods. Values are mean ± SEM of three vesicle preparations. 100% represents 149 ± 47 pmol/mg.
Preparation of [32P]AMP
[α-32P]ATP was converted to [32P]AMP by use of apyrase. The reaction mixture (0.18 ml) contained 1 mM [α-32P]ATP (1.4 Ci/mmol), 0.1 unit of apyrase, 3.8 mM CaCl2, and 38 mM succinate (pH 6.7). After incubation at 30° C for 10 min, the mixture was filtered with an Ultrafree-MC filter unit (0.1 μm, Millipore). Aliquots were subjected to high pressure liquid chromatography (HPLC) on Whatman Partisil 10 SAX (4.6 × 250 mm); flow rate: 1 ml/min); 10-sec fractions were collected. The column had been pre-equilibrated with 6 mM sodium phosphate (pH 7.5). Three minutes after sample application, the buffer concentration was increased to 56 mM to last additional 23 min, then to 200 mM to last further 40 min. This method provided base line resolutions for ATP, ADP and AMP; their retention times (min) were 35, 20.4, and 10.4, respectively. Authentic nucleotides were monitored at 260 nm. [32P]AMP peak fractions were pooled. [α-32P]ATP was found to be quantitatively converted to [32P]AMP under the incubation conditions used.
Assay for Formation of [α-32P]ADP and [α-32P]ATP from [32P]AMP
The pyruvate kinase-rich vesicle fraction (40 μg) was incubated at 30° C for 6 min in a solution (final volume, 0.3 ml) containing 0.5 mM [32P]AMP (11.6 mCi/mmol), 20 mM Hepes-Tris (pH 7.4), 4 mM MgSO4, 4 mM KCl, 2 mM aspartate, 0.1 mM ADP, 100 mM potassium gluconate, and 50 mM sucrose, in the absence or presence of 1 mM PEP. The mixture was frozen on dry ice, thawed, and filtered first with a glass microfiber GF/C syringe filter (1.2 μm, Whatman), then with an Ultrafree-MC filter unit (0.1 μm, Millipore). Aliquots (0.1 ml) were subjected to HPLC for product analysis, as described above for the preparation of [32P]AMP.
Treatment of [32P]AMP-derived Reaction Products with Hexokinase
The pyruvate kinase-rich vesicle fraction (40 μg) was first incubated at 30° C for 30 min in a solution (final volume, 0.2 ml) containing 0.1 mM [32P]AMP (64 mCi/mmol), 0.3 mM PEP, 20 mM Hepes-Tris (pH 7.4), 4 mM MgSO4, 4 mM KCl, 2 mM aspartate, 0.2 mM ADP, 100 mM potassium gluconate, and 50 mM sucrose. Glucose (final concentration, 10 mM) with or without hexokinase (0.1 unit) was then added to the mixture, followed by an additional 10 min incubation. The mixture was frozen on dry ice, thawed, and double-filtered, and aliquots (0.05 ml) subjected to HPLC for product analysis, as described above.
Statistical analysis
Data are presented as the mean ± SEM. Statistical analysis was performed with student t-test for difference between two values. Significance was set at p < 0.05.
3. Results
ATP Generated by Synaptic Vesicles from PEP and ADP is More Effective than Exogenous ATP in Fueling Vesicular Glutamate Uptake
Ishida et al. [32] reported evidence suggesting that ATP generated endogenously by synaptic vesicles in the presence of PEP and ADP is preferentially used compared to exogenous ATP; exogenous hexokinase has less access to the former ATP than to the latter. This supports the notion that ATP locally produced at the synaptic vesicle site is important in efficient fueling glutamate loading into synaptic vesicles. In an effort to provide further supporting evidence, we have directly compared the effectiveness of ATP synthesized endogenously by synaptic vesicles (in the presence of PEP and ADP) with that of exogenous ATP in fueling vesicular glutamate uptake.
As shown in Fig. 1, glutamate uptake in the presence of PEP and ADP was found to exceed that in the presence of exogenous ATP. Uptake in the presence of ADP alone is most likely fueled by ATP produced by adenylate kinase. After subtraction of the latter, uptake activity attained in the presence of PEP and ADP is still higher than that observed in the presence of exogenous ATP. Pyruvate kinase-mediated uptake in the presence of 1 mM PEP and 0.8 mM ADP would not exceed uptake in the presence of exogenous 1 mM ATP, since that enzyme alone cannot produce ATP more than 1 mM under these conditions; the concentration of the phosphate donor PEP is 1 mM. This suggests that the augmented uptake involves an additional mechanism involving an enzyme distinct from pyruvate kinase and adenylate kinase.
PEP-dependent ATP Formation Exceeds PEP-dependent ADP Consumption
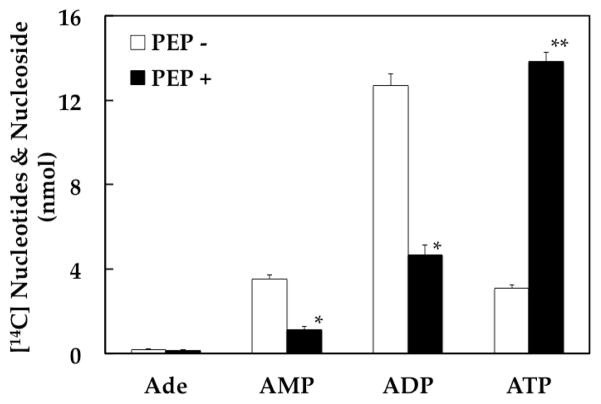
In an effort to elucidate the additional mechanism, we have incubated synaptic vesicles with [14C]ADP in the presence or absence of PEP, and analyzed reaction products (Fig. 2). In the absence of PEP, ATP and AMP were produced in an equal amount. Conversion of ADP to these nucleotides is most likely achieved by adenylate kinase. Upon addition of PEP, a large amount of ADP was converted to ATP by pyruvate kinase, as expected. Of interest, however, the amount of ATP produced in a PEP-dependent manner was greater than that of ADP apparently disappeared in the presence of PEP (Fig. 2). Moreover, AMP produced in the absence of PEP disappeared upon addition of PEP, presumably being converted to ADP initially, then to ATP. When the PEP-dependent disappearance of AMP is added to the difference in steady-state ADP levels between the presence and absence of PEP, this is equivalent to the PEP-dependent production of ATP. This indicates that a portion of ATP produced in a PEP-dependent manner is derived from AMP. This portion increased to about 50% upon a longer incubation (30 min). The other portion is presumably made by pyruvate kinase directly from ADP. The AMP-derived ATP production is likely through PEP-dependent formation of ADP from AMP. We postulate that this process is mediated by a new enzyme (which could be designated PEP:AMP phosphotransferase), here simply referred to as ADP synthase.
Fig. 2.
PEP-dependent formation of [14C]ATP from [14C]ADP. Synaptic vesicles (40 μg) were incubated at 30°C for 10 min with 0.2 mM [14C]ADP (0.66 mCi/mmol) in the absence or presence of 1 mM PEP in the standard medium (final volume, 0.3 ml) described in Materials and Methods. The mixture was double-filtered, and aliquots (0.1 ml) analyzed by HPLC, as described in Materials and Methods. The data represent mean ± SEM of three vesicle preparations. PEP − vs. PEP +: * p < 0.05; ** p < 0.01.
PEP-dependent ATP Formation via PEP-dependent ADP Formation from AMP
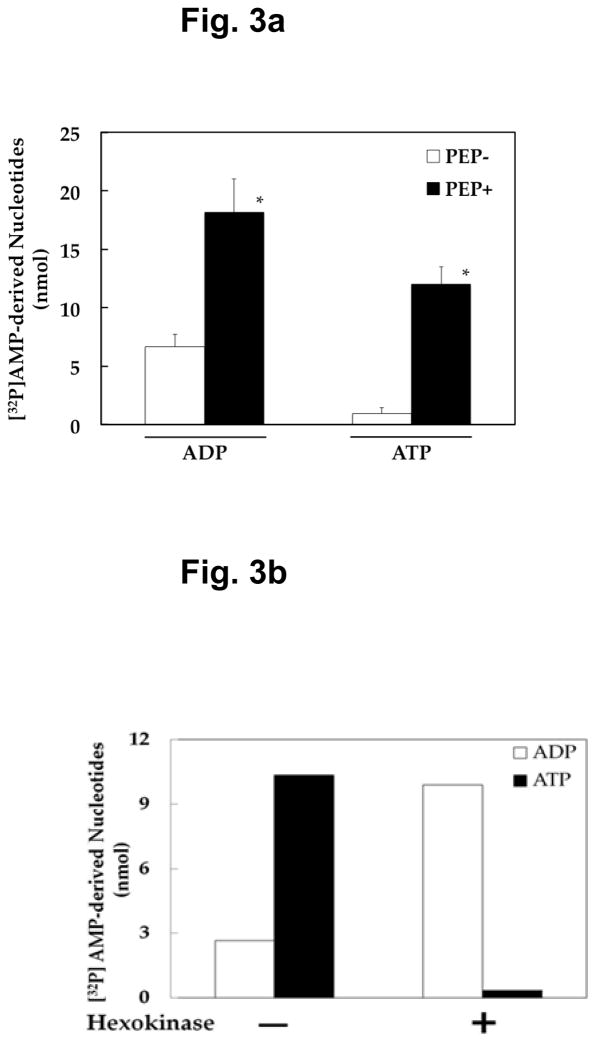
In order to provide direct evidence to support this notion, we synthesized [32P]AMP, as described in Material and Methods, and incubated it with synaptic vesicles in the absence or presence of PEP. Figure 3a shows that [32P]AMP is indeed converted to [32P]ATP as well as to [32P]ADP in a PEP-dependent manner. Similar results were observed with 0.1 mM [32P]AMP and 0.3 mM PEP (data not shown). Figure 3b indicates that 32P of [32P]ATP resides in the ADP moiety. When [32P]ATP was treated with hexokinase in the presence of glucose, it yielded [32P]ADP, not glucose-6-[32P]phosphate. This is consistent with ADP being [α-32P]ADP. These results support the notion that [32P]AMP is first converted, at the expense of PEP, to [α-32P]ADP, which is then transformed to [α-32P]ATP by pyruvate kinase. These results raise the possibility of a new pathway leading to ATP production from AMP, suggesting a novel recycling mechanism for ATP regeneration. Phosphorylation of AMP at the expense of the high-energy glycolytic intermediate PEP represents a novel biochemical reaction, since the only reaction presently known for conversion of AMP to ADP is AMP phosphorylation at the expense of the most common cellular energy ATP. This reaction could contribute to re-synthesis as well as de novo synthesis of ATP, by providing this new biochemical route to ATP synthesis: AMP plus PEP to ADP, then to ATP, as opposed to AMP plus ATP to ADP, then to ATP.
Fig. 3. PEP-dependent formation of [32P]ADP and [32P]ATP from [32P]AMP, and treatment of reaction products with hexokinase.
a Synaptic vesicles (40 μg) were incubated for 6 min with 0.5 mM [32P]AMP in the absence or presence of 1 mM PEP, and the reaction mixture double-filtered and aliquots (0.1 ml) analyzed for products by HPLC, as described in Materials and Methods. Data indicate values calculated to represent the amount of ADP and ATP formed in the total incubation volume (0.3 ml), with mean ± SEM of three vesicle preparations. − PEP vs. + PEP: * p < 0.05. b 32P in [32P]AMP-derived [32P]ATP resides in the ADP moiety. Reaction products generated after incubation of synaptic vesicles (40 μg) for 30 min with 0.1 mM [32P]AMP (64 mCi/mmol) and 0.3 mM PEP were treated with hexokinase (0. 1 unit) in the presence of glucose (10 mM), and aliquots (0.05 ml) analyzed for [32P]ADP and [32P]ATP, as described in Materials and Methods. Data indicate values calculated to represent the amount of ADP and ATP found after hexokinase treatment in the total incubation volume (0.2 ml).
Mention might be made that purified pyruvate kinase (cloned M2) itself did not catalyze synthesis of [32P]ADP or [32P]ATP directly from [32P]AMP and PEP, as expected (data not shown). Moreover, we have obtained direct evidence that the phosphoryl group of PEP is transferred to AMP, using [32P]PEP we synthesized (data not shown).
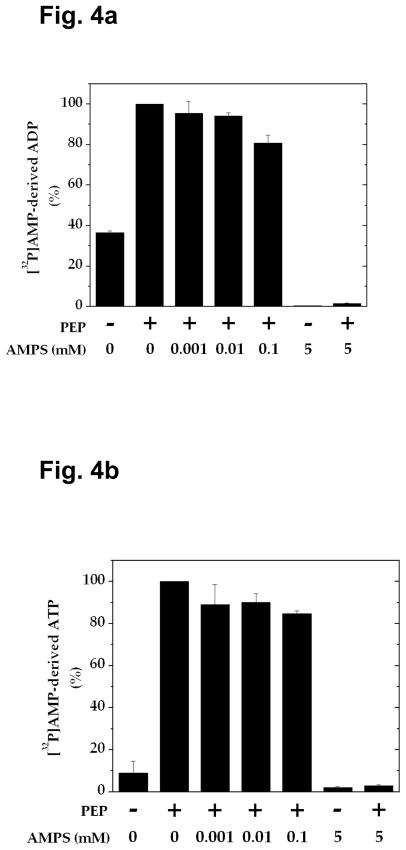
PEP-dependent ADP Formation Is Inhibited by AMPS
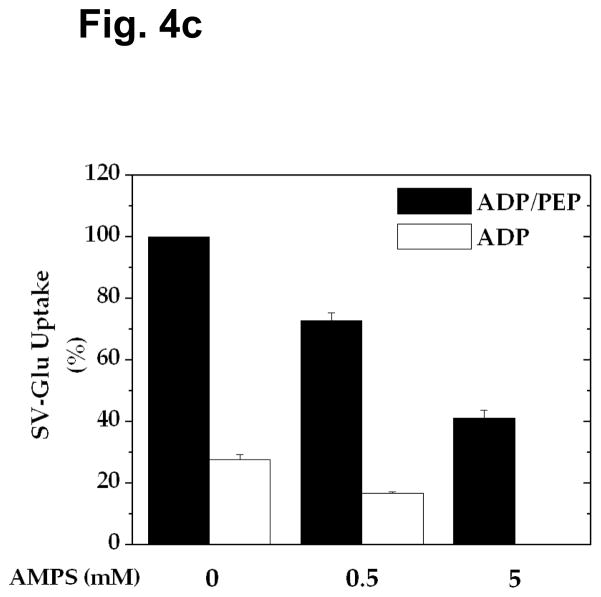
The experiments described above altogether suggest that vesicular glutamate uptake in the presence of PEP and ADP is fueled by ATP made on synaptic vesicles, not only by pyruvate kinase and adenylate kinase, but also by a mechanism involving ADP synthase coupled to pyruvate kinase. In an effort to assess the contribution of each of these mechanisms, we have sought ADP synthase inhibitors. As shown in Figs. 4a and 4b, we have found that the AMP analog AMPS inhibits formation of [32P]ADP and [32P]ATP from [32P]AMP almost completely at 5 mM. When this compound was tested for the ability to inhibit vesicular glutamate uptake in the presence of PEP and ADP, it inhibited adenylate kinase-mediated glutamate uptake entirely at 5 mM (Fig. 4c). However, it inhibited adenylate kinase-independent uptake only partially even at 5 mM, the concentration which caused almost total inhibition of ADP synthase activity. This suggests that AMPS-resistant vesicular glutamate uptake is mediated by pyruvate kinase alone. Based upon the percent inhibition of each of these mechanisms, one could calculate that 41, 27, and 32% of vesicular glutamate uptake observed in the presence of PEP and ADP are attributed to pyruvate kinase, adenylate kinase, and ADP synthase, respectively. This indicates that ATP produced by a mechanism involving ADP synthase significantly contributes, as an energy source, to vesicular glutamate occurring under these conditions. Thus, ATP generated in this manner could be responsible for the enhanced uptake beyond that fueled by pyruvate kinase and adenylate kinase.
Fig. 4.
Effect of AMPS on formation of [32P]AMP-derived ADP and ATP and on vesicular glutamate uptake. a, b Synaptic vesicles (40 μg) were incubated for 6 min in the presence of various concentrations of AMPS under the same conditions as described in Fig. 3. Aliquots (0.1 ml) of the double-filtered mixture were analyzed for [32P]ADP (a) and [32P]ATP (b). The data represent mean ± SEM of three vesicle preparations. c Synaptic vesicles (10 μg) were incubated for 10 min with various concentrations of AMPS, in the presence of 0.8 mM ADP or 0.8 mM ADP plus 1 mM PEP, as described in Fig. 1. The data represent average of two vesicle preparations.
AMP Augments Vesicular Glutamate Uptake Fueled by PEP and Low Concentrations of ADP
Our results (as in Fig. 5) support the importance of re-synthesis of extra ADP from AMP, at the expense of PEP, in facilitating synthesis of the neurotransmitter pool of glutamate. Thus, one would expect that AMP, with PEP, would augment vesicular glutamate uptake, particularly in the presence of low, but not saturating, concentrations of ADP.
Discussion
We have shown that ATP generated by synaptic vesicles from ADP and PEP can exceed exogenous ATP in fueling glutamate transport into synaptic vesicles. This could be in part due to better access of H+-ATPase to endogenously made ATP by vesicle-bound pyruvate kinase than to exogenous ATP, and also in part to ATP made from ADP by adenylate kinase. Moreover, our experiments presented here suggest that extra ATP derived from AMP via PEP-dependent ADP synthesis could significantly contribute to the enhanced glutamate uptake. ATP produced by each of these three mechanisms seems to equally contribute to generating the driving force for VGLUT. We propose that this ADP synthesis is catalyzed by ADP synthase, a novel enzyme. As to the third mechanism, we put forward the following working hypothesis:
Synaptic vesicles are capable of recycling AMP (made from ADP by adenylate kinase) back to ADP, harnessing the glycolytic high-energy intermediate PEP; this re-generated ADP is then converted to ATP by both pyruvate kinase (at the expense of PEP) and adenylate kinase. This pool of ATP derived from re-generated ADP from AMP would account for enhanced vesicular glutamate uptake observed in the presence of ADP and PEP, the enhanced uptake beyond the uptake caused simply by pyruvate kinase and adenylate kinase alone. This PEP/AMP-mediated enhanced uptake could play a role in efficient synaptic glutamate transmission, and contribute to the mechanism underlying glycolysis-dependent synaptic transmission. Thus, observations presented here could provide fresh insight into understanding the importance of glucose metabolism in synaptic transmission.
ADP synthase may not be limited to synaptic vesicles. PEP-dependent AMP-derived ATP formation was also observed in the brain cytosol fraction; ATP formation in nerve ending cytosol was twice as high as that in cell body cytosol. Moreover, PEP-dependent AMP-derived ATP formation was also observed in the cytosol fraction of heart, liver, and lung as well; activity in the heart was approximately the same as in the brain, but activity in the liver and lung was about 50% and 25% of that in the brain, respectively. This suggests that high-energy-demanding tissues are more enriched with this enzyme. ADP synthase activity could be enriched by affinity chromatography on Blue Sepharose (about 135-fold increase in specific activity), further supporting the existence of ADP synthase.
Cellular ADP does not just serve as an important substrate for ATP synthesis. It also affects bioenergetics by activating certain enzymes. It acts as an allosteric activator of the oxidative enzymes glutamate dehydrogenase [34–36] and isocitrate dehydrogenase [37–39] and the respiratory enzyme cytochrome c oxidase [40], all present in mitochondria. Moreover, mitochondrial oxidative phosphorylation is stimulated by cytosolic ADP concentration, with a second order kinetic function with respect to its concentration [41, 42]. Hence, influx of cytosolic ADP into mitochondria could play a critical role in modulation of mitochondrial ATP production by activating these enzymes. It would be of interest to see if changes in ADP synthase activity lead to alteration of activation of these enzymes and affect energy homeostasis.
In post-ischemic muscles, the free ADP concentration is decreased compared to non-ischemic control [43]. In brain tissue, we have observed that ADP synthase activity in synaptosomes is markedly reduced (by 72–74%) under acute aglycemic conditions (30–60 min). This observation could have implications for study of the regulatory mechanism of this enzyme, as well as of stroke and some of the other CNS pathophysiologies involving abnormal glucose metabolism. AMPS or, better yet, a more potent, membrane permeant inhibitor derivative of this compound, could be of use in such studies, as well as in determining a role for this enzyme in synaptic transmission.
Acknowledgments
This work was supported by National Institutes of Health NIMH grant MH 071384 (T.U.). We thank D. Minor J. Coon for reading the manuscript.
Abbreviations
- PEP
phosphoenolpyruvate
- HPLC
high pressure liquid chromatography
- AMPS
Adenosine 5′-O-thiomonophosphate
References
- 1.Siesjo BK. Brain Energy Metabolism. J. Wiley & Sons; NY: 1978. pp. 101–130. [Google Scholar]
- 2.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci USA. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci USA. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamport DJ, Lawton CL, Mansfield MW, Dye L. Impairments in glucose tolerance can have a negative impact on cognitive function: a systematic research review. Neurosci Biobehav Rev. 2009;33:394–413. doi: 10.1016/j.neubiorev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, Petersen RC, Shaw LM, Trojanowski JQ, Jack CR, Jr, Weiner MW, Jagust WJ. Alzheimer’s disease neuroimaging initiative: Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–8. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis LD, Ljunggren B, Ratcheson RA, Siesjo BK. Cerebral energy state in insulin-induced hypoglycemia, related to blood glucose and to EEG. J Neurochem. 1974;23:673–679. doi: 10.1111/j.1471-4159.1974.tb04390.x. [DOI] [PubMed] [Google Scholar]
- 7.Dirks B, Hanke H, Krieglstein J, Stock R, Wickop G. Studies on the linkage of energy metabolism and neuronal activity in the isolated perfused rat brain. J Neurochem. 1980;35:311–317. doi: 10.1111/j.1471-4159.1980.tb06266.x. [DOI] [PubMed] [Google Scholar]
- 8.Ghajar JBG, Plum F, Duffy TE. Cerebral oxidative metabolism and blood flow during acute hypoglycemia and recovery in unanesthetized rats. J Neurochem. 1982;38:397–409. doi: 10.1111/j.1471-4159.1982.tb08643.x. [DOI] [PubMed] [Google Scholar]
- 9.Bachelard HS, Cox DWG, Drower J. Sensitivity of guinea-pig hippocampal granule cell field potentials to hexoses in vitro: an effect on cell excitability? J Physiol. 1984;352:91–102. doi: 10.1113/jphysiol.1984.sp015279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleck MW, Henze DA, Barrionuevo G, Palmer AM. Aspartate and glutamate mediate excitatory synaptic transmission in area CA1 of the hippocampus. J Neurosci. 1993;13:3944–3955. doi: 10.1523/JNEUROSCI.13-09-03944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox DWG, Bachelard HS. Attenuation of evoked field potentials from dentate granule cells by low glucose, pyruvate + malate, and sodium fluoride. Brain Res. 1982;239:527–534. doi: 10.1016/0006-8993(82)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Cox DWG, Morris PG, Feeney J, Bachelard HS. 31P-n.m.r. studies on cerebral energy metabolism under conditions of hypoglycaemia and hypoxia in vitro. Biochem J. 1983;212:365–370. doi: 10.1042/bj2120365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanatani T, Mizuno K, Okada Y. Effects of glycolytic metabolites on preservation of high energy phosphate level and synaptic transmission in the granule cells of guinea pig hippocampal slices. Experientia. 1995;51:213–216. doi: 10.1007/BF01931098. [DOI] [PubMed] [Google Scholar]
- 14.Shoji S. Glucose regulation of synaptic transmission in the dorsolateral septal nucleus of the rat. Synapse. 1992;12:322–332. doi: 10.1002/syn.890120409. [DOI] [PubMed] [Google Scholar]
- 15.Spuler A, Endres W, Grafe P. Glucose depletion hyperpolarized guinea pig hippocampal neurons by an increase in potassium conductance. Exp Neurol. 1988;100:248–252. doi: 10.1016/0014-4886(88)90217-8. [DOI] [PubMed] [Google Scholar]
- 16.Ikemoto A, Bole DG, Ueda T. Glycolysis and glutamate accumulation into synaptic vesicles. Role of glyceraldehyde phosphate dehydrogenase and 3-phosphoglycerate kinase. J Biol Chem. 2003;278:5929–5940. doi: 10.1074/jbc.M211617200. [DOI] [PubMed] [Google Scholar]
- 17.Ueda T. Glutamate transport in the synaptic vesicle. In: Roberts PJ, Storm-Mathisen J, Bradford HF, editors. Excitatory Amino Acids. Macmillan; London: 1986. pp. 173–195. [Google Scholar]
- 18.Maycox PR, Hell JW, Jahn R. Amino acid neurotransmission: spotlight on synaptic vesicles. Trends Neurosci. 1990;13:83–87. doi: 10.1016/0166-2236(90)90178-d. [DOI] [PubMed] [Google Scholar]
- 19.Özkan ED, Ueda T. Glutamate transport and storage in synaptic vesicles. Jpn J Pharmacol. 1998;77:1–10. doi: 10.1254/jjp.77.1. [DOI] [PubMed] [Google Scholar]
- 20.Fonnum F, Fykse EM, Roseth S. Uptake of glutamate into synaptic vesicles. Prog Brain Res. 1998;116:87–101. doi: 10.1016/s0079-6123(08)60432-x. [DOI] [PubMed] [Google Scholar]
- 21.Reimer RJ, Fremeau RT, Jr, Bellocchio EE, Edwards RH. The essence of excitation. Curr Opin Cell Biol. 2001;13:417–421. doi: 10.1016/s0955-0674(00)00230-1. [DOI] [PubMed] [Google Scholar]
- 22.Otis TS. Vesicular glutamate transporters in cognito. Neuron. 2001;29:11–14. doi: 10.1016/s0896-6273(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 23.Naito S, Ueda T. Adenosine triphosphate-dependent uptake of glutamate into Protein I-associated synaptic vesicles. J Biol Chem. 1983;258:696–699. [PubMed] [Google Scholar]
- 24.Bellocchio EE, Reimer RJ, Fremeau RTJ, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 25.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- 26.Nelson N. Structure and pharmacology of the proton ATPases. Trends Pharmacol Sci. 1991;12:71–75. doi: 10.1016/0165-6147(91)90501-i. [DOI] [PubMed] [Google Scholar]
- 27.Naito S, Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem. 1985;44:99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- 28.Maycox PR, Deckwerth T, Hell JW, Jahn R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem. 1988;263:15423–15428. [PubMed] [Google Scholar]
- 29.Fykse EM, Christensen H, Fonnum F. Comparison of the properties of γ-aminobutyric acid and L-glutamate uptake into synaptic vesicles isolated from rat brain. J Neurochem. 1989;52:946–951. doi: 10.1111/j.1471-4159.1989.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 30.Tabb JS, Kish PE, Van Dyke R, Ueda T. Glutamate transport into synaptic vesicles. Roles of membrane potential, pH gradient, and intravesicular pH. J Biol Chem. 1992;267:15412–15418. [PubMed] [Google Scholar]
- 31.Wolosker H, de Souza DO, de Meis L. Regulation of glutamate transport into synaptic vesicles by chloride and proton gradient. J Biol Chem. 1996;271:11726–11731. doi: 10.1074/jbc.271.20.11726. [DOI] [PubMed] [Google Scholar]
- 32.Ishida A, Noda Y, Ueda T. Synaptic vesicle-bound pyruvate kinase can support vesicular glutamate uptake. Neurochem Res. 2009;34:807–818. doi: 10.1007/s11064-008-9833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda T, Ikemoto A. Cytoplasmic glycolytic enzymes. Synaptic vesicle-associated glycolytic ATP-generating enzymes: Coupling to neurotransmitter accumulation. In: Gibson G, Dienel G, editors. Handbook Neurochem. Mol. Neurobiol., 3rd ed., Brain Energetics, Cellular and Molecular Integration. Springer; Heidelberg: 2007. pp. 241–259. [Google Scholar]
- 34.Frieden C. Glutamate Dehydrogenase VI. Survey of purine nucleotide and other effects on the enzyme form from various sources. J Biol Chem. 1965;240:2028–2037. [PubMed] [Google Scholar]
- 35.Fang J, Hsu BY, Macmullen CM, Poncz M, Smith TJ, Stanley CA. Expression, purification and characterization of human glutamate dehydrogenase (GDH) allosteric regulatory mutations. Biochem J. 2002;363:81–87. doi: 10.1042/0264-6021:3630081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Li M, Chen P, Narayan S, Matschinsky FM, Bennett MJ, Stanley CA, Smith TJ. Green tea polyphenols control dysregulated glutamate dehydrogenase in transgenic mice by hijacking the ADP activation site. J Biol Chem. 2011;286:34164–34174. doi: 10.1074/jbc.M111.268599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen RF, Plaut GWE. Activation and Inhibition of DPN-linked isocitrate dehydrogenase of heart by certain nucleotides. Biochemistry. 1963;2:1023–1032. doi: 10.1021/bi00905a020. [DOI] [PubMed] [Google Scholar]
- 38.Cohen PF, Colman RF. Diphosphopyridine nucleotide dependent isocitrate dehydrogenase from pig heart. Characterization of the active substrate and modes of regulation. Biochemistry. 1972;11:1501–1508. doi: 10.1021/bi00758a027. [DOI] [PubMed] [Google Scholar]
- 39.Dange M, Coleman RF. Each conserved active site Tyr in the three subunits of human isocitrate dehydrogenase has a different function. J Biol Chem. 2010;285:20520–20525. doi: 10.1074/jbc.M110.115386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold S, Kadenbach B. The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett. 1999;443:105–108. doi: 10.1016/s0014-5793(98)01694-9. [DOI] [PubMed] [Google Scholar]
- 41.Jeneson JAL, Wiseman RW, Westerhoff HV, Kushmerick M. The signal transduction function for oxidative phosphorylation Is at least second order in ADP. J Biol Chem. 1996;271:27995–27998. doi: 10.1074/jbc.271.45.27995. [DOI] [PubMed] [Google Scholar]
- 42.Cieslar JH, Dobson JP. Free [ADP] and aerobic muscle work follow at least second order kinetics in rat gastrocnemius in vivo. J Biol Chem. 2000;275:6129–6134. doi: 10.1074/jbc.275.9.6129. [DOI] [PubMed] [Google Scholar]
- 43.Greenfield RA, Swain JL. Distribution of myofibrillar energy use: dual mechanisms that may contribute to postischemic dysfunction in stunned myocardium. Circ Res. 1987;60:283–289. doi: 10.1161/01.res.60.2.283. [DOI] [PubMed] [Google Scholar]