Abstract
Purpose
A mouse mutant identified during a recessive ENU mutagenesis screen exhibited ocular haemorrhaging resulting in a blood filled orbit, and hence was named ‘redeye’. We aimed to identify the causal mutation in redeye, and evaluate it as a model for Diabetic Retinopathy (DR).
Methods
The causative gene mutation in redeye was identified by haplotype mapping followed by exome sequencing. Glucose tolerance tests, detailed histological and immunofluorescence analyses, and vascular permeability assays were performed to determine the affect of redeye on glucose metabolism, pericyte recruitment and the development of the retinal vasculature and the blood retinal barrier (BRB).
Results
A mutation was identified in the Pdgfrb gene at position +2 of intron 6. We show that this change causes partial loss of normal splicing resulting in a frameshift and premature termination, and therefore a substantial reduction in normal Pdgfrb transcript. The animals exhibit defective pericyte recruitment restricted to the central nervous system (CNS) causing basement membrane and vascular patterning defects, impaired vascular permeability and aberrant BRB development, resulting in vascular leakage and retinal ganglion cell apoptosis. Despite exhibiting classical features of diabetic retinopathy redeye glucose tolerance is normal.
Conclusions
The Pdgfrbredeye/redeye mice exhibit all of the features of non-proliferative DR including retinal neurodegeneration. In addition, the perinatal onset of the CNS-specific vascular phenotype negates the need to age animals or manage diabetic complications in other organs. Therefore they are a more useful model for diseases involving pericyte deficiencies such as DR than those currently being used.
INTRODUCTION
Platelet derived growth factors (Pdgfs) are powerful mitogens and important regulators of embryological development, cell proliferation, migration and survival. First described 1, 2, as stimulating cell growth and proliferation, Pdgfs have two chains, A and B. Their receptors Pdgfrα and Pdgfrβ are transmembrane tyrosine kinases 3-7. Pdgfrα is able to bind both PdgfA and B isoforms whereas Pdgfrβ can only bind PdgfB with high affinity 4, 5.
Pericytes are perivascular cells located on the abluminal surface of endothelial cells and are embedded within the basement membrane 8. PdgfB is secreted by endothelial tip cells at the front of the extending vessel, which recruits Pdgfrβ-expressing mural cells such as vascular smooth muscle cells and pericytes to developing blood vessels during angiogenesis 9, 10. These mural cells play an important role in the remodelling and stabilisation of blood vessels 11, 12.
The functions of pericytes include roles in vascular development, immune and phagocytic functions and haemostasis (reviewed in 8, 13). More recently, pericytes have been shown to induce the formation of tight junctions between central nervous system (CNS) endothelial cells to form the blood brain barrier (BBB) and blood retinal barrier (BRB) 14, 15.
PdgfB/Pdgfrβ signalling is crucial for pericyte recruitment and survival 16, with mutations in these molecules resulting in pericyte recruitment deficiencies 12. PdgfRβ null mice are usually lethal due to severe haemorrhaging either in utero or at birth 17, however mice harbouring specific mutations in Pdgfrβ are viable 18, 19.
A major complication of diabetes mellitus is diabetic retinopathy (DR) which is one of the leading causes of blindness worldwide. DR is characterised by ‘pericyte drop-out’ which has severe irreversible pathological consequences for the retina characterised by microvascular abnormalities such as endothelial dysfunction and haemorrhage, and neuronal abnormalities including retinal ganglion cell (RGC) death 20, 21. Prolonged hyperglycemia in the retina activates PKC-δ and SHP-1 inhibiting Pdgfrβ signalling resulting in pericyte apoptosis 22. Some of the features of DR have also been identified in mice with diminished Pdgfrβ signalling 23-25, indeed PdgfB has been implicated in DR for many years 26.
Here we present a mouse mutant, redeye, with decreased pericyte coverage in the CNS caused by an N-ethyl-N-nitrosourea (ENU)-induced mutation in Pdgfrb. Most other DR mouse models currently in use do not exhibit all of the features of DR, namely pericyte and RGC loss, BRB breakdown, vascular leakage, and acellular capillaries 20, 27-31. The redeye strain exhibits all the features of DR within weeks of birth, negating the need to age experimental animals and without the multiple organ involvement 12, 16, 18, 23, 24, 26, 32-34 seen in other PdgfB/Pdgfrβ mutants. This enables easier isolation of the vascular defects in the redeye mice and makes them a less complicated disease model. Of particular note is that this strain and DR have the same underlying cause for pericyte loss, as hyperglycemia can result in decreased signalling from Pdgfrβ causing subsequent pericyte apoptosis 22. Furthermore, the defect in BRB development makes them a useful tool for dissecting the role of pericytes in BRB formation and vascular development.
MATERIALS AND METHODS
Experimental animals
The redeye strain, referred to as Pdgfrbredeye in this manuscript, was maintained on a sighted C3H background 35. The following primers were used for sequencing for genotyping. 5′-CATTGTGATGGGCAATGATG-3′ and 5′-ATAGGTGCCCGAATCACTCA-3′. All experiments complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the relevant local animal welfare conditions.
Mutation identification
Initial mapping by SNP array analysis (University of Edinburgh, Wellcome Trust Clinical Research Facility) identified an 8 Mb region of interest. All exons and splice junctions in this region were captured on a custom oligonucleotide-array, amplified and sequenced, identifying a mutation in the Pdgfrb gene which was confirmed by Sanger sequencing. To characterise the mutation we extracted RNA from enucleated eyes and performed quantitative real-time PCR using the Mouse Universal ProbeLibrary Set (Roche, UK) with probes for Pdgfrβ and TBP on a Roche Lightcycler LC480. The Lightcycler LC480 software was used to normalise results to TBP controls and calculate relative expression values.
Tissue preparation and Immunofluorescence
Mice were sacrificed at the appropriate age and wholemount retinae were prepared and stained as described 36. We stained mutant and control retinae in the same well to control for changes in staining efficiency, retinae were distinguished by different numbers of radial incisions.
Embryonic hindbrains were dissected as previously described 37 and all other organs were dissected from animals of the appropriate age and fixed in 4% PFA overnight at 4°C. Postnatal day 5 (P5) brains were embedded in 4% agarose in PBS and 200 μm sections were cut on a Vibratome Series 1000 (Technical Products International Inc). Immunofluorescence for hindbrains and brain sections was performed as for retinae.
We embedded the kidneys in paraffin for sectioning on a Leica RM 2235 microtome. Placentae and hearts were cryopreserved in 30% sucrose and embedded in OCT compound (VWR International, UK) for sectioning on a Leica CM30505 cryostat. Citrate buffer antigen retrieval was used for paraffin sections. We blocked all the sections in 10% heat inactivated donkey serum in PBST for an hour, and performed all antibody incubations at room temperature for an hour in block (antibody details are given in Supp Table S1). Haematoxylin and Eosin staining were performed according to standard procedures.
Tissue from at least five mice of each genotype from at least three different litters was used for all analysis. All tissues were mounted in Vectashield (Vector Laboratories, UK), imaged by confocal microscopy (Nikon A1R) and maximum intensity projections of z-stacks were created using NisElements AR Version 4.0 software. All images are representative of at least three animals.
Immunoblot
We extracted protein from all tissues using 1xRIPA buffer (Cell Signaling Technology, USA) containing protease and phosphatase inhibitors (Roche, UK) and followed manufacturer’s instructions. The NuPage gel electrophoresis and XCell II blotting systems (Invitrogen, UK) were used for protein separation and immunoblotting respectively. Blots were blocked in 5% milk/TBST and all antibodies (details given in Supp. Table S2) were incubated in block for 1 hour at room temperature. Immunolabelling was detected using the Amersham ECL plus Western blotting kit (GE Healthcare, UK). Quantification was performed using ImageQuant TL software.
Pericyte and Branchpoint Quantification
The MetaMorph® Angiogenesis Tube Formation application (Molecular Devices, UK) was used for quantification. Confocal images were used to determine the total area covered by vessels and pericytes in order to calculate percentage pericyte coverage of vessels and the total number of branchpoints/mm2. We imaged three areas of each retina; three different regions of the central retina each encompassing an artery and vein, and two images each of peripheral arteries and peripheral veins; a total of five images/retina. For pericyte quantification of the cerebral cortex capillaries, sections from the frontal, parietal and occipital regions of the brain were used and four images were taken from the cerebral cortex of each section. Threshold values were kept the same for analysis of samples of the same stage.
Avascular region measurement
As a measure of vascular branching we counted the number and area of the avascular regions between capillaries for each image, again for the three regions of each retina. Image analysis was performed using a custom macro written for the freeware image analysis package Fiji 38 which is based on ImageJ 39. The macro requires Gabriel Landini’s ‘Morphological Operators for ImageJ’ available from http://www.dentistry.bham.ac.uk/landinig/software/software.html.
Permeability assay
Fluorescence angiography was performed as previously described 40 with modifications. Mice were injected intraperitoneally at P10 with 40 μl of 50 mg/ml Fitc conjugated Dextran 20000S (Sigma, UK) w/v in PBS, sacrificed an hour later, the retinae dissected and imaged using a Zeiss Axioplan II fluorescence microscope and a Coolsnap HQ CCD camera (Photometrics Ltd, Tucson, AZ). IPLab Spectrum software (Scanalytics Corp, Fairfax, VA) was used to analyse retinal haemorrhages.
Retinal Ganglion Cell and Macrophage count
Volocity 3D Image analysis software (PerkinElmer) was used for both RGC and macrophage counts. Cells positively stained for the RGC marker Brn3 were counted in the centre of fully mature retinae (P28), which our preliminary data (not shown) suggested was the region most affected in our mutants.
Statistical Analysis
Statistical tests were performed using the ‘R’ statistics package, an open source software package based on the ‘S’ programming language (http://www.R-project.org). The appropriate parametric or non-parametric tests used are detailed in Supp. Table S3.
Glucose Tolerance Tests
Eight week old animals were fasted for five hours prior to intraperitoneal injection of 2 g glucose (Sigma, UK) per kilogram of body weight. Blood was drawn from the tail vein at 0, 30, 60, 90, and 120 minutes after injection and glucose values were monitored using an automatic glucometer (Accuchek, Roche, UK). Numbers of mice tested are females (4 WT, 6 Hom) and males (6 WT, 5 Hom).
RESULTS
redeye is a hypomorphic mutation in Pdgfrb
The redeye strain was identified during an ENU-induced mutagenesis screen for recessive eye mutations at MRC Harwell and initially presented with severe haemorrhaging in the eye (not shown). The severity of the haemorrhaging was reduced upon crossing the strain onto a congenic C3H strain lacking the Pde6brd1 mutation 35. Mutants show normal responses during optokinetic response drum testing (data not shown). No further defects were identified during phenotyping at MRC Harwell and the redeye mice have a normal lifespan.
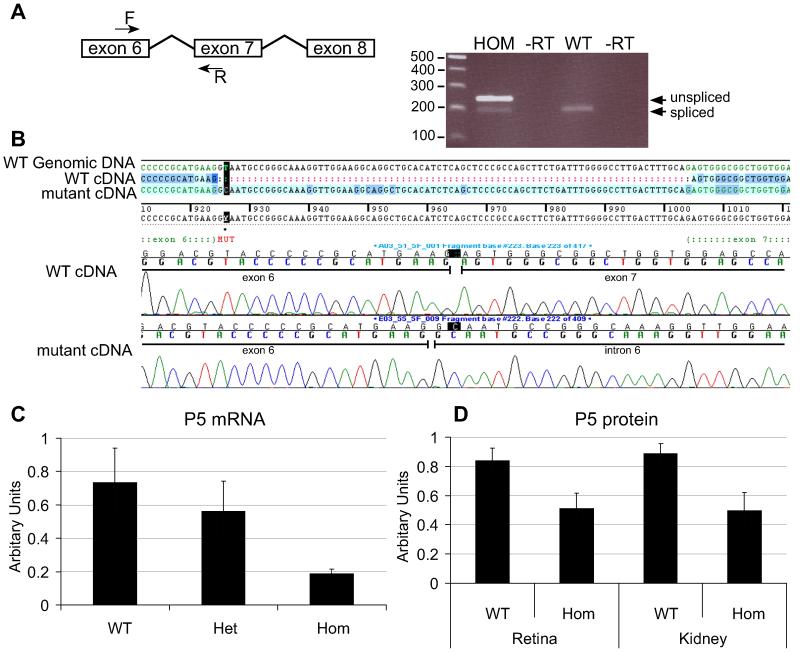
We identified a T to C mutation in the Pdgfrb gene, encoding Pdgfrβ, affecting the splice donor site at position +2 of intron 6 changing the consensus GT to the less efficiently utilised GC 41. To investigate the impact of this change on splicing we extracted RNA from P5 eyes and performed RT-PCR. In addition to the PCR product from the normally spliced transcript, we detected a product of increased size in homozygotes, sequencing of which confirmed the retention of intron 6 (Fig. 1A, B) in mutant mRNA. This additional sequence introduces 23 novel amino acids followed by premature termination. The normally spliced Pdgfrb transcript was present but quantitative RT-PCR showed that mutant eyes contain only approximately 25% of wildtype levels of Pdgfrb mRNA (Fig. 1C). This corresponds to a decrease in Pdgfrβ protein seen by immunoblotting and immunofluorescence of homozygote retinae (Fig. 1D, 2) confirming Pdgfrbredeye is a hypomorphic mutant allele of Pdgfrb.
Figure 1. redeye is a hypomorphic mutant of Pdgfrb.
A donor splice site mutation was identified in intron 6 of Pdgfrb, causing reduced splicing of the intron. RT-PCR between exons 6 and 7 results in a larger, unspliced RT-PCR product in homoygote animals (HOM) of 243 bp as well as the normally spliced product of 190 bp (A). Sequencing of these RT-PCR products shows that the mutant transcript contains intron 6 whereas the WT transcript does not (B). Quantitative analysis shows a decrease in Pdgfrb mRNA by quantitative RT-PCR (C) and protein by immunoblot (D). Error bars: standard deviation of the mean.
The redeye mutation causes decreased pericyte coverage restricted to the CNS
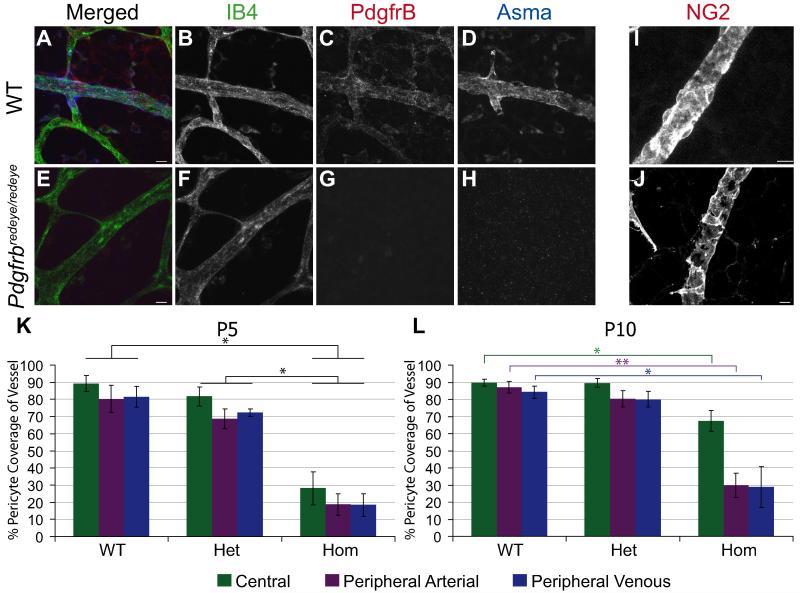
Pdgfrβ is required for pericyte recruitment so we performed immunofluorescence on perinatal wholemount retinae to determine whether decreased Pdgfrβ caused a corresponding decrease in pericyte coverage. As there is no definitive marker of pericytes a panel of markers is required to correctly identify these cells 42. This confirmed a decrease in pericytes as shown by a reduction in Pdgfrβ (Fig. 2G), alpha smooth muscle actin (Fig. 2H), proteoglycan NG2 (Fig. 2J), endosialin and desmin (Supp. Fig. S1). We found that proteoglycan NG2 (NG2) is the most robust of these markers in the mouse retina and therefore used it as a specific pericyte marker for our further investigations in the retina.
Figure 2. Pdgfrbredeye/redeye mutants have reduced pericyte coverage in the retina.
Representative images of P5 retinal vasculature from WT (A-D) and Pdgfrbredeye/redeye mice (E-H). Vessels are stained with Isolectin B4 (IB4) and pericytes with Pdgfrβ (C) and Alpha smooth muscle actin (Asma) (D). Staining is not detected in Pdgfrbredeye/redeye mice (G, H). Another marker of pericytes, Proteoglycan NG2 (NG2) was also used on P5 retinae which gives more robust staining. Wildtype vessels (I) have more complete coverage by pericytes than mutant vessels (J). IB4 and NG2 stained retinae were used to quantify the percentage of the vessel covered by pericytes. Perictye coverage in Pdgfrbredeye/redeye (Hom) is significantly lower than both WT and Pdgfrbredeye/+ (Het) at P5 and P10 (SRH test P < 0.001, Mann-Whitney U-test *P < 0.05, **P < 0.005) (K-L). All scale bars:10 μm. Error bars: standard deviation of the mean.
In order to quantify this reduction in pericytes, we stained wholemount retinae for NG2 to mark pericytes and isolectin B4 (IB4) to mark the endothelium. We analysed retinae at P5, when the primary vascular plexus has grown halfway between the optic nerve head and the periphery of the retina, and at P10 when the primary vascular plexus is fully mature. There is a significant decrease in pericyte coverage in all three retinal regions (central, peripheral vein and peripheral artery) in Pdgfrbredeye/redeye vs wildtype animals at P5 and P10 (Fig. 2K, L).
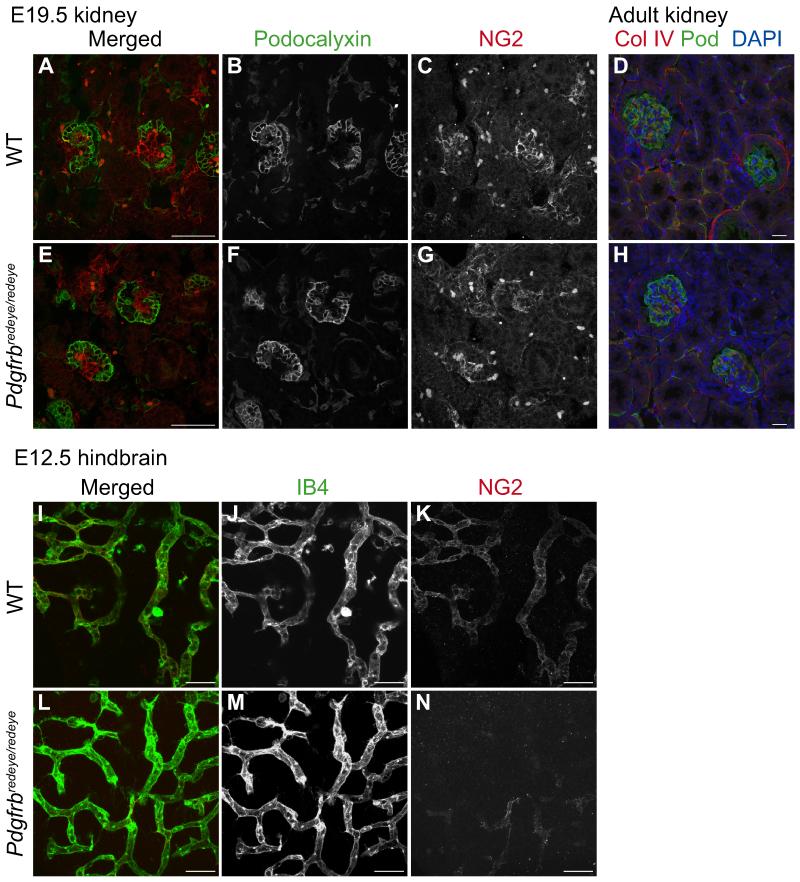
Other Pdgfrb mutants have defects in the recruitment of mesangial cells 26, the pericyte equivalent in the kidney, with subsequent developmental defects. We therefore harvested kidneys at embryonic day 17.5 (E17.5) when mesangial cells begin to invade the kidney glomerulus. Staining for podocytes and mesangial cells showed no apparent defect in mesangial cell recruitment (Fig. 3A-C, E-G). We also analysed adult Pdgfrbredeye/redeye kidneys which showed no histological defects (Fig. 3 D, H). Next we examined E18.5 placentae and hearts for the developmental abnormalities exhibited in other Pdgfrβ signalling mutants at this stage 23, and to determine whether pericyte recruitment was affected in these tissues. Our results show that both pericyte recruitment and histology of the embryonic heart and placenta are normal (Supp. Fig. S2), suggesting that the defect in pericyte coverage may be restricted to the CNS.
Figure 3. The pericyte recruitment deficiency is restricted to the CNS.
The pericyte equivalent in the kidneys are mesangial cells which are recruited to the developing glomerulus at around E17.5. Paraffin sections of E17.5 WT (A-C) and Pdgfrbredeye/redeye kidneys (E-G) stained for podocytes with Podocalyxin (B, F) and mesangial cells with Proteoglycan NG2 (C, G) show similar numbers of mesangial cells being recruited. Paraffin sections of adult kidneys stained for vessels with Collagen IV and podocytes with Podocalyxin (Pod) also show normal histology (D, H). E12.5 embryonic hindbrains were also stained with IB4 for vessels (J, M) and NG2 for pericytes (K, N) in the developing CNS. WT hindbrains recruit pericytes normally (I-K) but Pdgfrbredeye/redeye hindbrains show decreased pericyte recruitment (L-N). Scale bars: 100 μm (D, H), 50 μm (all other images).
To test this we studied another model of angiogenesis, the mouse hindbrain, which is vascularised between E9.5 and E12.5. Flatmounted E12.5 hindbrains from Pdgfrbredeye/redeye mutants showed reduced NG2 staining (Fig. 3L-N). We then examined pericyte coverage of cerebral vessels in P5 animals. As NG2 stains oligodendricytes as well as pericytes in the brain we used desmin as a marker for pericytes (Supp. Fig. S3A-F). Repeated analysis of P5 retinae with desmin staining showed pericyte coverage similar to that obtained with NG2 staining (Supp. Fig. S3G). We found a statistically significant decrease in pericyte coverage in Pdgfrbredeye/redeye mutants in the cerebral cortex (Supp. Fig. S3G) confirming that the reduction in pericyte coverage was widespread in, but limited to the CNS.
The Pdgfrbredeye/redeye mice exhibit basement membrane deposition and vascular patterning defects
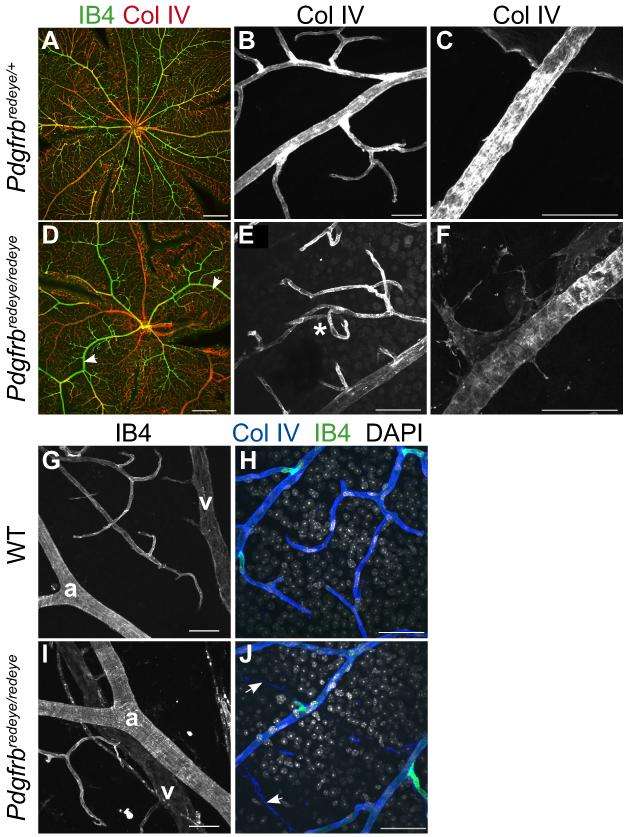
During this analysis we noted that there was a defect in vessel patterning (Fig. 4A, D), with increased vessel tortuousity in the mutants (Fig. 4E). Pericytes secrete their own basement membrane, so a reduction in pericytes would likely result in decreased basement membrane coverage. Immunofluorescence on the mutant retinae indeed shows less collagen IV coverage of the vessels with additional weaker expression on perivascular cells, which may be pericytes that have failed to adhere due to reduced Pdgfrβ signalling (Fig. 4C, F). Furthermore, artery-vein crossover events (Fig. 4I) and acellular capillaries or ‘ghost vessels’, (Fig. 4J) are present in mutant retinae.
Figure 4. Loss of pericytes results in vascular patterning defects.
Adult wholemount retinae stained with vessel markers IB4 and Collagen IV show regular patterning of vessels in a Pdgfrbredeye/+ retina with straight vessels (A, B). In the Pdgfrbredeye/redeye retina the vessels are irregular and the arteries in particular seem not to extend straight towards the periphery (arrowheads, D). At higher magnification these vessels appear to be tortuous (asterisk, E). A Normal level of Collagen IV is expressed by pericytes as well as endothelial cells in Pdgfrbredeye/+ retinae (C) and is decreased in Pdgfrbredeye/redeye retinae (F) arteries. WT retinae exhibit evenly spaced arteries (a) and veins (v) (G) but Pdgfrbredeye/redeye retinae show artery-vein crossover events (I) as well as acellular capillaries (arrows, J) that are characterised by positive Collagen IV staining and IB4 staining which are seen in WT retinae (H) but with the absence of nuclei, stained with DAPI. Scale bars: 200 μm (A, D), 50 μm (B, C, E-J).
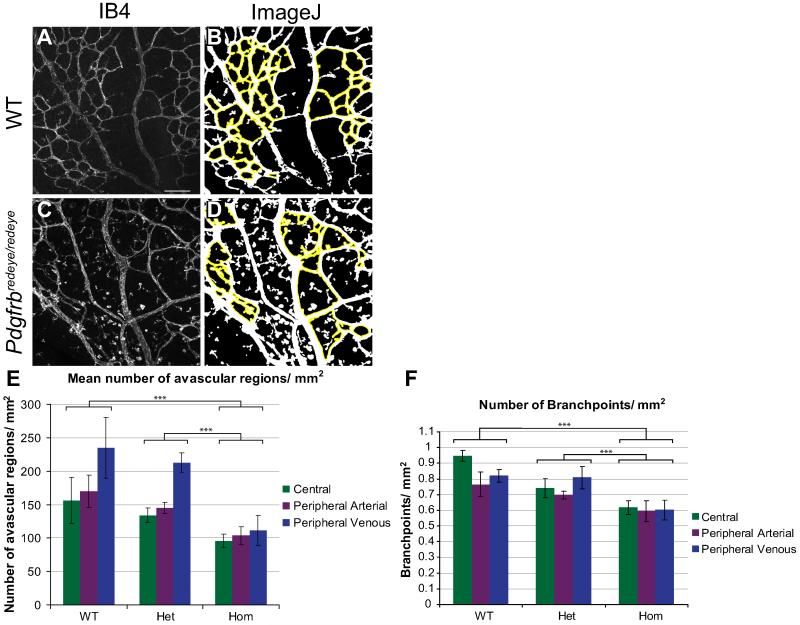
Analysis of Pdgfrbredeye/redeye retinae also revealed a defect in vessel branching (Fig. 5). To quantify this we used image analysis software to count the number of avascular regions in each image (Fig. 5A-D) which showed a significant decrease in the number of these regions in the Pdgfrbredeye/redeye animals (Fig. 5E). There is also a significant reduction in the number of branchpoints in Pdgfrbredeye/redeye animals (Fig. 5F).
Figure 5. Vascular branching is defective in Pdgfrbredeye/redeye mutants.
To characterise vessel patterning ImageJ software was used to quantify the avascular regions outlined in yellow (B, D) between vessels per mm2 of P5 retinae (E). MetaMorph® software was used to quantify branchpoint number per mm2 of P5 retinae (F). There are a greater number of smaller avascular regions and branchpoints (both central and peripheral) in WT and Pdgfrbredeye/+ (Het) retinae than in Pdgfrbredeye/redeye (Hom) retinae (ANOVA P < 0.001 in both cases, TukeyHSD ***P < 0.001) (E, F). Scale bars: 100 μm (A-D). Error bars: standard deviation of means.
Vascular permeability and Blood Retinal Barrier development are impaired in Pdgfrbredeye/redeye mice
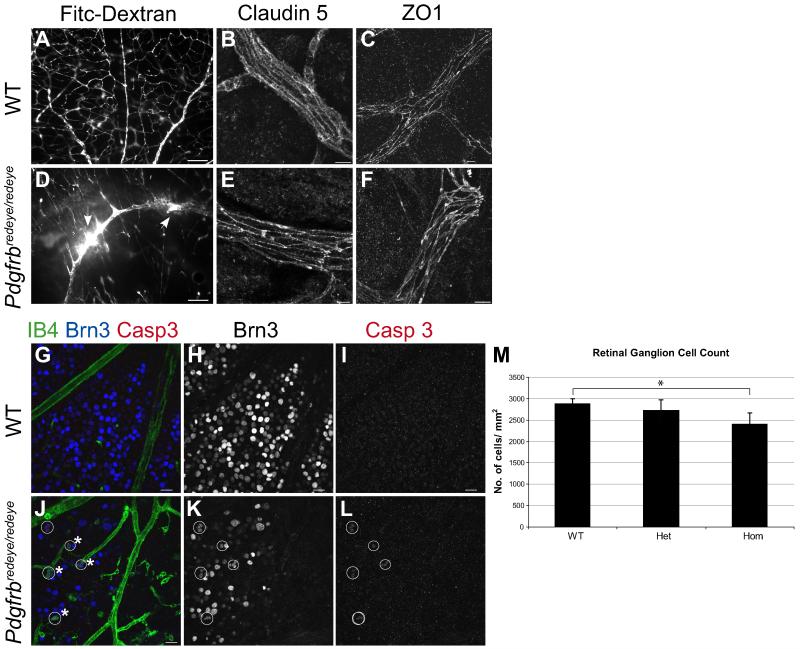
Initial phenotyping had shown ocular haemorrhage, consequently we investigated the vascular leakage further by using fluorescently labelled 2 kDa dextran which should not pass through the BRB. We found leakage from Pdgfrbredeye/redeye retinal vessels into the vitreous within an hour of injection (Fig. 6D). No haemorrhage or leakage was observed in control organs e.g. kidney (not shown) or in the retinae of wildtype mice (Fig. 6A). As pericytes have been linked to BRB induction14, 15, we next looked at the expression of BRB markers. The tight junction molecules claudin 5 and zona occludens 1 (ZO1) are expressed in both mutant and control retinae (Fig. 6B, C, E, F). BRB dysfunction can cause neurodegeneration, therefore we counted RGCs in the Pdgfrbredeye mice (Fig. 6H, K). Our results show that there is a significant decrease in the number of RGCs in the central retina of Pdgfrbredeye/redeye animals, relative to wildtypes by P28 (Fig. 6H, K, M). This is due to RGC apoptosis as seen by positive cleaved caspase 3 staining (Fig. 6I, L), confirming neurodegeneration in the Pdgfrbredeye/redeye mutants.
Figure 6. Pdgfrbredeye/redeye mutants exhibit BRB dysfunction.
Vascular permeability was tested by intraperitoneal injection of 2kDa Fitc-Dextran at P10 which does not leak from WT vessels in the retina due to the BRB (A), but Pdgfrbredeye/redeye retinae exhibit vascular leakage (arrows, D). Claudin 5 and ZO1 are expressed in the tight junctions between endothelial cells in P5 WT (B, C) and Pdgfrbredeye/redeye mutants (E, F). To determine if BRB dysfunction causes neurodegeneration, P28 retinae were stained with IB4 (vessels and macrophages), Brn3 (RGCs) and the apoptosis marker Cleaved caspase 3 (Casp3) showing apoptotic RGCs (circles) being engulfed by macrophages in Pdgfrbredeye/redeye (asterisk, J) but not WT retinae (G). RGCs stained with Brn3 were counted in the centre of the retina in P28 WT (H), Pdgfrbredeye/+ (Het) and Pdgfrbredeye/redeye (Hom) (K) animals. Pdgfrbredeye/redeye retinae had fewer ganglion cells than both WT and Pdgfrbredeye/+ retinae (Kruskal-Wallis test P = 0.002, Mann-Whitney U-test *P < 0.05) (K). Scale bars: 100 μm (A, D, G-L) and 10 μm (B, C, E, F). Error bars: standard deviation of the mean.
The Pdgfrbredeye/redeye mice have normal glucose tolerance
The features we describe in Pdgfrbredeye/redeye mice are symptomatic of DR, including decreased pericyte coverage, increased vascular permeability and BRB loss. It is therefore important to exclude diabetes as the cause of this retinopathy. We performed glucose tolerance tests which show that the Pdgfrbredeye/redeye mice were able to clear injected glucose (Supp. Fig. S4). The mutants also express insulin and glucagon at wildtype levels (not shown) and are therefore not diabetic.
DISCUSSION
redeye mutants exhibit a CNS-specific reduction in pericytes
Pdgfrbredeye is a Pdgfrb partial loss of function mutant expressing approximately 58% of the wildtype levels of Pdgfrβ. Much work has previously shown the requirement of Pdgfrβ signalling for the recruitment of pericytes to the developing vasculature 9, 10, 17, 18, 33, 37, 43-45. We confirm that a reduction in Pdgfrβ results in decreased pericyte coverage in retinae at P5 and P10 (Fig. 2) due to its requirement for the initial expansion and propagation of pericytes.
Pdgfrb null mice die at/shortly after birth from severe haemorrhaging due to pericyte deficiency 16-18. However, the Pdgfrbredeye/redeye mice are viable and occur at Mendelian ratios (not shown) with only the CNS vasculature exhibiting a reduction in pericytes (Fig. 2, 3, Supp. Fig. S1-S3). Previous reports describe similar variations in pericyte deficiency between different organs in other Pdgfrb mutants 13. This could be due to Pdgfrα compensating for the loss of Pdgfrβ in these tissues 18. However, our data shows that Pdgfrα is not upregulated in the retina in response to decreased Pdgfrβ expression (Supp. Fig. S1). It is more likely that the CNS-restricted phenotype is due to the higher pericyte:endothelial cell ratio in the CNS which is as high as 1:3 in the brain, compared with 1:100 in striated muscle, and is reportedly even higher in the retina 46, 47. Our results support previous findings that even within the CNS, pericyte coverage of capillaries can vary 48. We found approximately 40% of vessels are covered by pericytes in wildtype P5 cerebral cortex compared to approximately 80% coverage in the wildtype retina (Supp. Fig. S3G).
This increased pericyte density results in higher expression of Pdgfrβ in wildtype adult CNS tissues. Pdgfrβ expression in the brain is much higher than in other organs with a dramatic decrease in the expression of Pdgfrβ in Pdgfrbredeye/redeye mutants in the CNS (Supp. Fig. S5). Interestingly, Pdgfrβ is expressed at the same level in Pdgfrbredeye/redeye mutant and wildtype kidneys (Supp. Fig. S5A), which may explain why Pdgfrbredeye/redeye kidneys are unaffected. The reduced expression of Pdgfrβ in the Pdgfrbredeye/redeye hypomorphs may be sufficient to support a subpopulation of pericytes that are less sensitive to decreased Pdgfrβ signalling and enable vascular function outside the CNS. Also, as pericytes have a specific role in BRB formation14, 15 the CNS may be more sensitive to changes in Pdgfrβ.
Pericytes are crucial for vessel patterning and endothelial maturation
Our work confirms that a decrease in pericyte numbers affects vessel patterning. It has been established that during angiogenesis the initial vascular plexus is remodelled to form a fully functional vascular network 9, 49. Pericytes prevent excessive remodelling by conferring vascular stability 11, 12, 49. A delay or reduction in pericyte recruitment as seen in the Pdgfrbredeye/redeye mice would thus enable increased vascular pruning to occur resulting in a sparser vascular network with fewer branchpoints and fewer, but larger avascular regions between the vessels (Fig. 5). This further highlights the importance of pericytes during angiogenesis.
redeye mutants model Diabetic Retinopathy
Previous findings show that loss of pericytes in the CNS and the resultant vascular permeability is due to an increase in transcytosis 14. We can confirm this in the Pdgfrbredeye/redeye mutants in which BRB dysfunction manifests as increased vascular permeability with normal tight junction formation (Fig. 6B, C, E, F). Pdgfrbredeye/redeye mutants also exhibit the classic DR features of acellular capillaries (Fig. 4J) and neurodegeneration in the form of a reduced number of RGCs (Fig. 6G-K), though pericyte ghosts are not seen. Acellular capillaries arise as a consequence of endothelial cell apoptosis due to the loss of pericyte-derived survival signals 50 or a loss of the BRB 51, leaving an acellular basement membrane (Fig. 4J). As is the case in diabetes, the remaining endothelium is oxidatively stressed and produces less neurotrophic factors 52, resulting in neurodegeneration (Fig. 6G-K). RGCs are particularly sensitive to retinal ischemia and neurotoxicity 53, 54 and are often damaged in ischemic retinopathies such as DR. RGC apoptosis is seen much later than the vascular defects and we conclude that vascular dysfunction preceeds neurodegeneration in this model. This is a particularly important finding given the controversy regarding whether diabetic mouse models exhibit RGC loss 20, 27, 55.
The early perinatal onset of these vascular changes is particularly useful as most other DR models currently in use do not exhibit vascular pathology until at least 8 weeks of age 27-31 and do not show the full range of vascular and neuronal defects exhibited in this model. Though the Pdgfrbredeye mutants do not exhibit hyperglycaemia, they have the same cause for reduced pericytes i.e. diminished Pdgfrβ signalling, resulting in the same phenotype. As the Pdgfrbredeye/redeye mice do not exhibit neovascularisation we propose that the Pdgfrbredeye strain is a useful model for non-proliferative DR. The insights gained by studying this mutant will not only be valuable for understanding how pericytes are involved in maintaining a normal retinal vasculature but also their role in BBB/BRB formation.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank David Black and animal staff for animal husbandry, Matthew Pearson and Paul Perry for imaging assistance, Allyson Ross for histology assistance and Alexi Balmuth at the Genepool for exome sequencing. We are also grateful to Clare Isacke, BBCRC, London, UK for the kind gift of the anti-Endosialin antibody.
SUPPORT:
This research was supported by Eumodic; the European Mouse Disease Clinic, an EU Integrated Research Programme and MRC Core Support to the MRC Human Genetics Unit and the MRC Mammalian Genetics Unit.
Footnotes
DISCLOSURES/DISCLAIMERS: None
REFERENCES
- 1.Kohler N, Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974;87:297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974;71:1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronwald RG, Grant FJ, Haldeman BA, et al. Cloning and expression of a cDNA coding for the human platelet-derived growth factor receptor: evidence for more than one receptor class. Proc Natl Acad Sci U S A. 1988;85:3435–3439. doi: 10.1073/pnas.85.10.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart CE, Forstrom JW, Kelly JD, et al. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988;240:1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- 5.Heldin CH, Backstrom G, Ostman A, et al. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. Embo J. 1988;7:1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heldin CH, Hammacher A, Nister M, Westermark B. Structural and functional aspects of platelet-derived growth factor. Br J Cancer. 1988;57:591–593. doi: 10.1038/bjc.1988.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui T, Heidaran M, Miki T, et al. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989;243:800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- 8.Allt G, Lawrenson JG. Pericytes: Cell Biology and Pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 9.Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 10.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 12.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte Loss and Microaneurysm Formation in PDGF-B-Deficient Mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 13.Armulik A, Genové G, Betsholtz C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Developmental cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 15.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 17.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 18.Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003;1:E52. doi: 10.1371/journal.pbio.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2011;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS. Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Disease Models & Mechanisms. 2012;5:444–456. doi: 10.1242/dmm.009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. The Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 22.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, et al. Activation of PKC-[delta] and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjarnegard M, Enge M, Norlin J, et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 24.Enge M, Bjarnegard M, Gerhardt H, et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. Embo J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammes H-P, Lin J, Renner O, et al. Pericytes and the Pathogenesis of Diabetic Retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 26.Lindblom P, Gerhardt H, Liebner S, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber AJ, Antonetti DA, Kern TS, et al. The Ins2Akita Mouse as a Model of Early Retinal Complications in Diabetes. Investigative Ophthalmology & Visual Science. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 28.Cheung AKH, Fung MKL, Lo ACY, et al. Aldose Reductase Deficiency Prevents Diabetes-Induced Blood-Retinal Barrier Breakdown, Apoptosis, and Glial Reactivation in the Retina of db/db Mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 29.Kador PF, Zhang P, Makita J, et al. Novel Diabetic Mouse Models as Tools for Investigating Diabetic Retinopathy. PLoS ONE. 2012;7:e49422. doi: 10.1371/journal.pone.0049422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of Retinal Neurons in Streptozotocin-Induced Diabetic Mice. Investigative Ophthalmology & Visual Science. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 31.Rakoczy EP, Rahman ISA, Binz N, et al. Characterization of a Mouse Model of Hyperglycemia and Retinal Neovascularization. The American Journal of Pathology. 2010;177:2659–2670. doi: 10.2353/ajpath.2010.090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes & Development. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 33.Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 34.Uemura A, Ogawa M, Hirashima M, et al. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. The Journal of Clinical Investigation. 2002;110:1619–1628. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hart AW, McKie L, Morgan JE, et al. Genotype-phenotype correlation of mouse pde6b mutations. Invest Ophthalmol Vis Sci. 2005;46:3443–3450. doi: 10.1167/iovs.05-0254. [DOI] [PubMed] [Google Scholar]
- 36.West H, Richardson WD, Fruttiger M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development. 2005;132:1855–1862. doi: 10.1242/dev.01732. [DOI] [PubMed] [Google Scholar]
- 37.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Developmental Dynamics. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 38.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Meth. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez M, Wu Z, Moreno-Carranza B, et al. Vasoinhibin Gene Transfer by Adenoassociated Virus Type 2 Protects against VEGF- and Diabetes-Induced Retinal Vasopermeability. Investigative Ophthalmology & Visual Science. 2011;52:8944–8950. doi: 10.1167/iovs.11-8190. [DOI] [PubMed] [Google Scholar]
- 41.Sheth N, Roca X, Hastings ML, Roeder T, Krainer AR, Sachidanandam R. Comprehensive splice-site analysis using comparative genomics. Nucleic Acids Res. 2006;34:3955–3967. doi: 10.1093/nar/gkl556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehler E, Karlhuber G, Bauer HC, Draeger A. Heterogeneity of smooth muscle-associated proteins in mammalian brain microvasculature. Cell Tissue Res. 1995;279:393–403. doi: 10.1007/BF00318497. [DOI] [PubMed] [Google Scholar]
- 43.Hellstrom M, Kaln M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 44.Klinghoffer RA, Mueting-Nelsen PF, Faerman A, Shani M, Soriano P. The two PDGF receptors maintain conserved signaling in vivo despite divergent embryological functions. Mol Cell. 2001;7:343–354. doi: 10.1016/s1097-2765(01)00182-4. [DOI] [PubMed] [Google Scholar]
- 45.Lindahl P, Hellstrom M, Kalen M, et al. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- 46.Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- 47.Stewart PA, Tuor UI. Blood-eye barriers in the rat: correlation of ultrastructure with function. J Comp Neurol. 1994;340:566–576. doi: 10.1002/cne.903400409. [DOI] [PubMed] [Google Scholar]
- 48.Frank RN, Turczyn TJ, Das A. Pericyte coverage of retinal and cerebral capillaries. Investigative Ophthalmology & Visual Science. 1990;31:999–1007. [PubMed] [Google Scholar]
- 49.Ribatti D, Crivellato E. ‘Sprouting angiogenesis’, a reappraisal. Developmental Biology. 2012;372:157–165. doi: 10.1016/j.ydbio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barber AJ, Gardner TW, Abcouwer SF. The Significance of Vascular and Neural Apoptosis to the Pathology of Diabetic Retinopathy. Investigative Ophthalmology & Visual Science. 2011;52:1156–1163. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navaratna D, Guo SZ, Hayakawa K, Wang X, Gerhardinger C, Lo EH. Decreased cerebrovascular brain-derived neurotrophic factor-mediated neuroprotection in the diabetic brain. Diabetes. 2011;60:1789–1796. doi: 10.2337/db10-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidal-Sanz M, Lafuente M, Sobrado-Calvo P, et al. Death and neuroprotection of retinal ganglion cells after different types of injury. Neurotox Res. 2000;2:215–227. doi: 10.1007/BF03033795. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Mao D, Chen X, et al. Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice. Mol Vis. 2012;18:1411–1420. [PMC free article] [PubMed] [Google Scholar]
- 55.Feit-Leichman RA, Kinouchi R, Takeda M, et al. Vascular Damage in a Mouse Model of Diabetic Retinopathy: Relation to Neuronal and Glial Changes. Investigative Ophthalmology & Visual Science. 2005;46:4281–4287. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.