Abstract
Purpose
To assess for associations between hippocampal atrophy and measures of cognitive function, hippocampal magnetization transfer ratio (MTR), and diffusion measures of the fornix, the largest efferent white matter tract from the hippocampus, in patients with multiple sclerosis (MS) and controls.
Materials and Methods
A total of 53 patients with MS and 20 age- and sex-matched healthy controls participated in cognitive testing and scanning including high spatial-resolution diffusion imaging and a T1-MPRAGE scan. Hippocampal volume and fornicial thickness measures were calculated and compared to mean values of fornicial transverse diffusivity, mean diffusivity, longitudinal diffusivity, fractional anisotropy, mean hippocampal MTR, and scores on measures of episodic memory, processing speed, and working memory tasks.
Results
In patients with MS, hippocampal volume was significantly related to fornicial diffusion measures (P < 7 × 10−4) and to measures of verbal (P = 0.030) and visual spatial (P = 0.004) episodic memory and a measure of information processing speed (P < 0.037).
Discussion
These results highlight the role of the hippocampus in cognitive dysfunction in patients with MS and suggest that measures of hippocampal atrophy could be used to capture aspects of disease progression.
Keywords: Multiple Sclerosis, hippocampus, episodic memory, DTI, atrophy, fornix
INTRODUCTION
More than 40% of patients with the demyelinating disease multiple sclerosis (MS) suffer some form of cognitive decline[1]. Traditional imaging measures, such as assessment of macroscopic lesion burden, are weakly related to cognitive changes [2], leading some researchers to focus on the role of gray matter (GM) pathology in cognitive dysfunction [3, 4]. The hippocampus has emerged as a target for much of this research [5, 6].
The hippocampus plays an important role in episodic memory, one of the most frequently affected cognitive domains in MS [1, 7, 8]. Previous research has shown that hippocampal demyelination is common in postmortem MS and that demyelinated hippocampi show decreased expression of neuronal proteins involved in a number of biological processes, including learning and memory [9]. More recently, functional magnetic resonance imaging (MRI) studies have shown decreased functional connectivity to the hippocampus in patients with MS who have intact spatial memory [10], as well as functional activation changes in the hippocampal memory network during a visual spatial episodic memory task [11]. Even more straightforward measures such as hippocampal volume have been found to correlate with measures of verbal episodic memory [5, 12].
The current study assessed whether hippocampal volume is associated with cognitive performance and with imaging measures including hippocampal magnetization transfer ratio (MTR) and high spatial-resolution diffusion measures of the fornix, the largest efferent white matter (WM) tract from the hippocampus. We test the hypothesis that hippocampal volume in MS patients would be strongly related to fornicial diffusion measures and to MTR, and that damage to the hippocampus and fornix would correlate more strongly with episodic memory than other cognitive domains.
MATERIALS AND METHODS
A total of 53 patients with MS and 20 approximately age- and sex-matched controls were scanned using a Siemens TIM Trio 3 tesla scanner (Siemens Medical Solutions, Erlangen, Germany) with a standard 12-channel receive-only head coil. A bite bar was used to limit motion during anatomical scans but was removed during DTI scans because of scanner vibration. All data were acquired after informed consent was obtained, under a protocol approved by the Cleveland Clinic Institutional Review Board.
The following scans were performed for all study participants:
Whole-brain T1. T1-weighted inversion recovery turboflash (MPRAGE) with the following parameters: 120 axial slices; thickness = 1.2 mm; field-of-view (FOV) = 256 × 256 mm2; inversion time (TI)/echo time (TE)/repetition time (TR)/flip angle (FA) = 900/1.71/1900 ms/8°; matrix = 256 × 128; receiver bandwidth (BW) = 62 kHz.
2. Whole-brain field map. Axial gradient-recalled echo with the following parameters: 32 axial slices; thickness = 4 mm; FOV = 256 × 256 mm2; matrix = 64 × 64; TE1/TE2/TR/FA = 4.89/7.35/388 ms/60°; BW = 260 Hz/pixel.
3. High angular resolution diffusion imaging (HARDI). Single-shot echo-planar imaging readout; FOV = 192 × 192 mm2; matrix = 192 × 192; 45 1-mm thick slices; TE/TR = 90/7700 ms; 6/8 partial Fourier factor with GRAPPA acceleration factor = 2; readout BW = 930 Hz/pixel; 71 directions with b = 1000sec/mm2; 8 b = 0 acquisitions, 2 averages. High spatial resolution (1 mm isotropic) was used to avoid partial volume averaging between fornix and surrounding cerebrospinal fluid (CSF).
Repeat whole brain field map (scan 2)
In addition, all controls and a subset of 35 patients underwent two gradient-echo scans, one with (MT+) and one without (MT−) an off-resonance MT saturation pulse, with the following parameters: TR/TE = 3.81/24; 1 avg/sec; x–slice thickness = 1mm; 144 slices; FOV = 256mm2; matrix = 256 × 256, frequency offset=1250Hz.
HARDI Postprocessing
A previously described iterative algorithm was used for motion correction [13]. FSL FUGUE was used for unwarping [14], and the diffusion tensor was calculated on a voxel-wise basis using a log-linear fit [15]. The tensors were diagonalized to determine eigenvalues used in the calculations of fractional anisotropy (FA), mean diffusivity (MD), longitudinal diffusivity (LD), and transverse diffusivity (TD).
ROIs and Volumetric Analysis
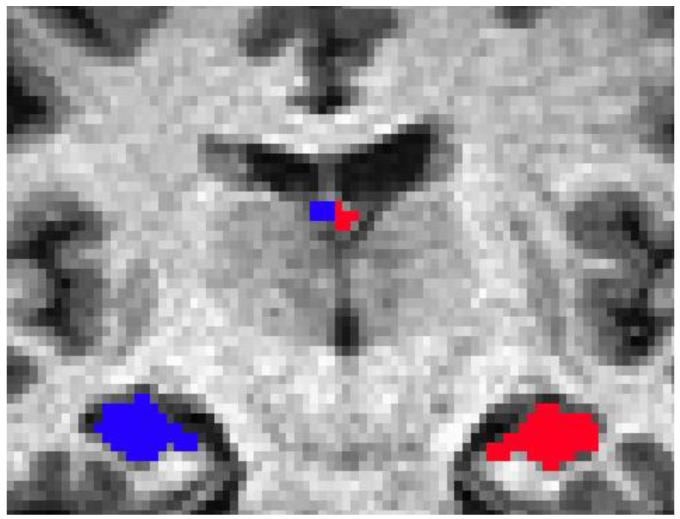
Fornix regions of interest (ROIs) were drawn manually. For the DTI analysis, the fornix was drawn in individual participants using the high-resolution T1-MPRAGE in Talairach space, starting at the posterior commissure and continuing to the fimbria. The ROIs were then warped into native space and checked for accuracy. The T1-MPRAGE was coregistered to the unwarped mean b = 0 image using FSL FLIRT [16], and the resultant transformation was applied to the ROIs to isolate the fornix on the FA, MD, LD, and TD images. Because the DTI images used a slightly smaller voxel size, ROIs were manually thinned in DTI space to ensure minimal effects of partial voluming. For the fornicial volume analysis, ROIs were manually drawn using the T1-MPRAGE in original space. Left and right fornix ROIs were drawn on five adjacent coronal slices, with the third slice approximately at the joining of the left and right crura (Figure 1).
Figure 1.
Representative example of hippocampal and fornicial ROIs.
Bilateral hippocampi were identified for each participant using the T1-MPRAGE and the automated program FSL FIRST [17]. ROIs were manually checked and corrected by a trained expert (Figure 1). Whole-brain WM and GM volumes were estimated using the FSL program SIENA [18-20]. SIENA uses an affine registration to MNI152 space to obtain a volumetric scaling factor, which is then used as a correction for head size [16, 21]. For each participant, the scaling factor was applied to GM, WM, hippocampal, and fornix volumes. Volume measures are defined as the number of voxels in the tissue mask multiplied by the scaling factor.
MTR Postprocessing
The MT− and MT+ scans were coregistered and the following formula was applied to each voxel:
Where MT− is the MR signal intensity in a given voxel for the non-MT data and MT+ is the signal in the data with the MT pulse. To exclude hippocampal WM, a trained expert manually drew bilateral fimbria ROIs on the T1-MPRAGE. Both fimbria and hippocampal ROIs as described above were registered to the MTR data using the AFNI program align_epi_anat.py [22]. For each participant, left and right hippocampal GM MTR values were histogrammed using 5% bins, with out-of-range values (MTR <0; MTR >100) excluded. Mean and mode MTR values were then calculated for each participant.
Behavioral Data
At the time of imaging, all participants were rated on the Expanded Disability Status Scale (EDSS) [23] and completed a variety of cognitive tests. The California Verbal Learning Test-II (CVLT-II) assesses verbal episodic memory and involves the recollection and identification of a series of words [24]. The Brief Visuospatial Memory Test, Revised (BVMT-R), is a measure of visual spatial episodic memory and requires participants to recall and reproduce simple line drawings [25]. Participants also completed the 3-second version of the Paced Auditory Serial Addition Test (PASAT), a measure of working memory, calculation, and speed of processing [26], and the oral version of the Symbol Digit Modalities Test (SDMT), also a measure of speed of processing and attention [27].
RESULTS
Demographics
All participants were right-handed (Edinburgh inventory > 80) [28]. The hippocampus and fornix were identified in all participants. One 49-year-old female patient was excluded from further analysis because of bilateral hippocampal volumes that were statistical outliers. Demographic information for the remaining participants is presented in Table 1. Unpaired Student’s t-tests were used to compare patient and control groups with respect to age and years of education. Control participants had significantly more education than patients did (P = 0.031).
Table 1. Demographic characteristics.
| Patients with MS (n = 52) |
Controls (n = 20) |
P | |
|---|---|---|---|
| Men, n | 16 | 7 | – |
| Mean age, years (SD) | 44.27 (8.9) | 41.35 (9.7) | 0.230 |
| Mean education, years (SD) | 15.09 (2.5) | 16.65 (3.1) | 0.032 |
| Mean MSFC (SD) | 0.31 (0.54) | 0.74 (0.24) | 0.001 |
| Median EDSS (range) | 1.5 (1–6.5) | – | – |
| Median disease duration (range) | 8(1–33) | – | – |
| Disease phenotype | 45 relapse remitting; 7 secondary progressive |
– | – |
| Mean raw cognitive task scores (SD) | |||
| SDMT | 54.4 (11.8) | 65.5 (13.5) | 5 × 10−4 |
| PASAT | 48.7 (9.9) | 53.9 (6.0) | 0.076 |
| CVLT-II | 49.5 (10.4) | 58.3 (10.5) | 0.002 |
| BVMT-R | 24.6 (5.9) | 29.2 (3.9) | 0.007 |
SDMT = Symbol Digit Modalities Test, PASAT = Paced Auditory Serial Addition Test, CVLT-II = California Verbal Learning Test-II, BVMT-R = Brief Visuospatial Memory Test-Revised
Behavioral Data
Raw scores for each cognitive measure were corrected using published norms. CVLT-II and BVMT-R total recall scores were converted to t-scores using age-corrected norms [24, 25], whereas SDMT scores were corrected for both age and level of education and converted to z-scores [29, 30]. PASAT scores were corrected for level of education and converted to z-scores [31]. Unpaired Student’s t-tests were used to compare cognitive performance in patients and controls. Patients scored significantly lower than controls on the CVLT-II, BVMT-R, and SDMT (P < 0.007) (Table 1). Uncorrected scores for all cognitive measures are reported in Table 1.
Volumetric Analysis and Imaging Measures
Unpaired t-tests were used to compare volumetric measures in patients and controls after correcting for head size. Corrected hippocampal volume was significantly lower in patients bilaterally (P < 0.038), whereas corrected fornix volume was significantly lower in patients only on the left (P < 0.001) (Table 2). Corrected GM and WM volumes were significantly lower in patients (P < 0.004). No sex differences were found after head size correction.
Table 2. Volumetric results.
| Patients with MS (n = 52) |
Controls (n = 20) |
P | |
|---|---|---|---|
| Volume measures, in voxels: mean (standard deviation) | |||
| Right hippocampus, corrected | 4192 (574) | 4501 (527) | 0.038 |
| Left hippocampus, corrected | 4040 (463) | 4296 (431) | 0.036 |
| Right fornix, corrected | 16.5 (6.9) | 19.9 (6.0) | 0.053 |
| Left fornix, corrected | 16.2 (6.4) | 22.2 (7.2) | 0.001 |
| White matter, corrected | 664,464 (48,658) | 702,676 (48,117) | 0.004 |
| Gray matter, corrected | 765,240 (39,773) | 805,979 (37,916) | 2 × 10−4 |
The relationship between imaging measures and hippocampal volumes was assessed with Pearson correlation. In patients, hippocampal volume was significantly related to all fornicial DTI measures. This relationship remained significant even after using a linear partial correlation to control for fornix volume (Table 3). Controls showed no correlation between hippocampal volumes and DTI measures. Bilaterally, patients showed significantly lower FA and significantly higher MD, TD, and LD than controls (P < 7 × 10−5).
Table 3. Correlation of hippocampal volume with fornicial DTI measures in patients with MS.
| Corrected for fornix volume |
||||
|---|---|---|---|---|
| Right hippocampal volume | r | P | r | P |
| Fractional anisotropy | 0.485 | 2 × 10−4 | 0.462 | 6 × 10−4 |
| Mean diffusivity | −0.482 | 2 × 10−4 | −0.448 | 9 × 10−4 |
| Transverse diffusivity | −0.519 | 8 × 10−5 | −0.488 | 2 × 10−4 |
| Longitudinal diffusivity | −0.373 | 6 × 10−3 | −0.328 | 0.019 |
| Left hippocampal volume | ||||
| Fractional anisotropy | 0.538 | 3 × 10−5 | 0.509 | 1 × 10−4 |
| Mean diffusivity | −0.522 | 7 × 10−5 | −330.492 | 2 × 10-4 |
| Transverse diffusivity | −0.555 | 1 × 10−5 | −0.529 | 6 × 10−5 |
| Longitudinal diffusivity | −0.425 | 1 × 10−3 | −0.383 | 5 × 10−3 |
All controls and a subset of 34 patients (13 men; mean age, 44.23 ± 9.1 years; mean MSFC, 0.32 ± 0.59; median EDSS, 1.75 [range, 1–6.5]; median disease duration, 7.5 years [range, 1–33]; 30 with relapse-remitting disease and 4 with secondary progressive disease) completed the MT scans. Neither patients nor controls showed a significant relationship between MTR and hippocampal volume. An unpaired t-test showed that patients had a significantly lower mean and mode MTR in the left hippocampus versus controls (P < 0.039).
Pearson correlations were used to assess the relationship between imaging and cognitive measures. In patients, hippocampal volume was significantly correlated with SDMT performance (P < 0.037) and EDSS (p < 0.037) bilaterally and with CVLT-II and BVMT-R performance on the left (P < 0.030) (Table 4). Bilateral fornicial DTI measures were strongly related to the BVMT-R and SDMT (P < 0.006) but showed no significant relationship to CVLT-II and PASAT. Fornicial MD, TD, and LD were related to EDSS (P < 0.020) on the right only. Mean hippocampal MTR was significantly related to performance on the CVLT-II (P = 0.043) and PASAT (p = 0.034) on the left and to the SDMT bilaterally (P < 0.042). MTR was not related to EDSS. Hippocampal volume, diffusion measures, and MTR were not significantly related to age or education level.
Table 4. Pearson’s r for the correlation of cognitive measures with hippocampal volume, fornicial DTI measures, and MTR in patients with MS.
| CVLT-II | BVMT-R | SDMT | PASAT | EDSS | |
|---|---|---|---|---|---|
| Right | |||||
| Fractional anisotropy | 0.132 | 0.431** | 0.280* | 0.164 | −0.185 |
| Mean diffusivity | −0.204 | −0.439** | −0.295* | −0.181 | 0.334* |
| Transverse diffusivity | −0.206 | −0.459*** | −0.309* | −0.188 | 0.322* |
| Longitudinal diffusivity | −0.187 | −0.370** | −0.246 | −0.156 | 0.343* |
| Volume | 0.123 | 0.249 | 0.290* | 0.110 | −0.289* |
| Mean MTR (n = 34) | 0.161 | 0.158 | 0.350* | 0.293 | −0.196 |
| Left | |||||
| Fractional anisotropy | 0.166 | 0.484*** | 0.436** | 0.235 | −0.143 |
| Mean diffusivity | −0.232 | −0.531**** | −0.469*** | −0.245 | 0.223 |
| Transverse diffusivity | −0.234 | −0.533*** | −0.478*** | −0.243 | 0.215 |
| Longitudinal diffusivity | −0.220 | −0.504*** | −0.429** | −0.237 | 0.231 |
| Volume | 0.301* | 0.393** | 0.456*** | 0.077 | −0.289* |
| Mean MTR (n = 34) | 0.349* | 0.304 | 0.436** | 0.365* | 0.004 |
P <0.05,
P <0.01,
P <0.001,
P <0.0001
CVLT = California Verbal Learning Test-II, BVMT-R = Brief Visuospatial Memory Test-Revised, SDMT = Symbol Digit Modalities Test, PASAT = Paced Auditory Serial Addition Test
DISCUSSION
In this study, overall hippocampal volume was 6% to 7% smaller in patients than in controls. Measures of WM integrity in the fornix were strongly related to hippocampal volume in patients but not in controls. Measures of episodic memory were also related to hippocampal volume in patients, but only on the left, although a measure of attention and speed of processing was related to bilateral hippocampal volumes. These findings point to involvement of the hippocampus in cognitive decline in MS.
The finding of a relationship between hippocampal volume and fornicial DTI measures suggests that DTI measures of the fornix may be an indicator of hippocampal injury. The direction of this relationship is unclear, however; it is possible that injury of the fornicial WM results in subsequent damage to the hippocampus. In studies of anterior temporal lobectomy patients, long-term changes in fornicial DTI values were consistent with myelin degradation [32, 33], although acute measures were more variable [34]. Studies of hippocampal volume and fornicial integrity in patients with mild cognitive impairment have confirmed that hippocampal volume loss is related to reduced integrity of the fornix [35, 36], although fornicial abnormalities with no concurrent hippocampal atrophy have been found in patients with early mild cognitive impairment [37].
The current work did not demonstrate a relationship between MTR and hippocampal volume, although MTR was lower in the left hippocampus in patients with MS. Recent work has found that MTR is sensitive to cortical demyelination in patients with MS [38], and changes have been found in GM MTR and in the fornix specifically [39-41]. We did find modest positive correlations between MTR and performance on cognitive tasks, consistent with other studies that found associations between MTR and overall cognitive impairment [41-43].
We found clear relationships between cognition and both hippocampal volume and fornicial DTI measures in MS. Measures of verbal and visual spatial episodic memory were related to hippocampal volume only on the left, in contrast to results from a previous study that demonstrated a significant relationship between verbal episodic memory and hippocampal volume bilaterally [5], though Sicotte et. al found a much weaker correlation between verbal episodic memory and hippocampal volume on the right. The composition of the current sample may prevent us from detecting this relationship, as we have a more restricted EDSS and disease duration range. In our study, bilateral fornicial DTI measures were related to visual spatial memory but not verbal memory. This is in contrast to a previous study that demonstrated an association between verbal memory performance and FA in the fornix [44]. While we believe that the high spatial resolution in the current study leads to more accurate diffusion measurements, it is worth noting that both of the above studies used a similar test of verbal episodic memory, in contrast to our use of the CVLT-II.
We found a strong relationship between all imaging measures and performance on the SDMT, a measure of information processing speed and the task that showed the greatest difference between patients and controls in this sample. Although it is possible that the SDMT involves some element of working memory, SDMT performance is not thought to involve the hippocampal memory circuit [45]. This task is very sensitive to cognitive deterioration in MS [46], and the correlation with hippocampal volume suggests that hippocampal atrophy may be related to overall cognitive decline rather than a specific deficit in episodic memory.
A recent study showed a moderate relationship between fornicial MTR and diffusion imaging measures and performance on the PASAT [41]. While our fornicial diffusion measures showed no relationship to the PASAT, we did find a relationship between hippocampal MTR and PASAT performance on the left. Both studies found a relationship between EDSS and fornicial diffusion measures, though in our case this measure was significant on the right only. It is possible that an increased number of participants and inclusion of patients with a higher EDSS and longer disease duration in the Syc et al. study resulted in a stronger relationship with imaging measures, though it does not appear that the samples differed substantially on PASAT performance.
The results of the current study draw a clear contrast between diffusion measures and MTR. Bilateral fornicial diffusion measures showed highly significant between-group differences and were related to hippocampal volume. Conversely, hippocampal MTR was not related to hippocampal volume, and showed group differences only on the left. A possible cause of these differences is the nature of pathology captured by DTI and MTR. Post-mortem studies confirm that lower MTR is sensitive to severe degrees of demyelination, but not intermediate levels [38]. DTI may be more sensitive to earlier and more subtle degeneration of nerve fibers [47]. As mentioned above, our low disease burden and small sample size may result in MTR changes that are insufficient for disease detection.
The SDMT has been shown to be one of the most sensitive measures for detecting cognitive decline in MS [46, 48]. Not surprisingly, the SDMT showed a bilateral and relatively strong relationship with MTR and DTI. The BVMT-R demonstrated strong, bilateral correlations with DTI, but not with MTR. In contrast, the PASAT and CVLT-II did not correlate with most MTR and DTI measures, the exception being their correlations with the left MTR. Further studies with a larger sample and a wider range of disease burden will be needed to determine if these differences in clinical-MRI correlations are meaningful with regard to specific cognitive processes or simply represent different degrees of sensitivity of the cognitive and MR measures.
This study had a number of limitations. The patient sample had relatively low disease burden; we expect that the addition of patients with a greater degree of cognitive impairment would reveal a relationship between hippocampal volume and MTR and greater differences between patients and controls in MTR measures. Additionally, because of the resolution of our anatomical scan, we were unable to reliably segment the hippocampus and measure regional volume loss.
We found that hippocampal volume is strongly related to fornicial diffusion measures but not to MTR. Hippocampal volume and MTR were correlated with episodic memory on the left only, whereas fornicial diffusion measures were strongly related to visual spatial episodic memory bilaterally. Most strikingly, all imaging measures showed a degree of correlation with performance on a speed of processing task. These results suggest that measurements of hippocampal atrophy can capture aspects of disease progression and that SDMT performance may be one of the more informative cognitive measures in MS.
ACKNOWLEDGMENTS
This work was supported by grants from the National Multiple Sclerosis Society and the National Institutes of Health (NS035058). The authors would like to thank John Cowan, Tami Gaebelein, Sarah Gallucci, Blessy Mathews, Katie Murphy, Christine Reece, and Derrek Tew for their contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–91. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- [2].Zivadinov R, De Masi R, Nasuelli D, Bragadin LM, Ukmar M, Pozzi-Mucelli RS, et al. MRI techniques and cognitive impairment in the early phase of relapsing-remitting multiple sclerosis. Neuroradiology. 2001;43:272–8. doi: 10.1007/s002340000500. [DOI] [PubMed] [Google Scholar]
- [3].Rovaris M, Judica E, Gallo A, Benedetti B, Sormani MP, Caputo D, et al. Grey matter damage predicts the evolution of primary progressive multiple sclerosis at 5 years. Brain. 2006;129:2628–34. doi: 10.1093/brain/awl222. [DOI] [PubMed] [Google Scholar]
- [4].Bergsland N, Horakova D, Dwyer MG, Dolezal O, Seidl ZK, Vaneckova M, et al. Subcortical and cortical gray matter atrophy in a large sample of patients with clinically isolated syndrome and early relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiology. 2012;33:1573–78. doi: 10.3174/ajnr.A3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sicotte N, Kern K, Giesser B, Arshanapalli A, Schultz A, Montag M, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–41. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- [6].Roosendaal S, Moraal B, Vrenken H, Castelijns J, Pouwels P, Barkhof F, et al. In vivo MR imaging of hippocampal lesions in multiple sclerosis. J Magn Reson Imaging. 2008;27:726–31. doi: 10.1002/jmri.21294. [DOI] [PubMed] [Google Scholar]
- [7].Yanike M, Wirth S, Suzuki W. Representation of well-learned information in the monkey hippocampus. Neuron. 2004;42:477–87. doi: 10.1016/s0896-6273(04)00193-x. [DOI] [PubMed] [Google Scholar]
- [8].DeLuca J, Gaudino E, Diamond B, Christodoulou C, Engel R. Acquisition and storage deficits in multiple sclerosis. J Clin Exp Neuropsychol. 1998;20:376–90. doi: 10.1076/jcen.20.3.376.819. [DOI] [PubMed] [Google Scholar]
- [9].Dutta R, Chang A, Doud M, Kidd G, Ribuado M, Young E, et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann. Neurol. 2011;69:445–54. doi: 10.1002/ana.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roosendaal S, Hulst H, Vrenken H, Feenstra H, Castelijns J, Pouwels P, et al. Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology. 2010;255:595–604. doi: 10.1148/radiol.10091433. [DOI] [PubMed] [Google Scholar]
- [11].Hulst H, Schoonheim M, Roosendaal S, Popescu V, Schweren L, van der Werf Y, et al. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp. 2012;33:2268–80. doi: 10.1002/hbm.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19:238–53. doi: 10.1111/j.1750-3639.2008.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sakaie K, Lowe M. Quantitative assessment of motion correction for high angular resolution diffusion imaging. Magn Reson Imaging. 2010;28:290–6. doi: 10.1016/j.mri.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jenkinson M. A fast, automated, n-dimensional phase unwrapping algorithm. Magnetic Resonance in Medicine. 2003;49:193–7. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- [15].Basser P, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- [16].Jenkinson M, Bannister P, Brady aJ, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- [17].Patenaude B, Smith S, Kennedy D, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Smith S, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- [19].Smith S, De Stefano N, Jenkinson M, Matthews P. Normalised accurate measurement of longitudinal brain change. J Comput Assist Tomogr. 2001;25:466–75. doi: 10.1097/00004728-200105000-00022. [DOI] [PubMed] [Google Scholar]
- [20].Smith S, Zhang Y, Jenkinson M, Chen J, Matthews P, Federico A, et al. Accurate, robust and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002;17:479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- [21].Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- [22].Saad Z, Glen D, Chen G, Beauchamp M, Desai R, Cox R. A new method for improving functional-to-structural alignment using local Pearson correlation. Neuroimage. 2009;44:839–48. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kurtzke J. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- [24].Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- [25].Benedict RH. Brief visuospatial memory test - revised: professional manual. Psychological Assessment Resources; Odessa, Florida: 1997. [Google Scholar]
- [26].Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT) Arch Clin Neuropsychol. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [27].Lewandowski L. The Symbol Digit Modalities Test: a screening instrument for brain-damaged children. Percept Mot Skills. 1984;59:615–8. doi: 10.2466/pms.1984.59.2.615. [DOI] [PubMed] [Google Scholar]
- [28].Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- [29].Smith A. Symbol Digit Modalities Test: Manual. Western Psychological Services; Los Angeles: 1982. [Google Scholar]
- [30].Tombaugh T, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–77. [PubMed] [Google Scholar]
- [31].Rao S. A manual for the brief, repeatable battery of neuropsychological tests in multiple sclerosis. Medical College of Wisconsin; Milwaukee, WI: 1990. [Google Scholar]
- [32].McDonald C, Hagler D, Jr., Girard H, Pung C, Ahmadi M, Holland D, et al. Changes in fiber tract integrity and visual fields after anterior temporal lobectomy. Neurology. 2010;75:1631–8. doi: 10.1212/WNL.0b013e3181fb44db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Concha L, Beaulieu C, Wheatley BM, Gross DW. Bilateral white matter diffusion changes persist after epilepsy surgery. Epilepsia. 2007;48:931–40. doi: 10.1111/j.1528-1167.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- [34].Liu L, Gross D, Wheatley B, Concha L, Beaulieu C. The acute phase of Wallerian degeneration: Longitudinal diffusion tensor imaging of the fornix following temporal lobe surgery. NeuroImage. 2013;74:128–39. doi: 10.1016/j.neuroimage.2013.01.069. [DOI] [PubMed] [Google Scholar]
- [35].Douaud G, Menke R, Gass A, Monsch A, Rao A, Whitcher B, et al. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer’s disease. J Neurosci. 2013;33:2147–55. doi: 10.1523/JNEUROSCI.4437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhuang L, Wen W, Trollor J, Kochan N, Reppermund S, Brodaty H, et al. Abnormalities of the fornix in mild cognitive impairment are related to episodic memory loss. J Alzheimers Dis. 2012;29:629–39. doi: 10.3233/JAD-2012-111766. [DOI] [PubMed] [Google Scholar]
- [37].Zhuang L, Sachdev P, Trollor J, Reppermund S, Kochan N, Brodaty H, et al. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PLoS One. 2013;8:e58887. doi: 10.1371/journal.pone.0058887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen J, Easley K, Schneider C, Nakamura K, Kidd G, Chang A, et al. Clinically feasible MTR is sensitive to cortical demyelination in MS. Neurology. 2013;80:246–52. doi: 10.1212/WNL.0b013e31827deb99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Audoin B, Davies G, Rashid W, Fisniku L, Thompson A, Miller D. Voxel-based analysis of grey matter magnetization transfer ratio maps in early relapsing remitting multiple sclerosis. Mult Scler. 2007;13:483–9. doi: 10.1177/1352458506070450. [DOI] [PubMed] [Google Scholar]
- [40].Vrenken H, Pouwels P, Ropele S, Knol D, Geurts J, Polman C, et al. Magnetization transfer ratio measurement in multiple sclerosis normal-appearing brain tissue: limited differences with controls but relationships with clinical and MR measures of disease. Mult Scler. 2007;13:708–16. doi: 10.1177/1352458506075521. [DOI] [PubMed] [Google Scholar]
- [41].Syc S, Harrison D, Saidha S, Seigo M, Calabresi P, Reich D. Quantitative MRI Demonstrates Abnormality of the Fornix and Cingulum in Multiple Sclerosis. Multiple Sclerosis International. 2013;2013:1–9. doi: 10.1155/2013/838719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rovaris M, Filippi M, Minicucci L, Iannucci G, Santuccio G, Possa F, et al. Cortical/subcortical disease burden and cognitive impairment in patients with multiple sclerosis. AJNR Am J Neuroradiol. 2000;21:402–8. [PMC free article] [PubMed] [Google Scholar]
- [43].Tur C, Penny S, Khaleeli Z, Altmann D, Cipolotti L, Ron M, et al. Grey matter damage and overall cognitive impairment in primary progressive multiple sclerosis. Mult Scler. 2011;17:1324–32. doi: 10.1177/1352458511410341. [DOI] [PubMed] [Google Scholar]
- [44].Kern K, Ekstrom A, Suthana N, Giesser B, Montag M, Arshanapalli A, et al. Fornix damage limits verbal memory functional compensation in multiple sclerosis. NeuroImage. 2012;59:2932–40. doi: 10.1016/j.neuroimage.2011.09.071. [DOI] [PubMed] [Google Scholar]
- [45].Forn C, Belenguer A, Belloch V, Sanjuan A, Parcet M, Ávila C. Anatomical and functional differences between the Paced Auditory Serial Addition Test and the Symbol Digit Modalities Test. J Clin Exp Neuropsychol. 2011;33:42–50. doi: 10.1080/13803395.2010.481620. [DOI] [PubMed] [Google Scholar]
- [46].Amato M, Portaccio E, Goretti B, Zipoli V, Iudice A, Della Pina D, et al. Relevance of cognitive deterioration in early relapsing-remitting MS: a 3-year follow-up study. Mult Scler. 2010;16:1474–82. doi: 10.1177/1352458510380089. [DOI] [PubMed] [Google Scholar]
- [47].Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- [48].Drake A, Weinstock-Guttman B, Morrow S, Hojnacki D, Munschauer F, Benedict R. Psychometrics and normative data for the Multiple Sclerosis Functional Composite: replacing the PASAT with the Symbol Digit Modalities Test. Multiple Sclerosis. 2010;16:228–37. doi: 10.1177/1352458509354552. [DOI] [PubMed] [Google Scholar]