Abstract
Background
The most accepted means of evaluating the response of a patient with cervical spondylomyelopathy (CSM) to treatment is subjective and based on the owner and clinician's perception of the gait.
Objective
To establish and compare kinetic parameters based on force plate gait analysis between normal and CSM-affected Dobermans.
Animals
Nineteen Doberman Pinschers: 10 clinically normal and 9 with CSM.
Methods
Force plate analysis was prospectively performed in all dogs. At least 4 runs of ipsilateral limbs were collected from each dog. Eight force platform parameters were evaluated, including peak vertical force (PVF) and peak vertical impulse (PVI), peak mediolateral force (PMLF) and peak mediolateral impulse, peak braking force and peak braking impulse, and peak propulsive force (PPF) and peak propulsive impulse. In addition, the coefficient of variation (CV) for each limb was calculated for each parameter. Data analysis was performed by a repeated measures approach.
Results
PMLF (P = .0062), PVI (P = .0225), and PPF (P = .0408) were found to be lower in CSM-affected dogs compared with normal dogs. Analysis by CV as the outcome indicated more variability in PVF in CSM-affected dogs (P = 0.0045). The largest difference in the CV of PVF was seen in the thoracic limbs of affected dogs when compared with the thoracic limbs of normal dogs (P = 0.0019).
Conclusions and Clinical Importance
The CV of PVF in all 4 limbs, especially the thoracic limbs, distinguished clinically normal Dobermans from those with CSM. Other kinetic parameters less reliably distinguished CSM-affected from clinically normal Dobermans.
Keywords: Cervical vertebral instability, Dog, Kinetic, Wobbler
Cervical spondylomyelopathy (CSM) is the most common cervical spinal disease of large and giant breed dogs, with Doberman Pinschers and Great Danes being overrepresented.1–7 This term collectively refers to several disorders of the caudal cervical verte-brae and intervertebral disks that result in static and dynamic compression of the spinal cord.5,8 Gait abnormalities are the hallmark of dogs suffering from CSM. Clinical signs often involve varying degrees of ataxia, paresis, or both affecting the pelvic limbs,5,9–11 with the most common initial presentation being a slowly progressive pelvic limb ataxia and paresis with less severe changes in the thoracic limbs.1,5,9,10 The diagnosis of CSM is not always straightforward and requires advanced imaging or myelography. In addition, the treatment for CSM is controversial, with several surgical techniques available to treat the disease.
Determining a CSM patient's response to treatment currently is highly subjective and based on client interpretation and gait examination in which patients are determined to be “improved, worse, or unchanged.” This assessment is very subjective because, in principle, there is no difference in the degree of improvement between a dog that demonstrated improvement after treatment, but still is severely ataxic and disabled, and a dog that returns to normal or almost normal function. Development of a more objective means of assessing an affected dog's neurologic status is essential to allow objective comparisons of treatment methods and outcome.
Force plate analysis is a noninvasive, objective measurement to evaluate limb loading in humans and animals,12,13 and it has been used to study normal gait as well as experimentally induced or naturally occurring lameness in dogs.14–27 In addition, ground reaction forces using force plate analysis have been evaluated as an assessment of recovery in dogs treated for lumbosacral stenosis.28,29 Force plate analysis also has been proven to be useful in differentiating between orthopedic and neurologic causes of gait abnormalities in horses.30 There have been no studies investigating the force plate characteristics of dogs with cervical spinal cord diseases causing weakness and ataxia.
The purpose of this study was to prospectively identify force plate parameter differences between Doberman Pinschers with CSM and normal Doberman Pinschers. We hypothesized that a subset of the force plate parameters, namely peak vertical force (PVF) and peak mediolateral force (PMLF), would be significantly altered in Doberman Pinchers with CSM because of ataxia, paresis, or a combination of both. Identification of consistent differences in kinetic parameters between normal and CSM-affected dogs will serve as an initial step in the development of objective outcome measures for monitoring response to medical or surgical treatment in dogs with CSM.
Materials and Methods
Animals
Nineteen client-owned mature Doberman Pinscher dogs were prospectively enrolled in this investigation. The study was conducted in accordance with the guidelines and with approval of the Clinical Research Advisory Committee and the Institutional Animal Care and Use Committee. Written owner consent was obtained before study enrollment.
Normal Dogs
Dogs were considered normal and eligible for study enrollment if they were 1 year of age and had no abnormalities identified on physical, orthopedic, and neurologic examinations. In addition, they could not have any previous orthopedic or neurologic disease.
Affected Dogs
Inclusion criteria for affected dogs included a history of pelvic limb ataxia, paraparesis, tetraparesis with or without neck pain in a mature Doberman Pinscher dog in addition to neurologic examination findings consistent with cervical myelopathy. All affected dogs had physical and neurologic examinations, CBC, serum biochemistry profile, cervical spinal radiographs, and magnetic resonance imaging (MRI) examination of the cervical spine. Neurologic status at the time of 1st examination was graded on a scale from 1 to 5 on the basis of a previously published grading scale.31,32 Dogs classified as Grade 1 had cervical hyperesthesia only. These patients were excluded from participation. Grade 2 dogs had mild pelvic limb ataxia or paresis with mild thoracic limb involvement. Thoracic limb involvement was defined as either short-strided or spastic gait with a floating appearance. Grade 3 dogs had moderate pelvic limb ataxia or paresis with thoracic limb involvement as described in Grade 2. Grade 4 was defined as marked pelvic limb ataxia or paresis with thoracic limb involvement, and Grade 5 was defined as nonambulatory tetraparesis. Any dog with Grade 5 neurologic status also was excluded from the study. The diagnosis of CSM was confirmed in all dogs by MRI, utilizing a modified standard protocol32 to support evidence of spinal cord compression with or without spinal cord signal change. Thoracic radiographs were performed in all dogs >7 years of age (n = 5). Neurologic examinations of all dogs (normal and affected) were performed by 2 of the investigators (K.F. and R.d.C.).
Gait Analysis
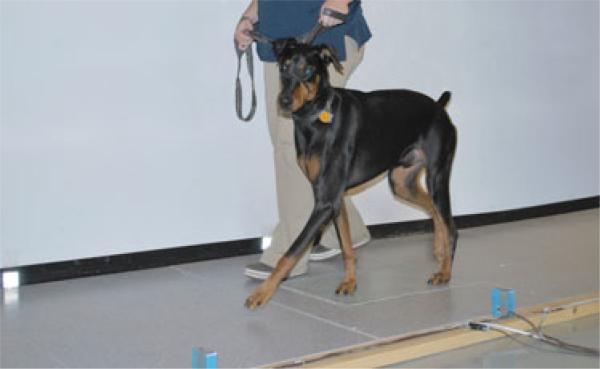
Force plate analysis was performed in all 4 limbs of all dogs by a stationary force platea and computer analysis system.b The force plate was 40 × 60 cm and mounted flush with the surface and in the center of a 6-m walkway (Fig 1). All dogs were weighed on the same digital scale before data collection and force plate data forces in Newton (N) were normalized to body weight (BW). Each dog was walked across the walkway for 5–10 minutes to acclimate the animal to the laboratory before data collection. Valid repetitions were recorded from the left and right sides of the body (minimum of 2 and maximum of 8 repetitions). A valid repetition was defined as a passage over the force plate by the dog in which the ipsilateral thoracic and pelvic limb fully contacted the surface of the plate and the gait velocity was within the range of 0.8–1.5 m/s. Dogs were walked in both directions, and starting position was standardized in each direction. Gait velocity was measured by the use of 3 photoelectric switches (spaced 1 m apart) connected to the computer system.
Fig 1.
Force plate gait analysis in a clinically normal Doberman Pinscher.
Three force-to-time curves were generated by the computer analysis system to compute 8 kinetic parameters in each limb. These parameters included PVF, peak vertical impulse (PVI), peak braking force (PBF), peak braking impulse (PBI), peak propulsive force (PPF), peak propulsive impulse (PPI), PMLF, and peak mediolateral impulse (PMLI). Force peaks and impulses were expressed as percentage of body weight and percentage of body weight per second by normalizing a dog's weight and multiplying it by time. Descriptive statistics (mean, standard deviation, range) were calculated for each force plate parameter. In addition, the CV for PVF and PMLF were calculated for each limb.
Data Analysis
A repeated measures analysis by commercial statistical softwarec was performed and the significance level was set at P < .05. First, all 8 parameters were compared between normal and CSM-affected dogs. Then, left and right side and thoracic and pelvic limbs were compared using all data as well as data from normal and CSM-affected dogs separately. To account for the nonindependent observations and measurements within limbs and limbs nested within dogs, compound symmetry covariance structure was used. Similarly, variability in PVF and PMLF between normal and CSM-affected dogs, as well as between left and right side, and thoracic and pelvic limbs was compared by the coefficient of variation (CV) as the outcome variable as follows:
Results
A total of 19 dogs were enrolled and placed into 1 of 2 groups. The 1st group consisted of 10 clinically normal Doberman Pinschers, of which 9 were male and 1 was female between 1 and 7 years old (mean, 4.2 years; SD, 1.8; median, 4 years) with body weights ranging from 31.3 to 43.6 kg (mean, 37.9; SD, 4.5; median, 39.3 kg). The affected group consisted of 9 Doberman Pinschers dogs that included 6 males and 3 females between 3 and 12 years old (mean, 7.7 years; SD, 3.5; median, 9 years). Clinical signs included mild pelvic limb ataxia or paresis with mild thoracic limb involvement (Grade 2; n = 4), moderate pelvic limb ataxia or paresis with thoracic limb involvement (Grade 3; n = 2), and marked pelvic limb ataxia or paresis with thoracic limb involvement (Grade 4; n = 3). The body weights of the dogs ranged from 27.2 to 55.8 kg (mean, 37.4 kg; SD, 6.7; median, 35.6 kg). All CSM-affected dogs had spinal cord compression in the caudal cervical spine. The main compression was at C5-6 in 3 dogs, and at C6-7 in 6 dogs. Kinetic gait analysis was performed in all dogs without any complications. The entire procedure required no more than 2 personnel to complete. Data collection time, including warm-up, ranged from 20 minutes to 2 hours (mean, 45 minutes) to acquire all valid passes. The collection time was approximately 1 hour for normal dogs and 1 hour for affected dogs. Time required for data collection did not appear to be associated with neurologic status. The mean number of valid passes collected during this process was 4.9 for the normal dogs (range, 3–8) and 4.5 (range, 2–6) for the CSM-affected dogs.
Descriptive statistics (mean ± SD) of all kinetic parameters are presented in Table 1. Based on the repeated measures analysis when adjusting for the correlated data structure, PVF was not significantly different between normal and affected dogs (P = .3031). However, PVF was significantly higher in the thoracic limbs than in the pelvic limbs (P < .0001) of all dogs. PMLF, using data from all 4 limbs, on the other hand, was significantly lower in CSM-affected dogs versus normal dogs (P = .0062) as were PVI (P = .0225) and PPF (P = .0408). No significant differences were detected in PPI, PBF, PBI, or PMLI when comparing normal and affected dogs.
Table 1.
Median (±SD) of force plate parameters from all 4 limbs in normal and CSM-affected dogs.
| Normal Dogs (n = 10) |
Affected Dogs (n = 9) |
|||||
|---|---|---|---|---|---|---|
| Force Plate Parameter | All Limbs | Thoracic Limbs | Pelvic Limbs | All Limbs | Thoracic Limbs | Pelvic Limbs |
| PVF (100 × N/N) | 57.72 ± 11.0 | 65.21 ± 6.02 | 47.08 ± 6.31 | 60.19 ± 15.9 | 65.52 ± 14.3 | 47.07 ± 11.05 |
| PVI (100 × Ns/N) | 20.04 ± 12.5 | 25.05 ± 13.4 | 15.855 ± 9.44 | 16.74 ± 5.9 | 21.3 ± 6.343 | 15.835 ± 3.63 |
| PPF (100 × N/N) | 7.2 ± 3.3 | 8.465 ± 3.3 | 6.375 ± 3.03 | 6.325 ± 3.8 | 6.88 ± 3.77 | 6.185 ± 3.74 |
| PPI (100 × Ns/N) | 1.075 ± 1.1 | 1.005 ± 1.03 | 1.15 ± 1.2 | 0.765 ± 1.1 | 0.52 ± 0.84 | 1.175 ± 1.25 |
| PBF (100 × N/N) | –8.57 ± 5.0 | –10.685 ± 4.97 | –6.7 ± 4.7 | –8.405 ± 4.6 | –9.24 ± 4.4 | –7.215 ± 4.55 |
| PBI (100 × Ns/N) | –1.015 ± 1.4 | –1.68 ± 1.14– | –0.64 ± 1.62 | –0.975 ± 1.1 | –1.205 ± 1.1 | –0.625 ± 0.97 |
| PMLF (100 × N/N) | –3.015 ± 7.3 | –3.015 ± 7.03– | –3.015 ± 7.53 | –2.73 ± 5.16 | –2.805 ± 5.24 | –2.71 ± 5.12 |
| PMLI (100 × Ns/N) | –0.46 ± 2.7 | –0.495 ± 2.65 | –0.39 ± 2.7 | –0.31 ± 1.4 | –0.31 ± 1.46 | –0.32 ± 1.52 |
PVF, peak vertical force; PVI, peak vertical force; PPF, peak propulsive force; PPI, peak propulsive impulse; PBF, peak braking force; PBI, peak braking impulse; PMLF, peak mediolateral force; PMLI, peak mediolateral impulse; SD, standard deviation.
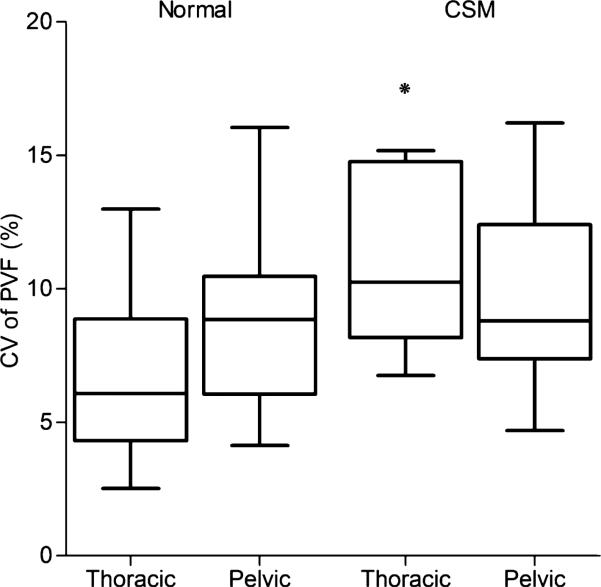
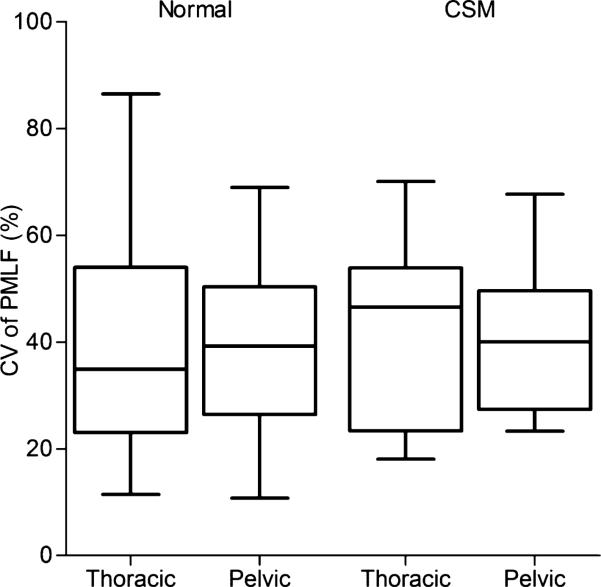
A summary of the data using CV as an outcome is presented in Table 2. When comparing CV of PVF between normal and affected dogs, the values were significantly higher in CSM-affected than normal dogs (P = .0045). When stratifying the data to compare the thoracic limbs of normal and affected dogs, the CV of PVF in the thoracic limbs of affected dogs also was much higher than that of normal dogs (P = .0019, Fig 2). However, no significant differences were found in the pelvic limbs between normal and affected dogs. The CV values of PMLF were not different between normal and affected dogs (P = .4926), or between thoracic and pelvic limbs (Fig 3).
Table 2.
Standard deviation, mean, and coefficient of variation for PVF and PMLF from all 4 limbs of normal and CSM-affected Doberman Pinschers.
| Normal Dogs (n = 10) |
Affected Dogs (n = 9) |
|||||
|---|---|---|---|---|---|---|
| Force Plate Parameter | All Limbs | Thoracic Limbs | Pelvic Limbs | All Limbs | Thoracic Limbs | Pelvic Limbs |
| PVF SD (100 × N/N) | 11.00562845 | 5.354030205 | 46.93352928 | 15.87375 | 14.26288648 | 10.70708963 |
| Mean PVF (100 × N/N) | 56.23964709 | 65.54576491 | 63.05106915 | 59.48801 | 69.1505131 | 49.82551286 |
| PVF CV (%) | 19.56916343 | 8.168384659 | 13.43412058 | 26.68395 | 20.62585777 | 21.48917094 |
| PMLF SD (100 × N/N) | 3.100395 | 3.028367 | 3.148396 | 2.499764 | 2.639914 | 2.123727 |
| Mean PMLF (100 × N/N) | 5.927 | 5.665176 | 6.188824 | 4.719936 | 4.946154 | 4.60141 |
| PMLF CV (%) | 52.30969 | 53.45583 | 50.87229 | 52.96182 | 53.37307 | 46.15383 |
PVF, peak vertical force; PMLF, peak mediolateral force; SD, standard deviation; CV, coefficient of variation.
Fig 2.
Boxplot of the coefficient of variation in the PVF in normal and CSM-affected dogs. The box represents the interquartile range (25–75%), and the line in each box delineates the median value. Overall, CSM-affected dogs had a significantly higher variability in PVF versus normal dogs, but more specifically, in the thoracic limbs. *P = .0045; PVF, peak vertical force; CV, coefficient of variation; CSM, cervical spondylomyelopathy.
Fig 3.
Boxplot of the coefficient of variation in the PMLF in normal and affected dogs. There was no significant difference in the CV of the PMLF between the normal and affected dogs. CV, coefficient of variation; PMLF, peak mediolateral force. See Figure 1 for remainder of key.
Discussion
This study is the first to use force plate analysis to investigate changes in gait pattern in dogs suffering from a cervical myelopathy. Of 26 different parameters evaluated, only 5 were found to be of significance, including PMLF, PVI, PPF, CV PVF, and the thoracic limb CV PVF. We found that dogs with CSM have much more variability in their PVF, in particular the PVF of the thoracic limbs, but not in the pelvic limbs. Previous force plate studies have shown that dogs have a different weight distribution between the thoracic limbs and the pelvic limbs. The thoracic limbs carry about 60% of the body weight, whereas the pelvic limbs carry 40% of the body weight during a walk.12,33 In addition, it has been observed that ataxic dogs have a tendency to shift their weight even more onto their thoracic limbs as a result of hind limb ataxia.34 These dogs also show some degree of ataxia in the thoracic limbs, which may play a role in the larger variability in PVF in affected dogs. For example, an ataxic dog may not be able to recognize its limb position and therefore cannot adjust the timing of foot landing based on visual perception. This then may lead to footfalls that occur early or late, leading to random weight distribution during each pass across the force plate, and greater variation in PVF with each step. Studies in humans have shown increased vertical forces when walking on an unbalanced surface with blocking of the lower visual field.13,35 When a patient has an unexpected footfall, the next step may be more hesitant, leading to a decrease in PVF of that limb in the subsequent foot placement.36 The combination of bearing more weight in the thoracic limbs and the incoordination associated with ataxia may lead not only to a larger PVF in the thoracic limbs but also to a greater variability in PVF in the affected dogs. Despite this finding, there was a great deal of overlap in the CV of the PVF in both CSM-affected dogs and normal dogs, suggesting that force plate analysis may be able to distinguish severely affected dogs from normal dogs, but may not be as clinically useful for discriminating between normal dogs and mildly affected dogs.
Dogs with CSM have upper motor neuron paresis (which typically is associated with increased muscle tone) and proprioceptive ataxia, versus a musculoskeletal weakness, which may explain why there were no differences detected in mean PVF between normal and affected dogs in either the thoracic or pelvic limbs. The mean PVI from all 4 limbs also was significantly lower in CSM-affected dogs compared with normal dogs. Normal dogs may have a longer stance phase to account for this difference, because the average velocity was not different between normal and affected dogs (1.08 and 1.07 m/s, respectively). PPF also was higher in all limbs of normal dogs. One explanation could be related possibly to a longer stance phase, because this phase of the gait often is broken into 2 parts: the braking forces, which are the impulses required to decrease momentum in the early stance phase; and the propulsion forces, which are the impulses required to increase momentum during the late stance phase.12,33 The thoracic limbs spend approximately 50% of the stance phase in braking and 50% in propulsion, whereas the pelvic limbs spend approximately 35% in braking and 65% in propulsion.12,33 Dogs with CSM can present with varying degrees of ataxia and weakness in the thoracic and pelvic limbs, which may alter the distribution of braking and propulsive forces, leading to decreased propulsion.
We expected that CSM-affected dogs also would show increased mediolateral forces as well as more variability in these forces. However, the mean peak MLF was not different between groups, nor was there a difference in the variability in the peak MLF. In fact, the peak MLF was found to be higher in all limbs of normal dogs, but not statistically different from the CSM dogs. This may be related to the fact that this force is based on measuring the estimate of mediolateral movement and not assessing stability of the patient. However, a study assessing force plate analysis in ataxic horses concluded that they had significantly larger mediolateral force peaks in the pelvic limbs than did normal or horses with musculoskeletal lameness.30 This may be explained by the observation that ataxic horses may have an elongated swaying motion resulting in exaggerated sideways movement, allowing detection of magnitude of force. This theory of exaggerated sideways movement most likely is true in dogs as well. However, horses are larger and theoretically may supply a larger amount of force, whereas mediolateral forces have been reported to be difficult to determine in dogs.15 This may be attributed to the fact that mediolateral forces are considered to be <6% of a dog's body weight.15 Perhaps a more reliable assessment of instability in ataxic dogs would be to assess the patient's center of pressure by means of stabilography. This method has been used widely in human medicine as a means of diagnosing a variety of neurologic disorders, including cervical spinal disorders.37–40 This method also has been applied in measuring postural sway in horses, but has not yet been evaluated in dogs.41,42 Another possible way to eliminate variability would be to calculate a symmetry index for the PVF and PMLF. This index has been used previously in studies on lameness in dogs to remove interdog variations because this calculation requires every dog to serve as its own control.19 In addition, the CV PMLF was quite large. Valid passes were collected from the ipsilateral limbs. A possible cause for the high CV may be attributed to using only 1 force plate to measure this value. For example, if an ataxic dog were to stumble when crossing the force plate, it may off-load the ataxic limbs during that pass and, during the next pass, bear more weight on the affected limbs. One possible way to address this issue would be to utilize 2 force plates to measure limbs simultaneously.
Limitations of this study include its small sample size. However, it is not uncommon for studies utilizing force plates to assess gait in veterinary medicine with small samples sizes,15,21,29,30,43,44 and our sample size actually was larger than some previous force plate studies.17,24,26,29,44 We also used a wide range for our velocity (0.8–1.5 m/s) to be able to obtain valid passes, especially in the more severely affected dogs. The standard speed for a walking dog is about 1.0 ± 0.3 m/s,29,43 but some studies have included a velocity of 2 m/s within the range of walking speeds.15 However, this large range of velocity may have lead to more variability in the value of the PVF in our cases.14,25 In addition, the data collection time was widely variable. Although we did not observe any difference in collection time between the 2 groups, we did observe that some dogs took longer than others, regardless of status, based solely on their behavior when being led on a leash. Furthermore, we attempted to collected data from ipsilateral limbs for a valid pass, which to our knowledge, has not been attempted previously. Most force plate studies, especially those assessing lameness, have focused on collecting data from 1 limb at a time, which may lead to more rapid collection times. Lastly, our sample size was small, which may have lead to the study being underpowered. Before data collection, a power analysis was performed to estimate the number of dogs necessary to detect a 20% difference between the groups to give a power of 0.8, which gave a number of 20 dogs (10 in each group). A post hoc power analysis was also performed and indicated that it would be necessary to enroll between 76 and 270 dogs in each group to have a power of 0.8 with a significance of 0.05 for PVF. To have a power of at least 0.8 and significance of 0.05 for PMLF between 29 and 87 dogs would be needed per group. Based on these results, it would have been impossible to enroll enough dogs of 1 breed to complete the study in a timely manner.
In conclusion, we found significantly more variability in the PVF of CSM-affected dogs compared with normal dogs, but we also observed substantial overlap between normal and affected dogs. In addition, several other parameters, including PMLF, were not found to be significantly different between Doberman Pinschers with and without CSM. The limited information provided by force plate analysis to detect differences between normal and affected dogs, coupled with the prolonged acquisition time, makes force plate analysis a method with limited applicability for use in the clinical setting for dogs with CSM. However, although there were not marked differences in variability between normal and affected dogs, there may be more substantial differences in variability detected within the same affected dog, which then may be used as a measure of outcome.
Acknowledgments
The authors thank Amanda Waln for her assistance with data collection. This study was supported by the Canine Funds of the College of Veterinary Medicine, The Ohio State University.
Abbreviations
- CSM
cervical spondylomyelopathy
- CV
coefficient of variation
- MRI
magnetic resonance imaging
- PBF
peak braking force
- PBI
peak braking impulse
- PMLF
peak mediolateral force
- PMLI
peak mediolateral impulse
- PPF
peak propulsive force
- PPI
peak propulsive impulse
- PVF
peak vertical force
- PVI
peak vertical impulse
Footnotes
Parts of this study will be presented in abstract form at the 2012 American College of Veterinary Internal Medicine Forum, New Orleans, LA.
Kistler Model 9687A force platform, Kistler Instrumente AG, Winterhur, Switzerland
Acquire 7.35, Sharon Software, Inc, Dewitt, MI
SAS 9.2, SAS Institute, Cary, NC
Conflict of Interest: Authors disclose no conflict of interest.
References
- 1.da Costa RC. Cervical spondylomyelopathy (wobbler syndrome) in dogs. Vet Clin North Am Small Anim Pract. 2010;40:881–913. doi: 10.1016/j.cvsm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Mason TA. Cervical vertebral instability (wobbler syndrome) in Doberman. Aust Vet J. 1977;53:440–445. doi: 10.1111/j.1751-0813.1977.tb05494.x. [DOI] [PubMed] [Google Scholar]
- 3.Seim HB, Withrow SJ. Pathophysiology and diagnosis of caudal cervical spondylomyelopathy with emphasis on the Doberman Pinscher. J Am Anim Hosp Assoc. 1982;18:241–251. [Google Scholar]
- 4.Lewis D. Cervical spondylomyelopathy (‘wobbler’ syndrome) in dogs. In Pract. 1992;14:125–130. [Google Scholar]
- 5.VanGundy TE. Disc-associated wobbler syndrome in the Doberman Pinscher. Vet Clin North Am Small Anim Pract. 1988;18:667–696. doi: 10.1016/s0195-5616(88)50061-x. [DOI] [PubMed] [Google Scholar]
- 6.Trotter EJ, deLahunta A, Geary JC, Brasmer TH. Caudal cervical vertebral malformation-malarticulation in Great Danes and Doberman Pinschers. J Am Vet Med Assoc. 1976;168:917–930. [PubMed] [Google Scholar]
- 7.Sharp NJ, Cofone M, Robertson ID, et al. Cervical spondylomyelopathy in the Doberman dog: A potential model for cervical spondylotic myelopathy in humans. J Invest Surg. 1989;2:333. [Google Scholar]
- 8.De Decker S, Gielen IM, Duchateau L, et al. Intraobserver and interobserver agreement for results of low-field magnetic resonance imaging in dogs with and without clinical signs of disk-associated wobbler syndrome. J Am Vet Med Assoc. 2011;238:74–80. doi: 10.2460/javma.238.1.74. [DOI] [PubMed] [Google Scholar]
- 9.De Decker S, Bhatti SFM, Duchateau L, et al. Clinical evaluation of 51 dogs treated conservatively for disc-associated wobbler syndrome. J Small Anim Pract. 2009;50:136–142. doi: 10.1111/j.1748-5827.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 10.Gray MJ, Kirberger RM, Spotswood TC. Cervical spondylomyelopathy (wobbler syndrome) in the Boerboel. J S Afr Vet Assoc. 2003;74:104–110. doi: 10.4102/jsava.v74i4.520. [DOI] [PubMed] [Google Scholar]
- 11.Shamir MH, Chai O, Loeb E. A Method for intervertebral space distraction before stabilization combined with complete ventral slot for treatment of disc-associated wobbler syndrome in dogs. Vet Surg. 2008;37:186–192. doi: 10.1111/j.1532-950X.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin RM. Kinetic and kinematic gait analysis in dogs. Vet Clin North Am Small Anim Pract. 2001;31:193–201. doi: 10.1016/s0195-5616(01)50045-5. [DOI] [PubMed] [Google Scholar]
- 13.Van der Linden MH, Marigold DS, Gabreels FJ, Duysens J. Muscle reflexes and synergies triggered by an unexpected support surface height during walking. J Neurophysiol. 2007;97:3639–3650. doi: 10.1152/jn.01272.2006. [DOI] [PubMed] [Google Scholar]
- 14.Voss K, Galeandro L, Wiestner T, et al. Relationships of body weight, body size, subject velocity, and vertical ground reaction forces in trotting dogs. Vet Surg. 2010;39:863–869. doi: 10.1111/j.1532-950X.2010.00729.x. [DOI] [PubMed] [Google Scholar]
- 15.Budsberg SC, Verstraete MC, Soutas-Little RW. Force plate analysis of the walking gait in healthy dogs. Am J Vet Res. 1987;48:915–918. [PubMed] [Google Scholar]
- 16.McLaughlin R, Jr, Roush JK. Effects of increasing velocity on braking and propulsion times during force plate gait analysis in greyhounds. Am J Vet Res. 1995;56:159–161. [PubMed] [Google Scholar]
- 17.Renberg WC, Johnston SA, Ye K, Budsberg SC. Comparison of stance time and velocity as control variables in force plate analysis of dogs. Am J Vet Res. 1999;60:814–819. [PubMed] [Google Scholar]
- 18.Fanchon L, Grandjean D. Accuracy of asymmetry indices of ground reaction forces for diagnosis of hind limb lameness in dogs. Am J Vet Res. 2007;68:1089–1094. doi: 10.2460/ajvr.68.10.1089. [DOI] [PubMed] [Google Scholar]
- 19.Voss K, Imhof J, Kaestner S, Montavon PM. Force plate gait analysis at the walk and trot in dogs with low-grade hind-limb lameness. Vet Comp Orthop Traumatol. 2007;20:299–304. doi: 10.1160/vcot-07-01-0008. [DOI] [PubMed] [Google Scholar]
- 20.Ballagas AJ, Montgomery RD, Henderson RA, Gillette R. Pre- and postoperative force plate analysis of dogs with experimentally transected cranial cruciate ligaments treated using tibial plateau leveling osteotomy. Vet Surg. 2004;33:187–190. doi: 10.1111/j.1532-950x.2004.04027.x. [DOI] [PubMed] [Google Scholar]
- 21.Kapatkin AS, Arbittier G, Kass PH, et al. Kinetic gait analysis of healthy dogs on two different surfaces. Vet Surg. 2007;36:605–608. doi: 10.1111/j.1532-950X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 22.Bennett RL, DeCamp CE, Flo GL, et al. Kinematic gait analysis in dogs with hip dysplasia. Am J Vet Res. 1996;57:966–971. [PubMed] [Google Scholar]
- 23.Budsberg SC, Chambers JN, Lue SL, et al. Prospective evaluation of ground reaction forces in dogs undergoing unilateral total hip replacement. Am J Vet Res. 1996;57:1781–1785. [PubMed] [Google Scholar]
- 24.McLaughlin RM, Jr, Miller CW, Taves CL, et al. Force plate analysis of triple pelvic osteotomy for the treatment of canine hip dysplasia. Vet Surg. 1991;20:291–297. doi: 10.1111/j.1532-950x.1991.tb01270.x. [DOI] [PubMed] [Google Scholar]
- 25.Budsberg SC, Jevens DJ, Brown J, et al. Evaluation of limb symmetry indices, using ground reaction forces in healthy dogs. Am J Vet Res. 1993;54:1569–1574. [PubMed] [Google Scholar]
- 26.McLaughlin RMJ, Roush JK. Effects of subject stance time and velocity on ground reaction forces in clinically normal Greyhounds at the trot. Am J Vet Res. 1994;55:1672–1676. [PubMed] [Google Scholar]
- 27.Mölsä SH, Hielm-Björkman AK, Laitinen-Vapaavuori OM. Force platform analysis in clinically healthy Rottweilers: Comparison with Labrador Retrievers. Vet Surg. 2010;39:701–707. doi: 10.1111/j.1532-950X.2010.00651.x. [DOI] [PubMed] [Google Scholar]
- 28.Suwankong N, Meij BP, Van Klaveren NJ, et al. Assessment of decompressive surgery in dogs with degenerative lumbosacral stenosis using force plate analysis and questionnaires. Vet Surg. 2007;36:423–431. doi: 10.1111/j.1532-950X.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Klaveren NJ, Suwankong N, De Boer S, et al. Force plate analysis before and after dorsal decompression for treatment of degenerative lumbosacral stenosis in dogs. Vet Surg. 2005;34:450–456. doi: 10.1111/j.1532-950X.2005.00068.x. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara A, Reed SM, Rajala-Schultz PJ, et al. Use of kinetic gait analysis for detection, quantification, and differentiation of hind limb lameness and spinal ataxia in horses. J Am Vet Med Assoc. 2009;234:644–651. doi: 10.2460/javma.234.5.644. [DOI] [PubMed] [Google Scholar]
- 31.da Costa RC, Parent JM. One-year clinical and magnetic resonance imaging follow-up of Doberman Pinschers with cervical spondylomyelopathy treated medically or surgically. J Am Vet Med Assoc. 2007;231:243–250. doi: 10.2460/javma.231.2.243. [DOI] [PubMed] [Google Scholar]
- 32.da Costa RC, Parent JM, Partlow G, et al. Morphologic and morphometric magnetic resonance imaging features of Doberman Pinschers with and without clinical signs of cervical spondylomyelopathy. Am J Vet Res. 2006;67:1601–1612. doi: 10.2460/ajvr.67.9.1601. [DOI] [PubMed] [Google Scholar]
- 33.DeCamp CE. Kinetic and kinematic gait analysis and the assessment of lameness in the dog. Vet Clin North Am Small Anim Pract. 1997;27:825–840. doi: 10.1016/s0195-5616(97)50082-9. [DOI] [PubMed] [Google Scholar]
- 34.Gordon-Evans WJ, Evans RB, Knap KE, et al. Characterization of spatiotemporal gait characteristics in clinically normal dogs and dogs with spinal cord disease. Am J Vet Res. 2009;70:1444–1449. doi: 10.2460/ajvr.70.12.1444. [DOI] [PubMed] [Google Scholar]
- 35.van Dieen JH, Spanjaard M, Konemann R, et al. Balance control in stepping down expected and unexpected level changes. J Biomech. 2007;40:3641–3649. doi: 10.1016/j.jbiomech.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Buckley JG, Heasley KJ, Twigg P, Elliott DB. The effects of blurred vision on the mechanics of landing during stepping down by the elderly. Gait Posture. 2005;21:65–71. doi: 10.1016/j.gaitpost.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Norre ME, Forrez G. Posture testing (posturography) in the diagnosis of peripheral vestibular pathology. Arch Otorhinolaryngol. 1986;243:186–189. doi: 10.1007/BF00470618. [DOI] [PubMed] [Google Scholar]
- 38.Kerr G, Morrison S, Silburn P. Coupling between limb tremor and postural sway in Parkinson's disease. Mov Disord. 2008;23:386–394. doi: 10.1002/mds.21851. [DOI] [PubMed] [Google Scholar]
- 39.Yoshikawa M, Doita M, Okamoto K, et al. Impaired postural stability in patients with cervical myelopathy: Evaluation by computerized static stabilometry. Spine (Phila Pa 1976) 2008;33:E460–E464. doi: 10.1097/BRS.0b013e318178e666. [DOI] [PubMed] [Google Scholar]
- 40.Nardone A, Galante M, Grasso M, Schieppati M. Stance ataxia and delayed leg muscle responses to postural perturbations in cervical spondylotic myelopathy. J Rehabil Med. 2008;40:539–547. doi: 10.2340/16501977-0214. [DOI] [PubMed] [Google Scholar]
- 41.Clayton HM, Nauwelaerts S. Is a single force plate adequate for stabilographic analysis in horses. Equine Vet J. 2011;44(5):550–553. doi: 10.1111/j.2042-3306.2011.00458.x. [DOI] [PubMed] [Google Scholar]
- 42.Clayton HM, Bialski DE, Lanovaz JL, Mullineaux DR. Assessment of the reliability of a technique to measure postural sway in horses. Am J Vet Res. 2003;64:1354–1359. doi: 10.2460/ajvr.2003.64.1354. [DOI] [PubMed] [Google Scholar]
- 43.Waxman AS, Robinson DA, Evans RB, et al. Relationship between objective and subjective assessment of limb function in normal dogs with an experimentally induced lameness. Vet Surg. 2008;37:241–246. doi: 10.1111/j.1532-950X.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 44.Nordquist B, Fischer J, Kim SY, et al. Effects of trial repetition, limb side, intraday and inter-week variation on vertical and craniocaudal ground reaction forces in clinically normal Labrador Retrievers. Vet Comp Orthop Traumatol. 2011;24:435–444. doi: 10.3415/VCOT-11-01-0015. [DOI] [PubMed] [Google Scholar]