Abstract
OBJECTIVES
To examine the association of maternal early pregnancy oxidative stress with risk of gestational diabetes mellitus (GDM).
DESIGN AND METHODS
A pilot prospective, nested case-control study was conducted. Study participants were recruited before 20 weeks gestation. Maternal urinary 8-hydroxydeoxyguanosine (8-OHdG), a biomarker of systemic oxidative DNA damage and repair, was measured using competitive immunoassays. Logistic regression was used to calculate odds ratio (OR) and 95% confidence intervals (95%CI).
RESULTS
Elevations in early pregnancy urinary 8-OHdG concentrations were associated with increased GDM risk. After adjusting for confounders, the OR for extreme quartiles (≥8.01 vs. <4.23ng/mg creatinine) of 8-OHdG was 3.79 (95%CI 1.03-14.00). The risk for GDM was highest for overweight women with urine 8-OHdG concentrations ≥ 8.01ng/mg creatinine (OR=5.36, 95%CI 1.33-21.55) when compared with lean women who had 8-OHdG concentrations < 8.01ng/mg creatinine.
CONCLUSIONS
Elevated urine 8-OHdG concentrations in early pregnancy appear to be associated with increased GDM risk.
Keywords: gestational diabetes mellitus, pregnancy, oxidative DNA damage, oxidative stress, urinary 8-OHdG concentrations
INTRODUCTION
Reactive oxygen species (ROS), including superoxide anion radicals, hydrogen peroxide and hydroxyl radical, are formed continuously in living cells as a consequence of normal metabolic reactions. Under normal physiological conditions, there is a balance between endogenous oxidants and various antioxidant defenses. The excessive generation of oxidants or a decrease of antioxidants results in an imbalance called oxidative stress, in which oxidants generated in vivo produce extensive oxidative damage to nucleic acids as well as lipids and proteins [1]. 8-Hydroxy-2′-deoxyguanosine (8-OHdG), produced by oxidation of the nucleoside deoxyguanosine and subsequently excreted directly into urine, has been identified as a sensitive marker for oxidative DNA damage [2-3]. Mounting evidence suggests that elevated urinary 8-OHdG, a biomarker of generalized, cellular oxidative stress, is associated with increased risks of cancer [2, 4], autoimmune disorders [5-6], atherosclerosis [7-8], and diabetes [9-12]. Leionen et al [9] in their study of Finnish diabetics and controls observed that total 24-hour urinary excretion of 8-OHdG was markedly elevated among non-insulin-dependent diabetes cases as compared with control subjects (35.9±16.4 vs. 24.3±15.2 ng/mg creatinine, p<0.001). Other investigators have also noted increased urinary 8-OHdG concentrations in diabetics with poor glycemic control [10, 13].
Increased oxidative stress in human pregnancy has also been implicated in the pathogenesis of preeclampsia [14-15], preterm birth [16], intrauterine growth retardation [14, 17] and low birth weight deliveries [18]. Stein et al [16] recently reported that elevated urine 8-OHdG concentrations measured in early pregnancy were associated with reduced infant birthweight and with shortened length of gestation. To our knowledge, no previous study has examined the extent to which, if at all, maternal urinary 8-OHdG in early pregnancy may be related with incident gestational diabetes mellitus (GDM). On the basis of an emerging literature documenting associations of 8-OHdG with hyperglycemia, type 2 diabetes and medical complications of pregnancy including preeclampsia, we hypothesized that maternal early pregnancy urinary 8-OHdG concentrations are positively associated with subsequent risk of GDM. Using information and urine specimens gathered as part of a prospective cohort study of women receiving prenatal care before 20 weeks gestation, we examined the association between early-pregnancy urine 8-OHdG concentrations, normalized using creatinine concentrations, with the subsequent risk of GDM.
MATERIALS AND METHODS
Study population
This nested case-control study was based on a prospective cohort study of pregnant women, the “Omega Study” [19]. In this cohort, participants were recruited from women attending prenatal care at clinics affiliated with Swedish Medical Center in Seattle and Tacoma General Hospital in Tacoma, Washington. Women were ineligible if they initiated prenatal care after 20 weeks gestation, were younger than 18 years of age, did not speak and read English, did not plan to carry the pregnancy to term, or did not plan to deliver at either of the two research hospitals. Participants completed a questionnaire administered in English by a trained interviewer at or near enrollment. These questionnaires were used to gather information on socio-demographic, anthropomorphic, and behavioral characteristics and reproductive and medical histories. After delivery, maternal and infant medical records were abstracted for information on the course and outcome of pregnancy. The procedures used in the Omega Study were in agreement with the protocols approved by the Institutional Review Boards of Swedish Medical Center and Tacoma General Hospital. All participants provided written informed consent.
The analytical population was selected from pregnant women who enrolled in the Omega Study between September 2002 and October 2004. During this period, a total of 953 pregnant women provided blood samples and completed interviews. Among them, we identified and sampled all 55 women who developed GDM and we randomly sampled 43 women who did not develop GDM as controls. The number of controls was limited by the availability of reagents for measuring urinary biological markers of interest. Power and sample size calculations indicated that with 43 GDM cases and an equal number of controls we had at least 85% power to detect a 25% difference in mean urinary 8-OHdG concentrations across the two study groups (α=0.05, two tailed). A difference in such magnitude is compatible with prior reports from pregnancy cohorts [18]. We had only 73% power to detect a 3.0-fold increased risk of GDM (α=0.05, two tailed) associated with elevated urinary 8-OHdG concentrations (defined as values above the highest quartile in controls).
Data collection
From structured questionnaire and medical records, we obtained information of covariates including maternal age, educational attainment, height, pre-pregnancy weight, reproductive and medical histories, and medical histories of first-degree family members. We also collected information on annual household income and maternal smoking before and during pregnancy. Pre-pregnancy body mass index (BMI) was calculated as pre-pregnancy weight in kilograms divided by height in meters squared. Maternal medical records were reviewed to collect detailed medical and clinical information. In our study settings, according to the recommendations from the American Diabetes Association (ADA) [20], pregnant women were screened at 24-28 weeks gestation using a 50 gram 1-hour oral glucose challenge test. Those patients who failed this screening test (glucose ≥7.8 mmol/l or 140 mg/dl) were then followed-up within 1-2 weeks with a 100g, 3-h oral glucose tolerance test (OGTT). We also abstracted laboratory results from participants’ 50 gram 1-hour glucose challenge test and from the diagnostic 100 gram 3-hour OGTT. Women were diagnosed with GDM if two or more of the 100 gram OGTT glucose levels exceeded the ADA criteria: fasting ≥5.3 mmol/l (≥95 mg/dl); 1-hour ≥10.0 mmol/l (≥180 mg/dl); 2-hour ≥8.6 mmol/l (≥155 mg/dl); 3-hour ≥7.8 mmol/l (≥140 mg/dl) [20].
Participants provided a clean-catch spot urine sample around 16 weeks of gestation. Immediately after collection, samples were separated into 2ml aliquots and stored at -80°C until analysis. We used urine levels of the oxidized base, 8-hydroxy-2’-deoxyguanosine (8-OHdG), as our biomarker of DNA oxidative stress. 8-OHdG was measured in duplicates using a highly sensitive 8-OHdG check enzyme-linked immunosorbent assay (ELISA) kit (Genox Corp., Baltimore, MD). This competitive ELISA is specifically designed for measurement of 8-OHdG in tissues expected to have low levels of cellular oxidative stress. This ELISA method offers a valid and comparatively simple alternative to more technically demanding HPLC-EC or GC-MS techniques for the quantitative assessment of oxidative DNA damage [21]. Moreover, investigators have reported excellent correlation between HPLC and ELISA methods (r = 0.96) [22]. The intra-assay coefficient of variation (CV) was 11.8% and the inter-assay CV was 8%. Urinary 8-OHdG concentrations were normalized using participants’ urine creatinine concentrations; and were expressed as ng/mg creatinine. Urine creatinine concentrations were measured using commercially available kits and reagents (R&D Systems, Minneapolis, MN).
Statistical analysis
Because the distribution of urine 8-OHdG concentrations was approximately normally distributed (Kolmogorov-Smirnov test P-value = 0.20), we examined differences in mean concentrations between cases and controls using the Student’s t test. However, given the observation of two potential outliers (high among cases), we also tested differences in median urine 8-OHdG concentrations between cases and controls using the Mann-Whitney test. We categorized urine 8-OHdG concentrations according to quartiles determined by the distribution among controls. We used logistic regression models to estimate odds ratios (OR) and 95% confidence interval (95% CI). We evaluated the covariates in Table 1 as potential confounders and included in the final model those that altered unadjusted ORs by 10% or more, including maternal age, race/ethnicity and pre-pregnancy body mass index. All analyses were performed using Stata 9.0 (Stata, College Station, TX). All reported confidence intervals were calculated at the 95% level and all reported p-values are two-tailed.
Table 1.
Socio-demographic and other characteristics of the study subjects, Seattle and Tacoma, Washington, USA
| Characteristics | GDM Cases (N=55) | Control Subjects (N=43) | P-value |
|---|---|---|---|
| Age (years) | 34.6 ± 4.6 | 32.6 ± 4.8 | 0.04 |
| Age (years) | |||
| 20-29 | 6 (10.9) | 9 (20.9) | 0.37 |
| 30-34 | 21 (38.2) | 19 (44.2) | |
| 35-39 | 21 (38.2) | 11 (25.6) | |
| ≥40 | 7 (12.7) | 4 (9.3) | |
| Race/ethnicity | |||
| Non-Hispanic White | 38 (69.1) | 37 (86.0) | 0.005 |
| African American | 2 (3.6) | 4 (9.3) | |
| Other | 15 (27.3) | 2 (4.7) | |
| Less than 12 years of education | 1 (1.8) | 3 (7.0) | 0.32 |
| Single marital status | 8 (14.6) | 3 (7.0) | 0.34 |
| Nulliparous | 26 (47.3) | 27 (62.8) | 0.13 |
| Smoked during pregnancy | 3 (5.5) | 0 (0.0) | --- |
| Consumed prenatal vitamins | 54 (98.2) | 41 (95.4) | 0.58 |
| No leisure time exercise during pregnancy | 9 (16.4) | 4 (9.3) | 0.38 |
| History of chronic hypertension | 6 (10.9) | 3 (7.0) | 0.73 |
| Family history of hypertension | 34 (61.8) | 24 (55.8) | 0.55 |
| Family history of diabetes | 13 (23.6) | 8 (18.6) | 0.55 |
| Pre-pregnancy body mass index (kg/m2) | 26.2 ± 6.3 | 23.2 ± 3.8 | 0.006 |
| Pre-pregnancy body mass index (kg/m2) | |||
| Lean (<18.5) | 1 (1.8) | 2 (4.6) | 0.11 |
| Normal (18.5-24.9) | 27 (49.1) | 30 (69.8) | |
| Overweight (25.0-29.9) | 20 (36.4) | 8 (18.6) | |
| Obese (≥30.0) | 7 (12.7) | 3 (7.0) | |
| Gestational age at urine collection (wks) | 16.4 ± 2.2 | 16.7 ± 2.4 | 0.51 |
| Gestational age at delivery (wks) | 38.2 ± 2.1 | 38.7 ± 1.8 | 0.24 |
| Infant birth weight (g) | 3486 ± 604 | 3273 ± 553 | 0.08 |
| Macrosomia (infant birth weight ≥4000g) | 14 (25.5) | 3 (7.0) | 0.03 |
| Multiple birth | 5 (9.1) | 3 (7.0) | 0.70 |
| Maternal early pregnancy urine 8-OHdG (ng/mg creatinine) | 7.98 ± 4.12 | 6.31 ± 2.49 | 0.02 |
Presented as in mean ± SD, or number (%)
RESULTS
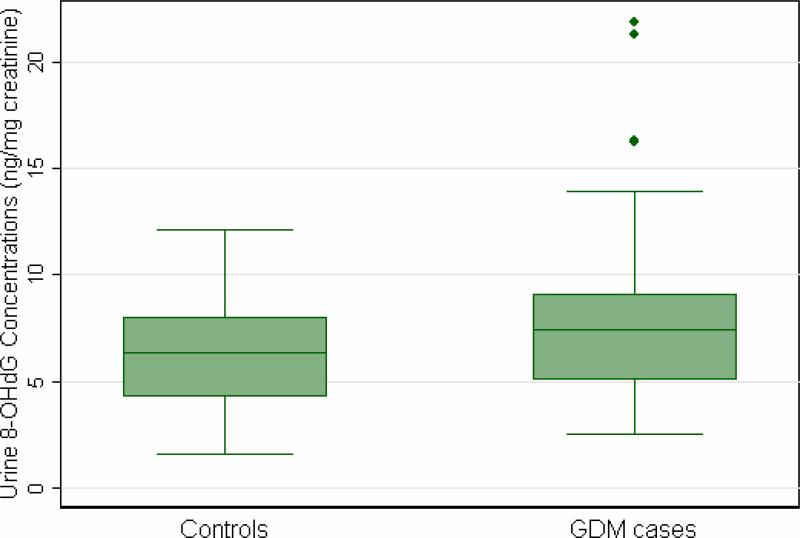
As shown in Table 1, women who developed GDM were older, heavier, more likely to have a positive family history of type 2 DM, and less likely to be non-Hispanic White as compared with controls. Maternal urinary concentrations of 8-OHdG were 26.5% higher (mean ± standard deviation: 7.98 ± 4.12 vs. 6.31 ± 2.49 ng/mg creatinine, P value = 0.02), on average, among women who subsequently developed GDM, as compared with those who did not develop GDM during the index pregnancy (Figure 1). Notably, this difference remained after we excluded two subjects with extremely high, potentially outlier, 8-OHdG concentrations (7.47 ± 3.79 vs. 6.31 ± 2.49 ng/mg creatinine, P value = 0.05). Urinary 8-OHdG concentrations remained statistically significantly higher, on average, among GDM cases as compared with controls after adjustment for maternal age, race/ethnicity and pre-pregnancy body mass index (geometric mean = 1.30 ng/mg creatinine, P value = 0.02).
Figure 1.
Boxplot of maternal early pregnancy urine 8-OHdG (ng/mg creatinine) concentrations among GDM cases and controls.
There was weak evidence of a linear trend in risk of GDM with increasing concentrations of maternal early pregnancy urine 8-OHdG concentrations (P for trend = 0.05) after control for confounding by maternal age, race/ethnicity and pre-pregnancy body mass index (Table 2). Women with urine 8-OHdG concentrations ≥ 8.01 ng/mg creatinine (i.e., the highest quartile) experienced a 3.79-fold increased risk of GDM (adjusted OR = 3.79; 95% CI 1.03-14.00) as compared with women whose concentrations were < 4.23 ng/ml creatinine (i.e., the referent group).
Table 2.
Odds ratios (OR) and 95% confidence intervals (CI) of the risk for gestational diabetes (GDM) according to maternal urine 8-OHdG concentrations in early pregnancy, Seattle and Tacoma, Washington, USA
| Urine 8-OHdG (ng/mg creatinine) | GDM Cases (N=55) | Control Subjects (N=43) | Unadjusted OR (95% CI) | *Adjusted OR (95% CI) |
|---|---|---|---|---|
| Quartiles | ||||
| Q1 (<4.23) | 8 (14.6) | 10 (23.2) | 1.00 (referent) | 1.00 (referent) |
| Q2 (4.23-6.34) | 15 (27.3) | 11 (25.6) | 1.70 (0.51-5.73) | 1.63 (0.41-6.40) |
| Q3 (6.35-8.00) | 8 (14.6) | 11 (25.6) | 0.91 (0.25-3.34) | 1.15 (0.27-4.97) |
| Q4 (≥8.01) | 24 (43.6) | 11 (25.6) | 2.73 (0.84-8.80) | 3.79 (1.03-14.00) |
| P for trend | 0.15 | 0.05 |
Adjusted for maternal age, race/ethnicity and pre-pregnancy body mass index
We further examined the independent and joint effect of maternal urine 8-OHdG concentrations and overweight status on the risk of GDM (Table 3). The association of GDM with elevated 8-OHdG concentrations appeared to be strongest for overweight (BMI ≥ 25kg/m2) women, though the p-value for the interaction term did not reach statistical significance (P for interaction = 0.46). The risk for GDM was highest for overweight women with early pregnancy urine 8-OHdG concentrations ≥ 8.01 ng/ml creatinine; they experienced a 5.36-fold increased risk as compared with lean women who had early pregnancy urine 8-OHdG concentrations <8.01 ng/mg creatinine (adjusted OR = 5.36, 95% CI 1.33-21.55). Inferences from these analyses should be made with caution as the sample sizes for each group was small and estimated ORs were statistically imprecise as evidenced by the wide 95% confidence intervals.
Table 3.
Odds ratios (OR) and 95% confidence intervals (CI) of the risk for gestational diabetes mellitus (GDM) according to maternal early pregnancy elevated urine 8-OHdG concentrations and pre-pregnancy overweight status, Seattle and Tacoma, Washington, USA
| Elevated 8-OHdG & Pre-pregnancy Overweight | GDM Cases (N=55) | Control Subjects (N=43) | Unadjusted OR (95% CI) | *Adjusted OR (95% CI) |
|---|---|---|---|---|
| No & No | 16 (29.1) | 25 (58.1) | 1.00 (referent) | 1.00 (referent) |
| Yes & No | 12 (21.8) | 7 (16.3) | 2.68 (0.87-8.24) | 3.63 (1.09-12.14) |
| No & Yes | 15 (27.3) | 7 (16.3) | 3.35 (1.12-10.01) | 3.06 (0.93-10.01) |
| Yes & Yes | 12 (21.8) | 4 (9.3) | 4.67 (1.29-17.10) | 5.36 (1.33-21.55) |
| P for interaction | 0.49 | 0.46 |
Elevated urine 8-OHdG is defined as maternal urine 8-OHdG ≥ 8.01 ng/mg creatinine (highest quartile in the controls). Pre-pregnancy overweight status is defined as maternal pre-pregnancy BMI≥ 25 kg/m2.
Adjusted for maternal age and race/ethnicity
DISCUSSION
In the pilot nested case-control study, maternal urine 8-OHdG concentrations in early pregnancy appeared to be positively associated with GDM risk. This association was statistically significant after controlling for established risk factors of GDM including maternal age, race/ethnicity and pre-pregnancy body mass index. The risk for GDM was highest for overweight women with urine 8-OHdG concentrations ≥ 8.01 ng/mg creatinine (OR=5.36) when compared with lean women who had 8-OHdG concentrations < 8.01 ng/mg creatinine.
Our findings are generally consistent with a relatively large body of literature documenting positive associations of urinary 8-OHdG with hyperglycemia, impaired glucose intolerance, and type 2 diabetes in men and non-pregnant women [9-10, 12-13, 23]. Our results are also in agreement with studies that assessed 8-OHdG concentrations in other tissues, including serum, mononuclear cells, and pancreatic β-cells, and found evidence of positive associations with hyperglycemia and impaired glucose tolerance [11, 23-25]. For example, Miyazaki et al [23] reported a positive association between serum 8-OHdG and acute hyperglycemia induced by oral glucose load. Our results are also consistent with several cross-sectional clinical studies and in vitro studies that have evaluated other indices of oxidative stress (e.g., measures of lipid and protein oxidation or measures of antioxidant defenses) in specimens collected from GDM cases and controls [26-30]. On balance, available data suggest that GDM is characterized by impaired enzymatic antioxidant activities [27-28, 31], reduced antioxidants in peripheral circulation [26], and enhanced measures of lipid [29, 32] and protein [30, 33] oxidative stress. For example Grissa et al [28], in their cross sectional study of GDM case and controls reported elevated serum thiobarbituric acid-reactive substances (TBARS) concentrations and superoxide dismutase (SOD) enzyme activity among cases versus controls. Investigators have shown that placental release of 8-isoprostane was 2-fold greater from women with GDM (P < 0.001) compared to healthy pregnant women [31]. These findings were recently corroborated and extended by a study that demonstrated increased lipid oxidative stress response (as measured by 8-isoprostane release) secondary to hypoxanthine (HX)/xanthine oxidase (XO) exposure of maternal omental and subcutaneous adipose tissues from GDM versus control subjects [29]. Others have reported lower mean maternal daily consumption of vitamin C (130.7±10.2 vs. 145±4.9 mg/d, P = 0.190) and lower plasma ascorbic acid concentrations (36±2.0 vs. 53±1.0 μmol/l, P < 0.001) among GDM cases as compared with controls [26]. Although we know of no other prospective studies of maternal urine 8-OHdG and GDM risk, our results are consistent with one prospective study in which investigators documented increased protein oxidation in amniotic fluid samples collected at 15 weeks gestation from women destined to develop GDM as compared with controls [30].
Our study has several strengths. First, determination of 8-OHdG concentrations using urine collected in early pregnancy served to define the temporal relationship between maternal oxidative stress and subsequent risk of GDM. Second, the high follow-up rate (> 95%) of women enrolled in our study minimized possible selection bias. However, several limitations also merit discussion and consideration. First, a single measurement of urinary 8-OHdG concentrations is not likely to provide a time-integrated measure of maternal cellular oxidative stress during the entire pregnancy. Longitudinal studies with serial measurements of urinary 8-OHdG concentrations along with indices of insulin sensitivity and secretion across gestation are needed to elucidate the mechanisms and pathophysiological consequences of maternal oxidative stress during pregnancy. Second, our study is relatively small and thus, larger prospective studies are needed to confirm our results and to provide more precise estimates of association. Third, as with all observational studies, although we adjusted for known and suspected confounders, we cannot exclude the possibility of residual confounding from unmeasured covariates. Fourth, universal glucose tolerance testing in early pregnancy is not part of the standard obstetric care. Hence, we cannot exclude the possibility that some subjects in our study had undiagnosed pre-gestational diabetes. Over 95% of study subjects reported having regular medical exams within a 24-month period before the index pregnancy; and the cumulative incidence of GDM in our study cohort is consistent with observations in other settings [20]. Finally, the generalizability of our finding is limited to a largely non-Hispanic White, well educated obstetric population.
Our results and those of others [9-13, 23-33] are supported by current understanding of the potential mechanisms that relate oxidative stress and subsequent impaired glucose tolerance and insulin resistance. Oxidative stress may induce systemic endothelial dysfunction that may directly or indirectly contribute to impaired pancreatic β-cell function and glucose intolerance. Pancreatic β-cells are particularly sensitive to ROS because they are low in free-radical quenching antioxidant enzymes such as catalase, glutathione peroxidase, and superoxide dismutase. Therefore, oxidative stress seems to damage mitochondria and markedly blunt insulin secretion [34-35]. Oxidative stress is known to impair insulin action through a change in the chemical-physical state of plasma membrane, an increase in intracellular calcium concentration or a reduction in nitric oxide availability [36]. Oxidative stress in muscle and adipose tissues can produce a state of insulin resistance which decreasing insulin-stimulated glucose uptake by those peripheral tissues and increasing the circulating glucose level [37]. Oxidative stress contributes, in vivo, to specifically alterthe early phase of insulin secretion because the latter can be restored by antioxidants [36]. Oxidative stress is convincingly the mediator of such damage characterized by a reversible defective insulin gene expression [38]. It is well recognized that inflammation is involved in the pathogenesis of diabetes and is one manifestation of oxidative stress and the pathways that generate the mediators of inflammation,such as adhesion molecules and interleukins, are all induced by oxidative stress [39].
CONCLUSIONS
In summary, this preliminary report extends the current literature by documenting a relation between early pregnancy urine 8-OHdG concentrations and GDM risk; and corroborates results from previous cross sectional studies. Our findings, coupled with an extensive literature, support a role of oxidative stress in the pathogenesis of impaired glucose homeostasis in pregnant and non-pregnant populations. Large-scale longitudinal studies, however, are warranted to further exam the extent to which early pregnancy urine 8-OHdG concentrations can be used as a risk marker for GDM.
ACKNOWLEDGEMENT
This research was supported, in part, by an award (HD/HL 355566) from the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST: None
There is no conflict of interest to declare.
REFERENCES
- 1.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed. Oxford Univ Press; Oxford: 1999. [Google Scholar]
- 2.Erhola M, Toyokuni S, Okada K, Tanaka T, Hiai H, Ochi H, Uchida K, Osawa T, Nieminen MM, Alho H, Kellokumpu-Lehtinen P. Biomarker evidence of DNA oxidation in lung cancer patients: Association of urinary 8-hydroxy-20-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS. Lett. 1997;409:287–291. doi: 10.1016/s0014-5793(97)00523-1. [DOI] [PubMed] [Google Scholar]
- 3.Loft S, Vistisen K, Ewertz M, Tjonneland A, Overad K, Poulsen HE. OxidativeDNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- 4.Chuma M, Hige S, Nakanishi M, Ogawa K, Natsuizaka M, Yamamoto Y, Asaka M. 8-Hydroxy-2′-deoxy-guanosine is a risk factor for development of hepatocellular carcinoma in patients with chronic hepatitis C virus infection. J. Gastroenterol. Hepatol. 2008;23:1431–1436. doi: 10.1111/j.1440-1746.2008.05502.x. [DOI] [PubMed] [Google Scholar]
- 5.Kageyama Y, Takahashi M, Ichikawa T, Torikai E, Nagano A. Reduction of oxidative stress marker levels by anti-TNF-alpha antibody, infliximab, in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2008;26:73–80. [PubMed] [Google Scholar]
- 6.Goodarzi MT, Navidi AA, Rezaei M, Babahmadi-Rezaei H. Oxidative damage to DNA and lipids: correlation with protein glycation in patients with type 1 diabetes. J. Clin. Lab. Anal. 2010;24:72–76. doi: 10.1002/jcla.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoud M, Mercer J, Bennett M. DNA damage and repair in atherosclerosis. Cardiovasc. Res. 2006;71:259–268. doi: 10.1016/j.cardiores.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Satoh M, Ishikawa Y, Takahashi Y, Itoh T, Minami Y, Nakamura M. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198:347–353. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 9.Leinonen J, Lehtimäki T, Toyokuni S, Okada K, Tanaka T, Hiai H, Ochi H, Laippala P, Rantalaiho V, Wirta O, Pasternack A, Alho H. New biomarker evidence of oxidative DNA damage in patients with non-insulin-dependent diabetes mellitus. FEBS Lett. 1997;417:150–152. doi: 10.1016/s0014-5793(97)01273-8. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa T, Sasahara T, Kiritoshi S, Sonoda K, Senokuchi T, Matsuo T, Kukidome D, Wake N, Matsumura T, Miyamura N, Sakakida M, Kishikawa H, Araki E. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care. 2003;26:1507–1512. doi: 10.2337/diacare.26.5.1507. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi S, Suzuki G, Kusunoki Y, Yamane K, Egusa G, Kohno N. Increasing of oxidative stress from mitochondria in type 2 diabetic patients. Diabetes. Metab. Res. Rev. 2004;20:399–404. doi: 10.1002/dmrr.469. [DOI] [PubMed] [Google Scholar]
- 12.Endo K, Miyashita Y, Sasaki H, Ebisuno M, Ohira M, Saiki A, Koide N, Oyama T, Takeyoshi M, Shirai K. Probucol and atorvastatin decrease urinary 8-hydroxy-2′-deoxyguanosine in patients with diabetes and hypercholesterolemia. J. Atheroscler. Thromb. 2006;13:68–75. doi: 10.5551/jat.13.68. [DOI] [PubMed] [Google Scholar]
- 13.Negishi H, Ikeda K, Kuga S, Noguchi T, Kanda T, Njelekela M, Liu L, Miki T, Nara Y, Sato T, Mashalla Y, Mtabaji J, Yamori Y. The relation of oxidative DNA damage to hypertension and other cardiovascular risk factors in Tanzania. J. Hypertens. 2001;19:529–533. doi: 10.1097/00004872-200103001-00002. [DOI] [PubMed] [Google Scholar]
- 14.Scholl TO, Stein TP. Oxidant damage to DNA and pregnancy outcome. J. Matern. Fetal. Med. 2001;10:182–185. doi: 10.1080/714904323. [DOI] [PubMed] [Google Scholar]
- 15.Rudra CB, Qiu C, David RM, Bralley JA, Walsh SW, Williams MA. A prospective study of early-pregnancy plasma malondialdehyde concentration and risk of preeclampsia. Clin. Biochem. 2006;39:722–726. doi: 10.1016/j.clinbiochem.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Peter Stein T, Scholl TO, Schluter MD, Leskiw MJ, Chen X, Spur BW, Rodriguez A. Oxidative stress early in pregnancy and pregnancy outcome. Free. Radic. Res. 2008;42:841–848. doi: 10.1080/10715760802510069. [DOI] [PubMed] [Google Scholar]
- 17.Karowicz-Bilinska A, Suzin J, Sieroszewski P. Evaluation of oxidative stress indices during treatment in pregnant women with intrauterine growth retardation. Med. Sci. Monit. 2002;8:CR211–216. [PubMed] [Google Scholar]
- 18.Min J, Park B, Kim YJ, Lee H, Ha E, Park H. Effect of oxidative stress on birth sizes: consideration of window from mid pregnancy to delivery. Placenta. 2009;30:418–423. doi: 10.1016/j.placenta.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A, Williams MA. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3:e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert committee on the diagnosis and classification of diabetes mellitus, Report of the expert committee on the diagnosis and classification of Diabetes Mellitus. Diabetes. Care. 2003;26:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 21.Cooke M, Evan MD, Herbert KE, Lunec J. Urinary 8-oxo-2′deoxyguanosine-source, significance, and supplements. Free. Radic. Res. 2000;32:381–397. doi: 10.1080/10715760000300391. [DOI] [PubMed] [Google Scholar]
- 22.Yin B, Whyatt RM, Perera FP, Randall MC, Cooper TB, Santella RM. Determination of 8-hydroxydeoxyguanosine by an immunoaffinity chromatography-monoclonal antibody-based ELISA. Free. Radic. Biol. Med. 1995;18:1023–1032. doi: 10.1016/0891-5849(95)00003-g. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki Y, Kawano H, Yoshida T, Miyamoto S, Hokamaki J, Nagayoshi Y, Yamabe H, Nakamura H, Yodoi J, Ogawa H. Pancreatic B-cell function is altered by oxidative stress induced by acute hyperglycaemia. Diabet. Med. 2007;24:154–160. doi: 10.1111/j.1464-5491.2007.02058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 25.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Williams MA, Frederick IO, King IB, Sorensen TK, Kestin MM, Dashow EE, Luthy DA. Vitamin C and the risk of gestational diabetes mellitus: a case-control study. J. Reprod. Med. 2004;48:257–266. [PubMed] [Google Scholar]
- 27.Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006;27:327–332. doi: 10.1016/j.placenta.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Grissa O, Atègbo JM, Yessoufou A, Tabka Z, Miled A, Jerbi M, Dramane KL, Moutairou K, Prost J, Hichami A, Khan NA. Antioxidant status and circulating lipids are altered in human gestational diabetes and macrosomia. Transl. Res. 2007;150:164–171. doi: 10.1016/j.trsl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Lappas M, Mitton A, Permezel M. In response to oxidative stress, the expression of inflammatory cytokines and antioxidant enzymes are impaired in placenta, but not adipose tissue, of women with gestational diabetes. J. Endocrinol. 2010;204:75–84. doi: 10.1677/JOE-09-0321. [DOI] [PubMed] [Google Scholar]
- 30.Boisvert MR, Koski KG, Skinner CD. Increased oxidative modifications of amniotic fluid albumin in pregnancies associated with gestational diabetes mellitus. Anal. Chem. 2010;82:1133–1137. doi: 10.1021/ac902322w. [DOI] [PubMed] [Google Scholar]
- 31.Coughlan MT, Vervaart PP, Permezel M, Georgiou HM, Rice GE. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25:78–84. doi: 10.1016/S0143-4004(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 32.Kinalski M, Sledziewski A, Telejko B, Kowalska I, Kretowski A, Zarzycki W, Kinalska I. Lipid peroxidation, antioxidant defense and acid-base status in cord blood at birth: the influence of diabetes. Horm. Metab. Res. 2001;33:227–231. doi: 10.1055/s-2001-14953. [DOI] [PubMed] [Google Scholar]
- 33.Mazzanti L, Nanetti L, Vignini A, Rabini RA, Grechi G, Cester N, Curzi CM, Tranquilli AL. Gestational diabetes affects platelet behavior through modified oxidative radical metabolism. Diabet. Med. 2004;21:68–72. doi: 10.1046/j.1464-5491.2003.01081.x. [DOI] [PubMed] [Google Scholar]
- 34.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 35.Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 36.Paolisso G, Giugliano D. Oxidative stress and insulin action: is there a relationship? Diabetologia. 1996;39:357–363. doi: 10.1007/BF00418354. [DOI] [PubMed] [Google Scholar]
- 37.Rudich A, Kozlovsky N, Potashnik R, Bashan N. Oxidant stress reduces insulin responsiveness in 3T3-L1 adipocytes. Am. J. Physiol. 272(1997):E935–940. doi: 10.1152/ajpendo.1997.272.5.E935. [DOI] [PubMed] [Google Scholar]
- 38.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappa B. Int. J. Mol. Med. 1999;4:223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]