Abstract
The major drawback hampering siRNA therapies from being more widely accepted in clinical practice is its insufficient accumulation at the target site mainly due to poor cellular uptake and rapid degradation in serum. Therefore, we designed a novel polymeric siRNA carrier system, which would withstand serum-containing environments and tested its performance in vitro as well as in vivo. Delivering siRNA with a system combining an arginine-grafted bioreducible polymer (ABP), microbubbles (MB), and ultrasound technology (US) we were able to synergize the advantages each delivery system owns individually, and created our innovative siRNA-ABP-MB (SAM) complexes. SAM complexes show significantly higher siRNA uptake and VEGF protein knockdown in vitro with serum-containing media when compared to naked siRNA, and 25k-branched-polyethylenimine (bPEI) representing the current standard in nonviral gene therapy. SAM complexes activated by US are also able to improve siRNA uptake in tumor tissue resulting in decelerating tumor growth in vivo.
Keywords: siRNA, Ultrasound, Microbubbles, RNAi, VEGF, cancer
1. Introduction
RNA interference (RNAi)-based gene therapy is a selective therapeutic approach allowing precise targeting of a specific gene or protein. To achieve the desired effect the necessary small interfering RNA (siRNA) sequence needs to be delivered in a therapeutically sufficient concentration to the right location [1]. For example, siRNA can be used to target a specific messenger RNA (mRNA), which leads to downregulation of the encoded protein. This approach has been investigated for the treatment of various cancers in which different proteins are found to be upregulated [2]. For example, the vascular endothelial growth factor (VEGF) is overexpressed in various malignancies such as ovarian, lung, breast, prostate cancer and is responsible for enhanced angiogenesis that leads to increased blood flow towards the tumor and consequently augments tumor growth [3,4].
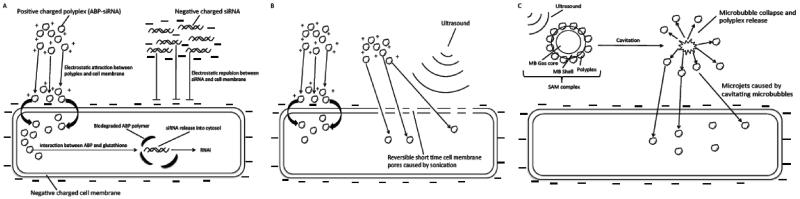
Delivering siRNA that targets VEGF mRNA and decreases the production of protein has been reported to be a suitable strategy to treat tumors displaying increased angiogenesis [4-6]. However, numerous obstacles for anticancer siRNA delivery have been identified. Naked siRNA is easily degraded by ribonucleases (RNases) present in serum and therefore, needs to be protected from degradation until reaching its target site [7,8]. Further, naked siRNA needs to reach the target site in a sufficiently high concentration to elicit the desired activity [9]. Lastly, naked siRNA only shows decreased cellular uptake due to the repulsion between the negatively charged siRNA backbone and the also negatively charged cellular membrane. Multiple strategies and approaches have been investigated to address the above-mentioned complications [10,11]. Cationic polymers (CP) like 25k-branched-polyethylenimine (bPEI) that bind siRNA through electrostatic interactions have been used extensively for gene delivery [12]. Because CP can form nanoparticles, called “polyplexes” when mixed with siRNA due to interaction of the positively charged amines in the CP backbone and the negatively charged phosphate backbone in the siRNA backbone, CP can protect siRNA from degradation in serum enhancing siRNA uptake compared to naked, uncomplexed siRNA which interacts between the positively charged polymer backbone and negatively charged cellular membrane (Scheme 1A). Yet, bPEI is limited by its cytotoxicity, due to the non-biodegradable backbone of the polymer [13]. Therefore, as described previously we synthesized an arginine-grafted bioreducible polymer (ABP) that has disulfide bonds in the polymer backbone undergoing intracellular reduction by glutathione. ABP showed higher transfection efficacy and lower cytotoxicity compared to bPEI because of its bioreducibility and the inclusion of arginine in the backbone that facilitates siRNA uptake into the cells [14-17]. However, most in vitro studies reported transfections in serum-free media showing decreased efficacy when repeated in serum containing media, thus, emphasizing the difficulty of designing an efficient and robust siRNA carrier [18]. One of the reasons for the decreased transfection in serum is that serum proteins interact with the CP backbone masking its positive charge, therefore, decreasing the interaction with the negatively charged cellular membrane [19].
Scheme 1.
Mechanisms of intracellular siRNA delivery using arginine grafted bioreducible polymer (ABP), Ultrasound and siRNA-ABP-Microbubble (SAM) Complexes. (A) siRNA delivery using ABP or naked siRNA. Positive charged polyplexes interact with negative charged cell membrane and facilitates cellular uptake of polyplexes due to electrostatic interaction. Biodegradation of ABP polymer due to reduction of disulfide bonds in ABP backbone by intracellular glutathione leads to siRNA release into cytosol and RNAi activity. Cellular uptake of naked siRNA is declined due to repulsion of negative charge of siRNA and cell membrane. (B) siRNA delivery using ABP and ultrasound (US). Ultrasound causes short time cell membrane pores that facilitates polyplex uptake in addition to the described mechanism in (A). (C) siRNA delivery using SAM complex and ultrasound. Cavitating microbubbles (MBs) release polyplexes from microbubble shell due to interaction with US. MBs cavitation causes microjets and jetstreams that shoot polyplexes through the cell membrane in addition to the described mechanisms in (A) and (B).
Another potential strategy to more effectively deliver siRNA delivery is the use of ultrasound (US) activating microbubbles (MB). Microbubbles have been used in the past to deliver DNA and siRNA in vitro as well as in vivo by sonoporation, however, the transfection efficacy was very low. Sonoporation is the rapid and reversible formation of small cell pores in the cell membrane that can be caused by US and is enhanced by combining it with MB that act as cavitation nuclei. The small cell pores then allow the uptake of DNA and siRNA, however, transfection efficacy is reduced if it occurs in serum-containing media due to degradation of genetic material by DNases and RNases [20-23].
To overcome aforementioned limitations, we combined ABP with and MB to deliver siRNA and formed siRNA-ABP-MB complexes (SAM). Our hypothesis is that current obstacles in siRNA delivery can be overcome by synergizing advantages of each delivery system by combining them into one application. We previously described the formation of SAM complexes by electrostatic interaction of negatively charged albumin MB and positively charged polyplexes (ABP-siRNA) [24]. SAM complexes have a size range of 1–5 μM and a flexible zeta-potential that can be tuned from positive to negative depending on the concentration of polyplexes loaded on the outer MB shell. Further, SAM complexes showed significant increase in siRNA uptake, transfection efficacy and improved VEGF protein knockdown in A2780 human ovarian cancer cells in vitro when compared to bPEI.
Here, we report our findings from US-assisted siRNA delivery experiments using SAM complexes to target ovarian cancer. VEGF/VEGF-receptors are one of the best-characterized therapeutic strategies in patients with ovarian cancer [25]. High VEGF expression occurs in a subset of patients predicting a very poor prognosis [26]. Therefore, we investigated how to improve siRNA uptake and VEGF protein knockdown in serum-containing media in vitro advanced our findings into a clinically relevant ovarian cancer animal model.
2. Materials and Methods
2.1 Materials
HEPES, human serum albumin (HSA), Dextrose, DMSO, branched polyethylenimine (bPEI, Mw 25,000), Fluorescein isothiocyanate (FITC) and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), were purchased from Sigma-Aldrich (St.Louis, MO). Arginine-grafted bioreducible polymer (ABP) was synthesized and characterized as described previously [16,17]. Human VEGF siRNA (sense, 5′-GGAGUACCCUGAUGAGAUCdTdT-3′; antisense, 5′-GAUCUCAUCAGGGUACUCCdTdT-3′), firefly luciferase siRNA (siRNA-Luc) (sense, 5′-GCUAUGAAACGAUAUGGGC-3′; antisense, 3′-CGAUACUUUGCUAUACCCG-5′), AlexaFluor555-labeled siRNA BLOCK-it™ and all cell culture products including Roswell Park Memorial Institute medium (RPMI), Dulbecco’s phosphate buffered saline (DPBS), penicillin-streptomycin and fetal bovine serum (FBS) were obtained from Invitrogen (GibcoBRL; Carlsbad, CA). The Health Sciences Center core research facilities at the University of Utah (Salt Lake City, UT) provided the Cy3-labeled siRNA. Perfluorocrownether (PCE) was obtained from Oakwood Products (West Columbia, SC).
2.2 siRNA complexes formation
Different delivery vehicles were used to transfect cancer cells with siRNA. Arginine-grafted bioreducible poly(disulfide amine) (ABP)–siRNA polyplexes, polyethyleneimine (PEI)–siRNA polyplexes, siRNA plus microbubbles (MB), naked siRNA and siRNA-ABP-Microbubble (SAM) complexes were prepared as described previously [20,24]. Briefly, polyplexes (ABP-siRNA, PEI-siRNA) were prepared by adding a HEPES buffer solution containing ABP to a HEPES buffer solution of siRNA at a weight ratio 10:1 (w/w; ABP to siRNA), or 1:1 (w/w; PEI to siRNA), followed by gentle shaking. After 15 min incubation polyplexes were agitated and allowed to rest for another 15 min at room temperature. MB were synthesized using a combination of three parts 5% dextrose solution, one part 5% HSA solution in HEPES buffer (20 mM, 5% dextrose, pH 7.4), and perfluorocrownether (1%). MB were obtained after sonication for 10 seconds with 60% amplitude of a 20 kHz ultrasonic processor (cuphorn 51mm probe diameter, Sonics Vibracell VCX-500 ultrasonic processor, Sonics, Newtown, CT). To form SAM complexes, MB and polyplexes were combined at desired ratios in HEPES buffer solution and incubated for 10 minutes.
2.3 Fluorescence microscopy
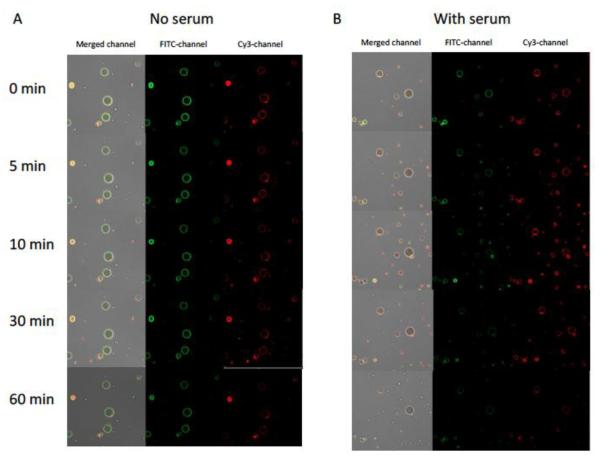
SAM stability in the presence of FBS was confirmed and visualized by a confocal laser scanning microscope (Olympus Fluoview FV300, Melville, NY) with a 60× water objective lens. ABP, HSA were labeled with FITC and siRNA with Cy3 dye at the 3′-terminal end of the sense strand as described previously [24]. SAM complexes were produced as described above. SAM complexes were incubated for 60 minutes in serum free and serum containing (10% FBS) cell culture media. Pictures were taken every ten minutes for the duration of one hour. SAM complexes were identified by FITC-labeled human serum albumin (HSA) microbubble shell (FITC-channel) and Cy3-labeled polyplexes siRNA-Cy3-ABP (Cy3-channel). The merged channel represents both, FITC plus Cy3-channel.
2.4 Cell culture
RPMI media containing 10% FBS and 1% penicillin-streptomycin was used to grow A2780 human ovarian carcinoma cells. Cells were seeded on 6-well plates at a density of 16.7 × 104 cells/well. Cells were cultured at 37° C in a humidified incubator and an atmosphere of 5% (v/v) CO2.
2.5 FACS analysis
Fluorescence-activated cell sorting (FACS) analysis was used to determine cellular uptake of siRNA. Cells were transfected with siRNA complexes (SAM, ABP-siRNA, PEI-siRNA, siRNA plus MB, naked siRNA) 24 hours post seeding with around 40% of cells being confluent. The cells were harvested for FACS analysis 24 hours after transfection. PEI-siRNA polyplexes were used as positive controls. Cells were analyzed by a flow cytometer (FACSCanto™ II, Becton-Dickinson, Mountain View, CA). Only transfected singlet cell population was gated for analysis of siRNA uptake.
siRNA uptake in serum-free cell culture media with and without US treatment; Cells were plated as described above and the cell media was replaced with serum free media prior to the transfection. siRNA complexes (SAM, ABP-siRNA, naked siRNA) were used to transfect cells with and without US treatment. A final concentration of 5 nM BLOCK-it™ siRNA and a MB:cell ratio of 50:1 per well in 2 ml cell media were used. siRNA complexes were added to the wells and incubated for 5 minutes prior to US treatment. An OmnisoundR 3000 Pro ultrasound device (ACP, Reno, NV) with a 3 cm diameter US probe was used. The cells were exposed to US conditions of 1MHz, 0.5 W/cm2, 50% duty. The US probe was fully rotated inside the well to guarantee equal exposure of cells for a duration of 60 seconds for each US treatment. After 10 min (Figure 2) or 4 hours (Figure 3-6) of incubation, the cell media was removed and replaced with fresh, serum-containing media.
Figure 2.
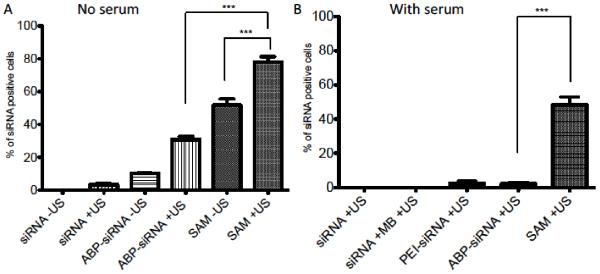
Comparison of cellular uptake delivering siRNA in serum free and serum containing media determined by FACS analysis in A2780 cell line. (A) Cellular uptake in serum free media with 5 nM siRNA complexed in polyplexes (ABP-siRNA), SAM complexes or as naked siRNA using 1 MHz US conditions with 0.5 Watt/cm2 and 50% duty or no US treatment. (B) Intracellular delivery in serum containing (10% FBS) cell culture media with with 5 nM siRNA complexed in polyplexes (ABP-siRNA, PEI-siRNA), SAM complexes, combined with MBs or as naked siRNA using 1 MHz US conditions with 0.5 Watt/cm2 and 50% duty. Data represent mean ± SD and significance tested (P < 0.0001) by one-way ANOVA and Tukey post test.
Figure 3.
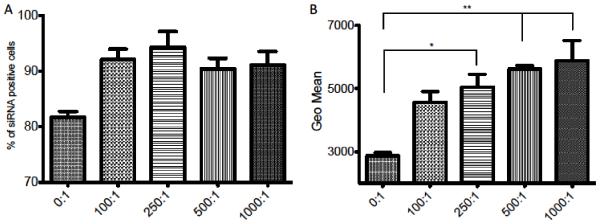
Cellular uptake of siRNA determined by FACS analysis in A2780 cell line using different MB:cell ratios. (A) Quantification of siRNA cellular uptake between siRNA positive and siRNA negative cells with 5 nM siRNA in SAM complexes and MB:cell ratios (0:1, 100:1, 250:1, 500:1, 1000:1, 0:1 = ABP-siRNA polyplex with no MBs) treated with 1 MHz US condition, 0.5 Watt/cm2 and 50% duty. (B) Quantification of siRNA per cell population using the GEO mean of groups from (A). Data represent mean ± SD and significance tested (P < 0.05 and 0.001) by one-way ANOVA and Tukey post test.
Figure 6.
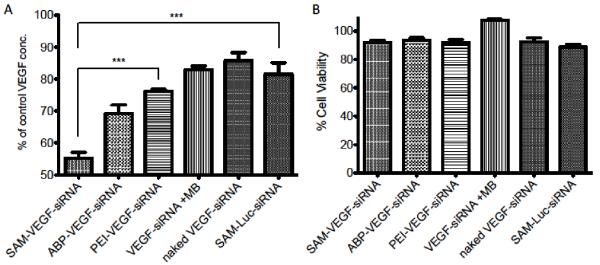
VEGF knockdown using SAM complexes. Transfection efficiency of VEGF-siRNA using polyplexes (ABP-VEGF-siRNA, PEI-VEGF-siRNA), SAM-VEGF complexes (MB:cell ratio 500), naked VEGF-siRNA, SAM-Luc (siRNA negative control, MB:cell ratio 500) and US. VEGF ELISA and MTT assay in A2780 cell line treated with US 1 MHz, 0.5 Watt/cm2 and 50% duty. (A) VEGF concentration after transfection of A2780 cells in serum containing media after US exposure with 100 nM siRNA targeting VEGF or luciferase (SAM-Luc). (B) Cell viability of (A) was determined by MTT assay and expressed as relative cell viability compared to the control. Data represent mean ± SD and significance tested (P < 0.0001-k) by one-way ANOVA and Tukey post test.
siRNA uptake in serum-containing media plus US treatment; siRNA complexes (SAM, ABP-siRNA, PEI-siRNA, siRNA plus MB, naked siRNA) and US treatment were used to transfect cells in serum containing media (10% FBS). Same siRNA concentration and US treatment as described above were used.
siRNA uptake in serum-containing media using various MB:cell ratios; SAM complexes with different MB:cell ratios (0, 100, 250, 500, 1000) were used to transfect cells in serum-containing media (10% FBS). MB:cell ratio of 0:1 equals only polyplexes (ABP-siRNA) without MB. The same siRNA concentration and US treatment as described above were used. siRNA uptake efficiency in serum containing media; siRNA complexes (SAM 500 (MB:cell ratio 500:1), SAM 1000 (MB:cell ratio 1000:1), ABP-siRNA polyplexes, PEI-siRNA polyplexes, siRNA plus MB, naked siRNA) and US treatment were used to transfect cells in serum containing media (10% FBS). The same siRNA concentration and US treatment as described above were used.
2.6 VEGF ELISA assay
VEGF protein silencing was quantified by using a human VEGF ELISA kit (Thermo Scientific, Rockford, IL). Cells were seeded as mentioned above. Briefly, siRNA targeting human VEGF mRNA was combined with ABP polymer at a w/w ratio 10:1 (ABP:siRNA) in HEPES buffer solution for a final concentration of 100 nM siRNA/well in 2 ml cell media. The siRNA targeting luciferase mRNA was used as negative siRNA sequence control and PEI complexed with VEGF siRNA at a w/w ratio (1:1) was used as positive control. Fourty-eight hours after transfection the cell media was collected to determine VEGF concentration in each well.
VEGF protein knock down in serum containing media using various MB:cell ratios; SAM complexes with different MB:cell ratios (0, 100, 500, 1000) were used to transfect cells in serum containing media (10% FBS). Same US conditions as described in the FACS assay were used.
VEGF protein knockdown efficiency in serum-containing media; siRNA complexes (SAM-VEGF-siRNA, ABP-VEGF-siRNA polyplexes, PEI-VEGF-siRNA polyplexes, VEGF siRNA plus MB, naked VEGF siRNA, SAM-Luc-siRNA) and US treatment were used to transfect cells in serum containing media (10% FBS). The MB:cell ratio was 500. Same US conditions as described above were used.
2.7 Cell viability assay
MTT assay was used to determine cell viability. Cells were plated, transfected and treated as described above. The cells were incubated for 24 (FACS) or 48 (ELISA) hours post transfection. MTT (200 μL/well at 5 mg/ml) was added to the cells. After 4 hours, media was removed and 2 ml DMSO was added into each well to dissolve the formazan crystals. The absorbance of MTT was determined using a UV-microplate reader at 570 nm. The MTT value for untreated cells (cells not exposed to transfection systems) was taken as 100% cell viability.
2.8 In vivo siRNA transfection
Six to eight week old, female nude mice (nu/nu) (Charles River, Wilmington, MA) were injected into the right flank with 5 × 106 A2780 human ovarian cancer cells (Sigma Aldrich, St. Louis, MO) to form subcutaneous xenografts. Tumor diameters were measured with digital calipers, and the tumor volume in mm3 was calculated by the formula: Volume = (a*b2)/2 with a>b. After tumors reached an average size of 70 mm3, mice were treated with siRNA complexes (SAM-VEGF, SAM-Luc, ABP-siRNA). Tumors were injected daily for five days. For each injection siRNA complexes were formed as described above using 108 MB, 3 μg VEGF or luciferase siRNA combined with ABP weight ratio 10:1 (w/w; ABP to siRNA) in a total of 50 μl injection volume. Prior exposing mice to ultrasound treatment, each animal was anaesthetized using isoflurane to sonicate tumors using a 3 cm diameter US probe, which was carefully placed onto the tumor directly contacting the skin. 0.5 mL of US-contact gel was used for each animal and tumors were sonicated with 3 MHz, 1.0 W/cm2 and 50% duty for 10 min. Using this frequency was save and did not cause any burns or other skin irritation. Tumor size and body weight were measured every other day for ten days. All animal experiments were carried out strictly adhereing to University of Utah IACUC guidelines and following approved protocols.
3. Results and Discussion
3.1 SAM complex stability in serum containing media
As described previously, SAM complexes can be formed in serum free media by combining positive charged polyplexes (ABP-siRNA) with negatively charged MBs due to electrostatic interaction [24]. Thus, it is a reasonable concern for SAM complex formation to be disrupted in serum-containing media. Therefore, we performed SAM complex formation in serum (10% FBS) and compared it to SAM formation in serum-free media. SAM stability was observed and evaluated for 60 minutes using a confocal microscope to guarantee stability for in vitro and in vivo experimentation assuming SAM complexes will reach their desired target site within this 60 minute time frame. SAM complexes have been observed for 60 minutes and pictures were taken at time points 0, 5, 10, 30 and 60 minutes. As seen in Figure 1A (SAM serum-free) polyplexes (ABP-siRNA-Cy3) remain colocalized at the microbubble shell (FITC) for an incubation time for at least 60 minutes. At the 60 minute time point mild, fading in fluorescence could be observed, which could be explained by photobleaching. SAM incubated in serum-containing media (Figure 1B) showed similar results as SAM incubated in serum-free media and indicates that SAM formation remains stable when performed in serum. However, more detailed stability studies of SAM and its use as siRNA carrier in serum containing media need to be conducted in vitro as well as in vivo.
Figure 1.
Confocal microscopy of SAM complexes incubated for 60 minutes in serum free (A) and serum containing (10% FBS) (B) cell culture media. SAM complexes with FITC labeled human serum albumin (HSA) microbubble shell (FITC-channel) and Cy3 labeled polyplexes siRNA-Cy3-ABP (Cy3-channel). Merged channel represents FITC channel plus Cy3-channel.
3.2 Cellular uptake of SAM complexes in vitro
Delivery of siRNA in serum-containing media is still a challenge and needs improvement [7,8]. Therefore, we evaluated siRNA uptake by A2780 human ovarian cancer cell line in serum-containing media using FACS assay. To compare siRNA uptake in serum-containing versus serum-free media we first studied the effect of US in serum-free media. Thus, cellular uptake efficiency using 5 nM siRNA (final concentration in 2 ml cell media) in SAM complexes, ABP-siRNA polyplexes or as naked siRNA with or without US (1 MHz US condition at 0.5 Watt/cm2 and a 50% duty cycle, MB:cell ratio; 50:1) was investigated. Transfection was carried out in serum-free media for 4 hours. At first, siRNA uptake using SAM complexes and US treatment was significantly higher with ~80% (P < 0.0001) compared to sonicated ABP-siRNA ~30% polyplexes and naked siRNA ~5%. In addition there was a significant difference (P < 0.0001) of siRNA uptake between SAM complexes with (~80%) and without (~50%), respectively US treatment (Figure 2A). Positively charged polyplexes facilitate the cellular uptake of siRNA by interaction with the negatively charged cellular membrane. The phosphate groups in the backbone being responsible for its negative charge, naked siRNA undergoes electrostatic repulsion while interacting with the negatively charged cellular membrane (Scheme 1A). Therefore, naked siRNA showed decreased uptake when compared to ABP-siRNA polyplexes or SAM complexes. The enhancement of siRNA uptake through the cell membrane using US can be described by the following phenomena. First, US treatment alone can create short-lived cell membrane pores that allow siRNA to enter the cell (Scheme 1B). Second, to activate MBs with US causes microjets and microstreams, which propel the siRNA through the cell membrane and pores (Scheme 1C) [23,27]. The use of US-activated SAM complexes as siRNA delivery tool takes advantage by combining several effects described above, therefore, showed the highest siRNA uptake when compared to ABP-siRNA polyplexes or naked siRNA.
Next, siRNA uptake efficiency was compared using US treatment in combination with siRNA complexes (naked siRNA, siRNA plus MBs, PEI-siRNA polyplexes, ABP-siRNA polyplexes and SAM complexes) in serum-containing media. In this experiment 5 nM (final concentration in 2 ml cell media) siRNA was delivered using US conditions of 1 MHz, 0.5 Watt/cm2, 50% duty cycle and a MB:cell ratio of 50. The addition of serum to the cell culture media during transfection significantly impacted, cellular uptake of siRNA, which was found to be drastically decreased in all groups when compared to siRNA uptake in serum-free media (Figure 2B). Naked siRNA +US and siRNA +MB and +US showed 0% siRNA uptake when transfected in serum-containing media. This was not surprising since naked siRNA can easily be degraded by RNases present in serum, underlining the necessity for the development of a safe and more efficient siRNA delivery vehicle [7,8]. Further, the transfection efficiency of ABP +US dropped from ~30% (in serum-free media) to < 5% (in serum-containing media) reflecting the extreme difficulty of siRNA delivery in the presence of serum containing media. In addition, even the use of 25k bPEI showed only a very modest transfection efficiency of < 5%. Lastly, cellular uptake of siRNA using SAM complexes dropped from ~80% (in serum-free media) to ~50% (in serum-containing media). As expected delivering sRNA with SAM complexes +US in serum-containing media was the only group that showed a significant higher siRNA uptake (P < 0.0001) when compared to all other controls including naked siRNA +US, PEI and ABP polyplexes each +US. These results indicate that our newly combined SAM system has promising potential to become a save and more successful siRNA delivery vehicle that can be used to deliver siRNA to human ovarian cancer cells in serum-containing media.
The MB:cell ratio is an important factor for siRNA delivery using SAM complexes. So far, a MB:cell ratio of 50 has been used successfully, but additional experiments are ongoing to optimize and possibly customize the MB:cell ratio based on the cell type for further, improved use. In conclusion, 5 nM (final concentration in 2 ml cell media) siRNA was delivered using US conditions of 1 MHz, 0.5 Watt/cm2, 50% duty cycle and MB:cell ratios 0:1 (polyplex without MBs), 100:1, 250:1, 500:1, 1000:1. FACS data was analyzed by using % of siRNA positive cells (Figure 3A) and the GEO mean (Figure 3B). The “GEO mean” data gives quantitative information about the amount of siRNA that was taken up per cell and the “percentage of siRNA positive cells” data helps to distinguish between cells with and without siRNA. First, there is a difference between the use of MB:cell ratios (100, 250, 500, 1000) and no MBs (MB:cell ratio 0) when the “% of siRNA positive cells” were compared between these two groups (Figure 3A). Second, there was a significant difference between MB:cell ratios 0:1 and 250:1 (P < 0.05) and when 0:1 was compared to ratios 500:1 and 1000:1 (P < 0.001) in regards of the GEO mean (Figure 3B). Hence, the use of MBs increased the uptake of siRNA to A2780 cells in two ways. On one hand, more cells of the total cell population showed positive siRNA uptake. On the other hand, the amount of siRNA uptake for each individual cell increased with higher MB:cell ratio. As described above, the use of SAM complexes facilitates siRNA delivery in serum containing media due to the combination of ABP, MBs and US. Thus one can safely conclude that ABP protects siRNA from RNase degradation, facilitates cellular uptake and releases siRNA into the cytosol (Scheme 1A). In addition, MBs activated by US enhance the siRNA uptake due to the formation of microstreams, shockwaves and microjets that are caused by cavitating MBs (Scheme 1C)[23,27]. The cavitating MBs are able to increase the porosity of the cell membrane and allow siRNA to enter into the cell [22,23,28-30]. Therefore, increasing the MB:cell ratio consequently increases microjets/microstreams/shockwaves forcing more siRNA into the cells.
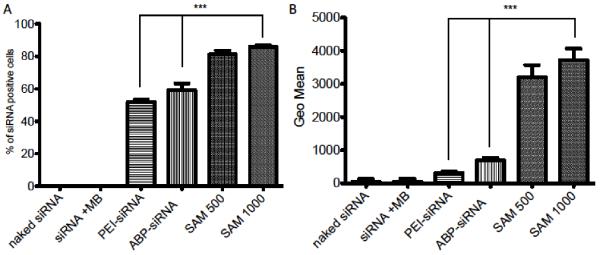
To identify the optimal MB:cell ratio for most efficient siRNA delivery using SAM complexes comparing it to the appropriate positive and negative controls is crucial to successfully translate preclinical findings. Thence, SAM complexes with the best MB:cell ratios 500 and 1000 were used and compared to polyplexes (ABP-siRNA, PEI-siRNA), siRNA plus MBs and naked siRNA with US conditions of 1 MHz, 0.5 Watt/cm2 and 50% duty cycle. There was a significant difference (P < 0.0001) between SAM complexes (MB:cell ratios 500, 1000) and polyplexes (ABP-siRNA, PEI-siRNA) when positive and negative siRNA cell populations were compared (Figure 4A). SAM complexes delivered siRNA to ~90% of the total cell population. However, polyplexes delivered to ~60% of cells and the naked siRNA controls showed no siRNA uptake at all. Further, SAM complexes (MB:cell ratio 1000) showed an eight times higher GEO mean (PEI ~500 vs SAM ~4000) when compared to the standard PEI with a significance of P < 0.0001 (Figure 4B), thus, indicates that SAM complexes are able to deliver an increased amount of siRNA per cell in serum containing media when compared to polyplexes and naked siRNA.
Figure 4.
Cellular uptake efficacy using SAM complexes. (A) Quantification of siRNA cellular uptake between siRNA positive and siRNA negative cells with 5 nM siRNA using naked siRNA, siRNA plus MB, polyplexes (ABP-siRNA, PEI-siRNA) and SAM complexes (MB:cell ratio 500 and 1000) treated with 1 MHz US condition, 0.5 Watt/cm2 and 50% duty. (B) Quantification of siRNA per cell population using the GEO mean of groups from (A). Data represent mean ± SD and significance tested (P < 0.0001) by one-way ANOVA and Tukey post test.
3.3 VEGF protein knockdown ability and cell viability using SAM complexes
The previous paragraph described how SAM complexes can be used to increase the siRNA uptake of A2780 cells analyzed by FACS assay. However, it is still unclear if US mediated siRNA delivery is harmful to the actual cargo, the siRNA. To investigate if actual functionally active siRNA is being delivered, we investigated the decrease of VEGF protein production. We performed VEGF ELISA studies to investigate the effect of US enhanced protein knockdown using SAM complexes with siRNA targeting VEGF mRNA. In addition, we further investigated which MB:cell ratio would lead to the highest VEGF protein knockdown. SAM complexes with different MB:cell ratios (100, 500 and 1000) were used to deliver 100 nM siRNA targeting VEGF (final concentration in 2 ml cell media). These were then compared to the polyplexes (ABP-siRNA). All groups were equally treated with US conditions of 1 MHz, 0.5 Watt/cm2 and 50% duty cycle. A known difference in VEGF protein knockdown is observed when SAM complexes are compared to polyplexes (P < 0.001 for polyplexes vs SAM 500 and 1000). VEGF protein knockdown increased significantly (P < 0.05) when we compared MB:cell ratios 100 with 500 and 1000 (Figure 5A). However, there was no difference between SAM complexes 500 and 1000 indicating that the maximum efficacy of MB:cell ratio of ~500 was attained. Thus, we used MB:cell ratio 500 for further experimentation. We also conducted a cell viability study using a MTT assay to confirm that SAM complexes and US are not harmful to the cells and that the VEGF protein knockdown is only caused by mRNA knockdown and not cell viability. Thus, as seen in Figure 5B there is no significant difference in cell viability between SAM complexes and polyplexes when compared to the untreated control.
Figure 5.
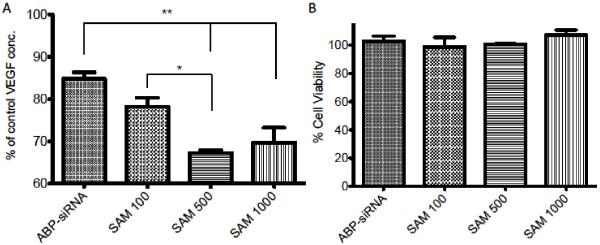
Optimization of MB:cell ratio for VEGF protein knockdown. Transfection efficiency of VEGF-siRNA using polyplexes (ABP-siRNA), SAM complexes (MB:cell ratio 500 and 1000) and US. VEGF ELISA and MTT assay in A2780 cell line treated with US 1 MHz, 0.5 Watt/cm2 and 50% duty. (A) VEGF concentration after transfection of A2780 cells in serum containing media after US exposure with 100 nM siRNA targeting VEGF, complexed in SAM or polyplexes. (B) Cell viability of (A) was determined by MTT assay and expressed as relative cell viability compared to the control. Data represent mean ± SD and significance tested (P < 0.05 and 0.001) by one-way ANOVA and Tukey post test.
Finally, we used SAM complexes with a MB:cell ratio 500 and US conditions as described above to deliver 100 nM of siRNA targeting VEGF mRNA (final concentration in 2 ml cell media) and compared them to appropriate controls including a siRNA that targets the luciferase protein (SAM-Luc-siRNA) as negative control. The negative control is a necessity in siRNA delivery experiments to exclude the possibility for off-targeting also confirming that the VEGF protein knockdown is solely caused by VEGF mRNA knockdown [31]. Only SAM complexes (~45% knockdown) delivered in serum containing media were able to reduce the concentration of VEGF protein significantly (P < 0.0001) when compared to the negative control SAM-Luc-siRNA (~20% knockdown) (Figure 6A). In some cases siRNA delivery to cells can cause off-targeting, a possible explanation for the VEGF protein knockdown caused by SAM-Luc-siRNA. Furthermore, only SAM complexes showed a significant difference in VEGF protein knockdown (P < 0.0001) when compared to the positive control and “gold standard” PEI (~25% knockdown). Naked VEGF-siRNA and VEGF siRNA plus MBs showed no significant difference in VEGF protein knockdown when compared to the negative control. As described, the synergistic effects using ABP, MBs and US (Scheme 1 A-C) are able to facilitate siRNA delivery in serum containing media and significantly enhance VEGF protein knockdown compared to both, positive and negative controls. Further, there was no significant difference in cell viability between each group (Figure 6B) indicating our system to be safe.
3.4 In vivo efficacy of SAM complexes
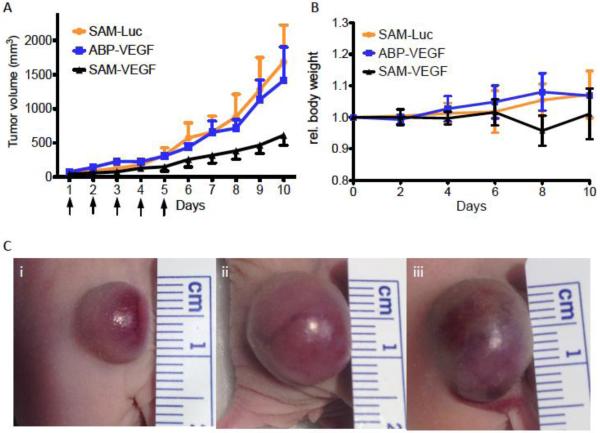
We evaluated the cellular uptake, transfection efficiency and protein silencing when siRNA was delivered in vitro in serum containing media using SAM complexes. However, we were interested, if our in vitro results also could be translated into in vivo. Therefore, we delivered SAM complexes to A2780 ovarian cancer xenografts in 6-8 week old, female nu/nu mice intratumorally (i.t.). Mice were injected daily with one injection for five days after the tumor reached a size of ~70 mm3 in diameter. SAM complexes that include a siRNA targeting VEGF m-RNA were compared to ABP-siRNA polyplexes also targeting VEGF, SAM-Luc complexes served as our negative control. Following i.t. injections, tumors were sonicated with US conditions using a 3 cm diameter US probe with 3 MHz, 1.0 W/cm2 and 50% duty for 10 min. Mice treated with SAM-VEGF showed a 3-4 fold decreased tumor size on day ten (day 1 being the 1st day of injections) when compared to negative controls SAM-Luc and ABP-VEGF polyplexes (Figure 7A). Thus, treating tumors with SAM-VEGF complexes showed a trend for decelerated tumor growth compared to controls indicating that siRNA targeting VEGF m-RNA was successfully delivered to the cells. No significant difference could be observed between the negative control SAM-Luc and ABP-VEGF polyplexes. Hence, the data indicates that MB plus US are able to enhance siRNA delivery to tumor tissues following i.t. injection. These findings concurr with our in vitro findings. Yet, tumor treatment with SAM complexes followed by sonication needs further research to optimize and standardize all variables involved. Dosing and frequency of siRNA injections, MB concentration and US conditions are just a few examples of variables that need to be optimized and possibly customized for each animal experiment based on cancer type studied and tumor location. Lastly, we did not observe a significant difference between all groups comparing total body weights (Figure 7B).
Figure 7.
Effect of intratumorally administered siRNA against VEGF using SAM complexes and ultrasound in tumor bearing nude mice. (A) Mice were injected five times total (see arrows) with SAM-VEGF-siRNA, SAM-Luc-siRNA or ABP-VEGF polyplexes intratumorally followed by ultrasound treatment. (B) Relative Body weight. (C) Tumor size pictures five days after the last injection for i) SAM-VEGF-siRNA, ii) ABP-VEGF polyplexes and iii) SAM-Luc-siRNA.
4. Conclusion
We investigated the complexation and stability of SAM in serum, SAM’s transfection efficacy, protein knockdown in vitro and siRNA delivery in vivo using SAM complexes in combination with US. We found SAM complexes to be stable in serum containing media for at least 60 minutes when compared to SAM stability in serum-free media. Second, siRNA delivery in serum-containing media is challenging and showed a wide range of variability when compared to siRNA delivery in serum-free media. Only SAM complexes in combination with US were able to successfully deliver sufficient amounts of siRNA in serum-containing media to treat A2780 cells when compared to the standard PEI and naked siRNA. Further, only the use of SAM complexes for VEGF protein knockdown in serum in A2780 cells showed a significant difference when compared to negative control SAM-Luc-siRNA and positive control PEI. Lastly, in vitro results could be translated and confirmed in vivo decreasing tumor size and decelerating tumor growth when compared to negative control SAM-Luc-siRNA and ABP-siRNA polyplexes.
Consecutive studies are planned to optimize and tailor in vivo US conditions and SAM complex delivery for local administration at the tumor site. Our results indicate that the difficulties and challenges of siRNA delivery in the presence of serum can be successfully overcome using our novel, combined delivery system and showed promising data in vitro as well as in vivo. Therefore, we are optimistic that the use of this innovative delivery tool may lead to new discoveries in the field of US enhanced and polymeric siRNA delivery in the area of tumor therapy.
Acknowledgments
This work was supported by NIH grants CA107070. We thank the University of Utah Core Facilities for assistance with fluorescence microscopy and FACS analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hammond SM, Caudy AA, Hannon GJ. Post-transcriptional gene silencing by double-stranded RNA. Nat. Rev. Genet. 2001;2:110–119. doi: 10.1038/35052556. [DOI] [PubMed] [Google Scholar]
- [2].Fougerolles A.d., Vornlocher H-P, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nature Reviews Drug Discovery. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- [4].Ferrara N. VEGF-A: a critical regulator of blood vessel growth. European cytokine network. 2009;20:158–163. doi: 10.1684/ecn.2009.0170. [DOI] [PubMed] [Google Scholar]
- [5].Wannenes F, Ciafré SA, Niola F, Frajese G, Farace MG. Vector-based RNA interference against vascular endothelial growth factor-A significantly limits vascularization and growth of prostate cancer in vivo. Cancer Gene Therapy. 2005;12:926–934. doi: 10.1038/sj.cgt.7700862. [DOI] [PubMed] [Google Scholar]
- [6].Kim WJ, Christensen LV, Jo S, Yockman JW, Jeong JH, Kim YH, Kim SW. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;14:343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- [7].Hickerson RP, Vlassov AV, Wang Q, Leake D, Ilves H, Gonzalez-Gonzalez E, Contag CH, Johnston BH, Kaspar RL. Stability Study of Unmodified siRNA and Relevance to Clinical Use. Oligonucleotides. 2008;18:345–354. doi: 10.1089/oli.2008.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl II, Toudjarska, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- [9].Ferrari M. Vectoring siRNA therapeutics into the clinic. nature reviews clinical oncology. 2010;7:485–486. doi: 10.1038/nrclinonc.2010.131. [DOI] [PubMed] [Google Scholar]
- [10].Higuchi Y, Kawakami S, Hashida M. Strategies for In Vivo Delivery of siRNAs. BioDrugs. 2010;24:195–205. doi: 10.2165/11534450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [11].de Fougerolles AR. Delivery Vehicles for Small Interfering RNAIn Vivo. Human Gene Therapy. 2008;19:125–132. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- [12].Nimesh S. Polyethylenimine as a Promising Vector for Targeted siRNA Delivery. Current Clinical Pharmacology. 2012;7:121–130. doi: 10.2174/157488412800228857. [DOI] [PubMed] [Google Scholar]
- [13].Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22:471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- [14].Kim SH, Jeong JH, Kim T.-i., Kim SW, Bull DA. VEGF siRNA Delivery System Using Arginine-Grafted Bioreducible Poly(disulfide amine) Molecular Pharmaceutics. 2008;6:718–726. doi: 10.1021/mp800161e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim T, Baek J, Zhebai C, Park J. Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier. Biomaterials. 2007;28:2061–2067. doi: 10.1016/j.biomaterials.2006.12.013. [DOI] [PubMed] [Google Scholar]
- [16].Kim T.-i., Ou M, Lee M, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30:658–664. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ou M, Wang X-L, Xu R, Chang C-W, Bull§ DA, Kim SW. Novel Biodegradable Poly(disulfide amine)s for Gene Delivery with High Efficiency and Low Cytotoxicity. Bioconjug Chem. 2008;19:626–633. doi: 10.1021/bc700397x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park K, Lee MY, Kim KS, Hahn SK. Target specific tumor treatment by VEGF siRNA complexed with reducible polyethyleneimine-hyaluronic acid conjugate. Biomaterials. 2010;31:5258–5265. doi: 10.1016/j.biomaterials.2010.03.018. [DOI] [PubMed] [Google Scholar]
- [19].Amiji MM. Polymeric gene delivery, Principles and Applications. CRC Press; 2005. [Google Scholar]
- [20].Porter TR, Iversen PL, Li S, XIie F. Interaction of diagnostic ultrasound with synthetic oligonucleotide labeled perfluorocarbon-exposed sonicated dextrose albumin microbubbles. J. Ultrasound Med. 1996;15:577–584. doi: 10.7863/jum.1996.15.8.577. [DOI] [PubMed] [Google Scholar]
- [21].Tsunoda S, Mazda O, Oda Y, Iida Y, Akabame S, Kishida T, Shin-Ya M, Asada H, Gojo S, Imanishi J, Matsubara H, Yoshikawa T. Sonoporation using microbubble BR14 promotes pDNA/siRNA transduction to murine heart. Biochem Biophys Res Commun. 2005;336:118–127. doi: 10.1016/j.bbrc.2005.08.052. [DOI] [PubMed] [Google Scholar]
- [22].Miller D, SV P, JE G. Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somat Cell Mol Genet. 2002;27:115–134. doi: 10.1023/a:1022983907223. [DOI] [PubMed] [Google Scholar]
- [23].Pitt WG, Husseini GA, Staples BJ. Ultrasonic Drug Delivery – A General Review. Expert Opin Drug Deliv. 2004;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Florinas S, Nam HY, Kim SW. Enhanced siRNA delivery using a combination of an arginine-grafted bioreducible polymer, ultrasound, and microbubbles in cancer cells. Mol Pharm. 2013;10:2021–2030. doi: 10.1021/mp400048p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sood AK, Coleman RL, Ellis LM. Moving beyond anti-vascular endothelial growth factor therapy in ovarian cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:345–347. doi: 10.1200/JCO.2011.38.8413. [DOI] [PubMed] [Google Scholar]
- [26].Duncan TJ, Al-Attar A, Rolland P, Scott IV, Deen S, Liu DT, Spendlove I, Durrant LG. Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies? Clin Cancer Res. 2008;14:3030–3035. doi: 10.1158/1078-0432.CCR-07-1888. [DOI] [PubMed] [Google Scholar]
- [27].Sirsi SR, Hernandez SL, Zielinski L, Blomback H, Koubaa A, Synder M, Homma S, Kandel JJ, Yamashiro DJ, Borden MA. Polyplex-microbubble hybrids for ultrasound-guided plasmid DNA delivery to solid tumors. J Control Release. 2012;157:224–234. doi: 10.1016/j.jconrel.2011.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ogawa K, Tachibana K, Uchida T, Tai T, Yamashita N, Tsujita N, Miyauchi R. High-resolution scanning electron microscopic evaluation of cell-membrane porosity by ultrasound. Med Electron Microsc. 2001;34:249–253. doi: 10.1007/s007950100022. [DOI] [PubMed] [Google Scholar]
- [29].Miller DL, Quddus J. Diagnostic ultrasound activation of contrast agent gas bodies induces capillary rupture in mice. Natl. Acad. Sci. 2000:10179–10184. doi: 10.1073/pnas.180294397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998:1264–1267. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- [31].Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nature reviews. Drug discovery. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]