Abstract
Clinical use of diagnostic ultrasound imaging during pregnancy has a long history of safety and diagnostic utility, as supported by numerous human case reports and epidemiological studies. However, there exist in vivo studies linking large but clinically relevant doses of ultrasound applied to mouse fetuses in utero to altered learning, memory, and neuroanatomy of those mice. Also, there exists a well-documented significant increase in the likelihood of non-right handedness in boys exposed to diagnostic ultrasound in utero, potentially relevant given the increased prevalence of autism in males, and some reports of excess non-right-handedness in this population. Motivated by these observations, we applied 30 minutes of diagnostic ultrasound to pregnant mice at embryonic day 14.5 and assayed the social behavior of their male pups three weeks after their birth. The ultrasound-exposed pups were significantly (p < 0.01) less interested in social interaction than sham-exposed pups in a 3-chamber sociability test. In addition, they demonstrated significantly (p < 0.05) more activity relative to the sham-exposed pups, but only in the presence of an unfamiliar mouse. These results suggest that fetal exposure to diagnostic ultrasound applied in utero can alter typical social behaviors in young mice that may be relevant for autism. There exist meaningful differences between the exposure of diagnostic ultrasound to mice versus humans that require further exploration before this work can usefully inform clinical practice. Future work should address these differences as well as clarify the extent, mechanisms, and functional effects of diagnostic ultrasound’s interaction with the developing brain.
Keywords: diagnostic ultrasound, mouse social behavior, autistic-like behavior, risk factor
Introduction
The incidence of diagnosed autism spectrum disorder (ASD) has increased over the past decade and is now estimated at upwards of 1% of the population (ADDM Network 2012, Saemundsen et al. 2013). The most recent update of the NIH Interagency Autism Coordinating Committee Strategic Plan for Autism Disorder Research (IACC, 2012) highlights the continuing likely importance of potential environmentally based risk factors and possible genetic contributions to the risk for ASD, especially during early stages of human embryonic development (IACC, 2012). Among the possible risk factors, recent work has drawn attention to diagnostic ultrasound (Abramowicz 2012; ter Haar et al. 2013; Williams and Casanova 2010, 2013).
Diagnostic ultrasound (dUS) imaging during pregnancy has been part of standard obstetrical care for decades. Imaging with diagnostic ultrasound has proved medically useful during the first trimester in screening for fetal abnormalities, detecting and tracking multiple embryos especially during in vitro fertilization, and determining chances for miscarriage, for example (Whitworth et al. 2010). In the second and third trimesters, ultrasound imaging can determine gender, assess growth, and assay for potential problems that may have arisen during fetal development (Whitworth et al. 2010). Medical professionals also use pulsed Doppler diagnostic ultrasound to assay for the presence and quality of the fetal heartbeat, starting in the first trimester. Appropriate weighing of the benefits over the risks governs the medical uses of dUS during pregnancy. However, these appropriate uses increase in association with factors also associated with an increase autism risk including advanced maternal age (Sandin et al. 2012; Lampi et al. 2013), maternal metabolic conditions (Krakowiak et al. 2012), and complications during pregnancy (Lyall et al. 2012).
Along with the medical assurances they generally receive, expectant parents like ultrasound imaging because they can see their baby before birth. Entrepreneurs now sell so-called “keepsake” ultrasound images (Rados 2004; Williams and Casanova 2013), where ~30 minutes of ultrasound yields a DVD ultrasound images for less than $200. Clinicians and the FDA recommend that pregnant women avoid keepsake ultrasounds yet these businesses remain unregulated in most US states (FDA; Rados 2004). While these ultrasound devices have passed FDA requirements for sale, their unlicensed use for non-medical reasons raises concerns. In addition to rising popularity of keepsake ultrasound, easy access to cheap (< $100) Doppler ultrasound heartbeat monitor devices on the Internet has increased their use in the home.
Given the increasing use of dUS, both medical and otherwise, and the over-all increase in its intensity since the original FDA limits on ultrasound were put in place (Gibbs et al. 2009) it remains important to ensure the safety of the procedure and to educate users and expectant parents about any potential adverse effects. This on-going assay of safety also remains relevant because there exist scientific studies showing that long but clinically relevant durations of dUS exposure to rodents in utero can alter their neurological function (early studies reviewed in Stewart et al. 1985). More recently Devi et al. (1995) and Suresh et al. (2002, 2008) found that mice exposed to at least 10 minutes of ultrasound in utero on embryonic day 14 (E14) of gestation exhibited evidence of impaired learning and memory. Suresh et al. (2008) also found that mice exposed to ultrasound in utero had significantly reduced hippocampal dopamine, noradrenalin, and serotonin levels relative to control animals, and a significant decrease in number of neurons in one region of the brain relative to controls. Finally, Ang et al. (2006) observed that mice exposed to 30 minutes or more of ultrasound on day E14.5 experienced significantly disrupted cortical neuronal migration relative to sham controls. (It is worth noting that there exist negative results: for example, Jensh et al. (1995) found no alterations of the memory and anxiety level of rats after 35 minutes of in utero ultrasound exposure.) To our knowledge, there do not exist any studies that assess possible changes in social behavior induced by ultrasound delivered in utero.
Published analyses of human data are not as clear as the rodent studies. As positive examples, several related, randomized studies (reviewed in Salvesen 2007) correlated use of dUS during pregnancy to a statistically significantly greater likelihood of non-right-handedness in boys, as has been reported for ASD (Soper et al. 1986; Dane and Balci 2007). Another study (Stalberg et al. 2009) correlated the number of dUS exams during the second trimester to school performance, finding a trend (without statistical significance) towards lower mean school grades for boys but not for girls. Two other studies that sought to directly assay at the population level the likelihood of ASD and proxies for dose of dUS dose failed to find a meaningful correlation (Grether et al. 2009, Stoch et al. 2012).
Because of the in vivo results and the increase in power of dUS since FDA standards were set (Gibbs et al. 2009), there remain concerns about the overall safety of diagnostic ultrasound and its potential link to increased risk of autism spectrum disorders (Abramowicz 2012; ter Haar et al. 2013; Williams and Casanova 2010, 2013). Here, we present preliminary data assessing if exposure of mice to thirty minutes of diagnostic ultrasound imaging in utero can alter their social behavior in an “autistic-like” way.
Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington under protocol #4084-07.
Animals
Eight standard C57BL/6J mice were purchased timed pregnant from Jackson Laboratories (Sacramento, CA). The mice arrived on gestational day E13 and were promptly placed in housing.
Application of Diagnostic Ultrasound to Pregnant Mice
On day E14.5 one group of four pregnant mice received 30 minutes of diagnostic ultrasound, described below while another group of four pregnant mice received 30 minutes of sham ultrasound. We applied ultrasound on day E14.5 because it is during the key stages of neural differentiation within the mouse brain (Kaufman and Bard 1999), and is a standard date for behavioral teratology studies. For ultrasound application mice were anesthetized using Isoflurane (4% for induction, 1.0-1.5% for maintenance), then fur was removed from each side of the abdomen. A circulating water-heating pad placed under the mouse maintained adequate body temperature during ultrasound application. Ultrasound gel was used to couple the transducer with the mouse’s abdomen and an acoustic absorber was placed opposite the transducer, also coupled to the mouse with gel to minimize internal ultrasound reflections. The 30-minute application was split into a 15-minute block on each side of the abdomen in order to distribute the exposure among the pups. The ultrasound device was turned on for the entire 30 minutes in the ultrasound group, but was turned off for the entire 30 minutes in the sham group. In this way the sham mice experienced the same procedure as the ultrasound group, differing only in the state of the ultrasound device. After the procedure, mice were returned to their cages and the pregnancies were carried out to term. At three weeks of age, the pups were weaned and the male pups were retained for the study.
Temperature measurements
To determine if ultrasound application might generate deleterious heating in the mouse, three non-pregnant mice were anesthetized and prepared for ultrasound delivery as above. A needle thermocouple (Applied Physics Laboratory, University of Washington) was inserted into the abdomen and ultrasound was applied for 30 minutes while a computer recorded the temperature from the thermocouple at 5 samples per second.
Ultrasound system
Our source of ultrasound consists of a Sonosite MicroMaxx, with a P17/5-1 transducer, general imaging mode with tissue harmonic imaging on, run at a mechanical index of 0.8, with its focus set to its minimum value of 4.7 cm. At Sonic Concepts (Woodinville, WA), we made direct measurements of the maximum value of the spatial peak, temporal average pressure within the ultrasound field emitted by our device under the use conditions just described, through standard use of a calibrated needle hydrophone (Precision Acoustics, Dorchester, England) and a tank filled with degassed water (Center, 1997). We found the maximum I_spta (spatial peak, temporal average intensity) of our device to equal 0.62 +/-0.22 W/cmˆ2, consistent with the FDA limit on intensity of 0.72 W/cmˆ2.
Behavioral Tests
All behavioral tests were conducted when the pups were 3-4 weeks of age, beginning 3 days after weaning.
Open Field Test
To assess the general activity of the mice, we placed a subject mouse in a Plexiglas open field (45 cm × 45 cm) and observed it for 10 minutes. Noldus tracking technology (Wageningen, Netherlands) was used to videotape the entire session and subsequently analyze the speed and distance travelled of each mouse.
Social Interaction Test
This test assesses a mouse’s interest in socializing with a novel “stranger” mouse (Yang et al. 2011). Noldus technology was used to videotape the mouse throughout a 30-minute trial within a three-chamber Plexiglas enclosure (the ‘arena’ – Figure 1), where each side chamber connects via a doorway to the central chamber, allowing access to the entire arena. We started with the first, acclimation phase of the study, where we placed the subject mouse in the center of the arena, allowing it to wander at will throughout the arena for 10 minutes. Next came the second, ‘stranger versus object preference’ phase. Immediately following the acclimation phase, the mouse was briefly confined to the center chamber while the researcher placed an object designed to contain a mouse in each of the side chambers. Into one of these objects we placed a “stranger” mouse. Note that we randomized between Object A and Object B so that the stranger mouse was not always located in the same chamber. The subject mouse was then allowed to roam the entire arena for 10 minutes, with video surveillance as before. The test concludes with the third ‘novel stranger versus familiar stranger preference’ phase. Immediately after the second phase we confined the mouse to the center chamber and placed a second stranger mouse within the remaining, empty object. The subject mouse then explored the entire arena for 10 minutes. We cleaned the entire arena and objects with 70% ethanol between testing of each new subject mouse.
Figure 1.
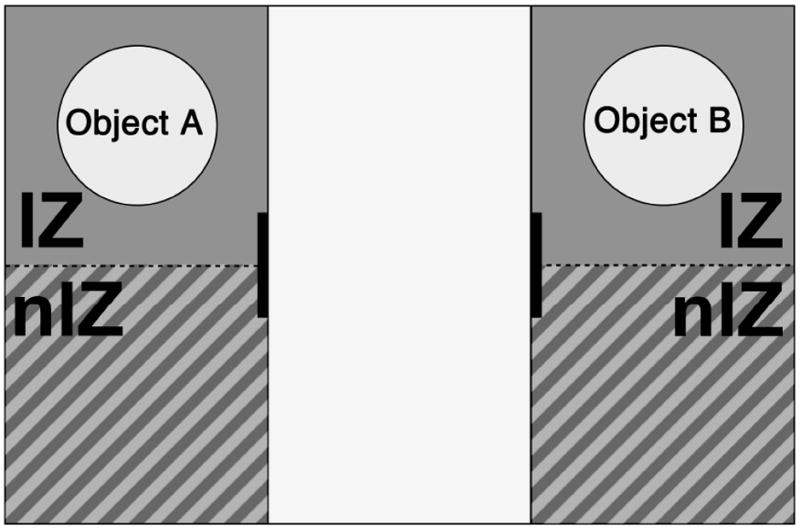
Schematic of the three-chamber social interaction testing set up showing the virtual zones analyzed by the Ethovision software and positioning of Object A or Object B, each representing a novel object, or a novel stranger mouse in a novel object, or a familiar stranger mouse in a novel object.
Data Analysis
We analyzed the open field and social interaction test data using Noldus Ethovision software. The software tracked the mouse, calculated the location, distance travelled, and speed of the mouse. For the speed calculations, the Ethovision software computed both the average speed of a given mouse and the amount of time it spent travelling at speeds greater than 4 cm/s and 10 cm/s. In the social interaction test, the software quantified the time spent by each mouse within a given zone in the three-chamber test, defined as follows (Figure 1): one zone consists of the center chamber while the other four zones reside in the side chambers. Specifically we divided each of the side chambers into an interaction zone (IZ) around the ‘object’ and a non-interaction zone (nIZ) that defined the remaining portion of the chamber.
We used this approach to analyze the data in three different ways (Table 1). Method 1 quantified the percentage of time spent by each group in a given chamber relative to the entire test phase. Method 2 quantified the percentage of time spent by each group in a given IZ relative to the entire test phase. Method 3 quantified the time spent in a given IZ relative to time spent within the corresponding chamber in a given test phase.
Table 1.
Equations for analysis methods of the activities of a given mouse during a given test phase.
| Analysis Method | Equation | |
|---|---|---|
| Method 1 |
|
|
| Method 2 |
|
|
| Method 3 |
|
In all cases where datasets were compared, we conducted 2-tailed, unpaired t-tests assuming equal variance using Microsoft Excel (Redmond, WA). We report all p-values less than or equal to 0.05 as statistically significant, and all p-values less than or equal to 0.1 as approaching or trending toward statistical significance.
Results
Sample size
In the US group, a total of 28 pups survived to three weeks of age from four mothers, of which 15 were male and were included in the study. In the sham group, a total of 26 pups survived to three weeks of age from four mothers, of which 13 were male. However due to poor mothering of one litter, two of the male pups in the sham group had to be removed from the study resulting in a sample size of 11 for the sham group.
Measurements of ultrasound induced heating
We observed an average basal temperature of 33°C +/-2 before application of ultrasound, which increased an average of 1.5°C +/-2 by the end of the time period of application of diagnostic ultrasound. This change in temperature was not statistically significant.
Open field test
We did not observe a difference or trend towards a difference in distance travelled or average speed between the ultrasound and sham groups in the open field test. There was also no difference or trend in the amount of time spent travelling at high speeds between the groups (Table 2).
Table 2.
Average and standard deviation for each category of activity for each treatment group in the open field test.
| Treatment Group | Distance travelled | Average speed | Time speed > 4 cm/s | Time speed > 10 cm/s |
|---|---|---|---|---|
| US | 3983 cm +/- 663 | 6.6 cm/s +/- 1.1 | 278.2 s +/- 42.1 | 145.6 s +/- 25.6 |
| Sham | 3995 cm +/- 768 | 6.7 cm/s +/- 1.3 | 276.6 s +/- 38.4 | 142.3 s +/- 31.2 |
Social interaction test
Acclimation phase
In the acclimation phase the mice were allowed to acclimate to the three-chamber arena without the presence of any other objects or mice for 10 minutes. We observed no significant difference between the US and sham groups with regard to how much time they spent in any of the chambers (Table 3). Interestingly, we found that the US group spent significantly more time in the right chamber than in the left chamber (p<0.01) while the sham group did not. We accounted for this difference in analysis of the latter two phases and found that it did not affect the results of those tests (data not shown).
Table 3.
Average and standard deviation of percentage time mice spent in each chamber during the acclimation phase for each group.
| Treatment Group | Left | Center | Right |
|---|---|---|---|
| US | 28.3% +/- 7.4 | 33.5% +/- 5.8 | 38.0% +/- 5.9 |
| Sham | 33.2% +/- 6.2 | 30.4% +/- 6.1 | 36.5% +/- 5.9 |
Stranger versus object preference phase
Each subject mouse spent 10 minutes in the arena where one chamber contained a novel object and the other contained an identical object holding a stranger mouse.
Within group analysis showed that the US mice did not spend significantly more time in the entire stranger chamber compared to the entire object chamber while the sham mice showed a significant preference for spending time in the entire stranger chamber compared to the entire object chamber (Figure 2A, p = 0.002). Between group analysis showed that the US mice spent less time in the stranger chamber than did the sham mice, with this difference approaching statistical significance (p = 0.07).
Figure 2. Analysis of Stranger versus Object preference phase.
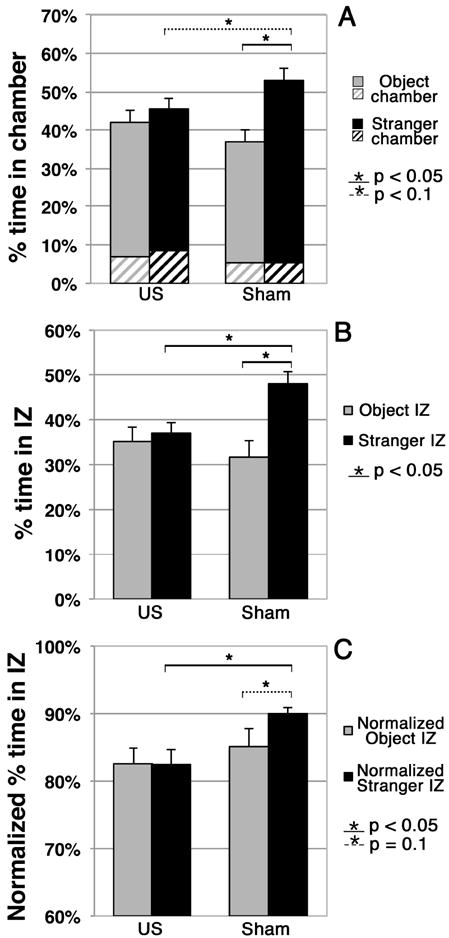
(A) Percentage of time that each group spent in each chamber during this Phase. Here, solid colors denote the time spent in the interaction zone (IZ) alone of a given chamber while crosshatching denotes the time spent in the non-interaction zone – see Figure 1.
(B) Percentage of time that each group spent in each IZ during this Phase. (C) Percentage of time that each group spent in the IZ of a given chamber while in that chamber.
Regarding the interaction zones (Figure 2B), within group analysis showed that the US mice had no preference for either interaction zone while the sham mice spent significantly more time in the stranger IZ than the object IZ (p = 0.002). Between group analysis showed that the US mice spent significantly less time in the stranger IZ than did the sham mice (p = 0.006). In addition, the difference between US within-group (non) preference and sham within-group (strong) preference for time spent in the mouse versus object interaction zones approached statistical significance (p = 0.08).
With regard to time spent within versus away from the interaction zone of a given chamber while in that chamber (Figure 2C), within group analysis showed that the sham mice tended to spend more of the time in the IZ of the stranger chamber than in the IZ of the object chamber (p = 0.1) while the US mice did not. Between group analysis showed that US mice, when within the stranger chamber, spent a significantly smaller portion of their time within its IZ than did the sham mice (p = 0.01).
Novel stranger versus familiar stranger preference phase
In this third phase of the social interaction test, we placed a second stranger mouse (called here the novel stranger) within the previously empty while leaving the first, now familiar stranger mouse within its original object. We then allowed the subject mouse to explore the entire arena for 10 minutes.
Within group analysis showed that the US group spent significantly more time in the entire novel stranger chamber than in the entire familiar stranger chamber (Figure 3A, p=0.002) while the sham group did not show a preference for either chamber. Between group analysis did not identify any significant nor near-significant differences between groups.
Figure 3. Novel stranger (Nov. Stranger) versus familiar stranger (Fam. Stranger) preference.
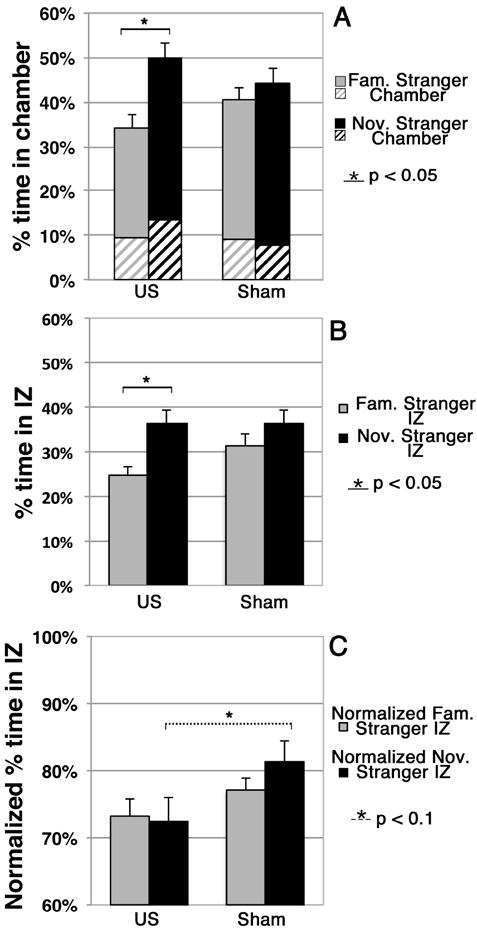
(A) Percentage of time that each group spent in each chamber during this Phase. Here, solid colors denote the time spent in the interaction zone (IZ) alone of a given chamber while crosshatching denotes the time spent in the non-interaction zone – see Figure 1.
(B) Percentage of time that each group spent in each IZ during this Phase. (C) Percentage of time that each group spent in the IZ of a given chamber while in that chamber.
Regarding the interaction zones (Figure 3B), within group analysis showed that the US mice spent significantly more time in the IZ of the novel stranger mouse than in the IZ of the familiar stranger mouse IZ (p = 0.002). The sham group did not show a preference. Between group analysis did not identify any difference between the US mice and sham mice with regard to the individual interaction zones; however, the US mice spent meaningfully less time in the two IZs combined than did the sham mice, with the difference approaching statistical significance (p = 0.1).
With regard to time spent within versus away from the interaction zone of a given chamber while in that chamber (Figure 3C), between group analysis showed that the US mice, when within the novel stranger chamber, spent a smaller portion of their time within its IZ than did the sham mice, with the difference approaching statistical significance (p = 0.09). No other comparisons between or within groups were statistically significant or approached significance.
Distance travelled
We did not observe a difference in the distance travelled within the entire arena between the US and sham groups during the acclimation phase of the test. During the other two phases of the social interaction test, however, the US group travelled greater distances than did the sham group – Figure 4. These differences were nearly statistically significant for the stranger versus object phase (p = 0.06), and statistically significant for the novel versus familiar stranger phase (p = 0.048).
Figure 4.
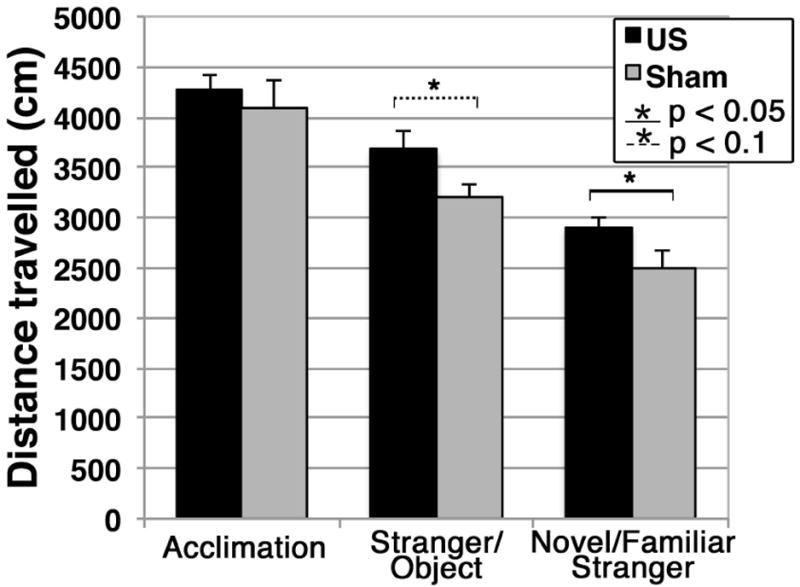
Distance travelled during the social interaction test within the entire experimental arena by each group of mice during each phase of the social interaction test.
Speed
We assessed the speed of the mice while they moved throughout the entire arena during the social interaction test, binning the data into the amount of time the mice spent moving at a speed greater than 4 cm/s (Figure 5A) or greater than 10 cm/s (Figure 5B). We did not observe a difference in time spent moving above these threshold speeds in the acclimation phase. During the other two phases of the social interaction test, however, we observed that the US mice spent significantly more time moving above the threshold speeds than did the sham mice (p = 0.047 and p = 0.02 during the stranger versus object phase; p = 0.04 and p = 0.049 during the familiar versus novel stranger phase). Moreover, during the stranger versus object phase, the US mice traveled faster on average than did the sham mice (6.2 +/-1.2 cm/s versus 5.5 +/-0.7 cm/s, respectively), with a difference that approached statistical significance (p = 0.09). In addition, during the novel versus familiar stranger phase, the US mice travelled significantly faster than did the sham mice (p = 0.03), with average speeds of 5.0 +/-0.6 cm/s and 4.3 +/-0.9 cm/s, respectively.
Figure 5.
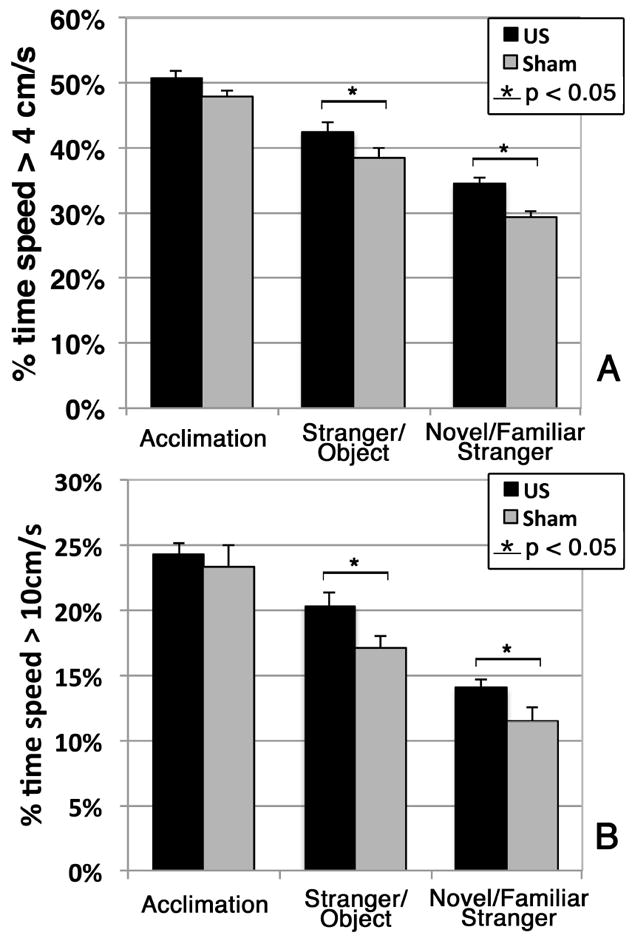
Percentage of time out of each of the three phases of the social interaction test that the mice were moving at speeds at or above (A) 4 cm/s or (B) 10 cm/s.
Discussion
Diagnostic ultrasound (dUS) has found increasing usage and clinical utility for monitoring human fetuses during pregnancy. Ample epidemiological evidence suggests that the benefits of current clinical dUS use outweigh the risks. Several animal-based studies demonstrate, however, that exposure of rodents to dUS in utero can alter learning and memory. Other work demonstrated changes within the cortical structure of mouse brains. These studies continue to raise concerns about the safety of dUS in this context, especially given increases in both acoustic power associated with these systems and the increase in non-clinical usage of dUS for imaging fetuses. In addition, the rise of ASD prevalence has occurred during a time period that overlaps with both increased usage and power of dUS for fetal studies (Gibbs et al. 2009, ADDM Network 2012), among an admittedly capacious list of other candidate environmental factors. These concerns motivated our study, where we sought to determine if mice exposed in utero to a large but clinically relevant dose of dUS (here, thirty minutes) would exhibit altered social behavior relative to sham-exposed mice.
Central to our work is the use of a three-chamber assay of social interaction (Yang et al. 2011). This assay allowed us to observe mice while they made a series of choices between more or less social circumstances: a new mouse versus a novel object; a familiar mouse versus a novel mouse.
In the acclimation phase of the social interaction test, where mice roamed freely through the empty arena, we observed that the ultrasound-exposed mice exhibited a significant preference for spending time in the right chamber of the testing apparatus while the sham-exposed mice did not display this preference. The experimenter sat near the left chamber throughout the experiment, suggesting that the US mice may have sought to move away from the experimenter during this phase while the sham mice did not. It is possible that this difference between groups suggests a difference in anxiety, but other more established anxiety measures did not distinguish ultrasound-treated animals from controls (data not shown). We therefore performed a post-hoc analysis on the results of the latter phases of our study. We found that our sham- and ultrasound-exposed mice behaved similarly regardless of whether the stranger mouse was placed in the left or right chamber (data not shown). We therefore analyzed all the data together, regardless of the location of the stranger mouse.
Results of the stranger versus object phase of the social interaction test (Figure 2) showed that ultrasound-exposed mice spent significantly less time interacting with a new mouse than did the sham-exposed mice. Furthermore, the ultrasound-exposed mice spent as much time interacting with a new mouse as they did with a new object. In contrast, the sham-exposed mice spent significantly more time interacting with a new mouse than a new object. These results support the hypothesis that ultrasound exposure in utero produced juvenile mice with reduced interest in social interaction.
Our results were less clear in the novel versus familiar stranger interaction phase of the social interaction test. This phase sought to gauge the preference of mice for social novelty, expecting that mice would spend comparable time with a new stranger mouse versus a familiar mouse and less time with a new mouse than would a sham exposed mouse. Inconsistent with the apparent induction of autistic-like social behavior discussed above, the ultrasound-exposed and sham-exposed mice spent comparable amounts of time with the novel stranger mouse (Figure 3B). Moreover, the ultrasound-exposed mice spent considerably more time with the novel stranger mouse than with the familiar stranger mouse, when analyzed in terms of time spent in the respective interaction zones during the entire experiment (p = 0.002 – Figure 3B). However, consistent with possible induction of autistic-like social behavior by diagnostic ultrasound exposure in utero, we observed that the ultrasound-exposed mice spent less time in both interaction zones added together than did the sham-exposed mice, with a difference that approached statistical significance (p = 0.1). This suggests that the ultrasound-exposed mice were less interested in social interaction with any mouse, regardless of their novelty, than were the sham-exposed mice. In addition, normalized interaction-zone analysis (Figure 3C) demonstrated that once in a chamber, the ultrasound-exposed mice were less interested in social interactions with a novel stranger than were the sham-exposed mice, with a difference that approached statistical significance (p = 0.09). Interestingly, this result did not hold for interactions with the familiar stranger mouse. Taken together these results are consistent with but do not definitively support the hypothesis that ultrasound exposure in utero can produce juvenile mice with reduced interest in social novelty.
Hyperactivity is so common in ASD that until recently the presence of ASD actually precluded concurrent diagnosis of ADHD. The current growing appreciation of the overlap of these conditions suggest that from 20% to 70% of individuals with ASD may also meet criteria for ADHD (Matson et al., 2013). Our observations of hyperactivity of our mice echo this phenomenon. In the open field test and the acclimation phase of the social interaction test, when the test mice were alone, the ultrasound- and sham-exposed mice traveled the same distance overall within the entire arena and with comparable distributions of speed. However, during those portions of the social interaction test involving interaction of test mice with other mice (the ‘stranger versus object’ and the ‘novel stranger versus familiar stranger’ phases) the ultrasound-exposed mice travelled significantly further throughout the arena and at faster speeds than did the sham-exposed mice (Figures 4 and 5).
Taken together, our results demonstrate that exposure of juvenile mice in utero to thirty minutes of diagnostic ultrasound can cause them to exhibit autistic-like behavior, specifically social deficits and hyperactivity in social circumstances.
Mechanisms
We did not study how dUS acted to change mouse social behavior. Here we suggest potential mechanisms that may guide future work. First, we consider how ultrasound interacts with tissue. Ultrasound can damage tissue if applied with sufficient intensity and/or duration above FDA limits through cavitation, acoustic radiation pressure, or heating (Mourad 2013). Of these effects, we sought to determine whether or not we unduly warmed the pregnant mother, hence her fetuses, during application of diagnostic ultrasound, either from the absorption of the ultrasound or due to warming by direct contact with the ultrasound scan head. We monitored the internal body temperature of three mice during ultrasound application and found that the temperature rose 1.5°C on average, and never rose above 39°C. Published studies (Hinoue et al. 2007) show that hyperthermia that raised the body temperature greater than 4 degrees centigrade above normal temperature (that is, to 43°C from 38°C) for more than 15 minutes was required to alter the neuro-development of the pups. This suggests that maternal heating did not play a role in our observations. Cavitation in vivo under these conditions is unlikely due to the absence of endogenous cavitation nuclei (Mourad 2013). This leaves the acoustic radiation force as the most likely physical means to produce these effects. This possible fetal irritant (Fatemi et al. 2001) requires further study. This becomes more plausible given that migrating axons in the developing cortex respond to a variety of exogenous factors via intra-cellular calcium-based signaling pathways (Kalil et al. 2011) and observations that dUS can centrally generate action potentials via calcium (and sodium) signaling (Tufail et al. 2010).
Second, we consider the vulnerability of the developing fetus to the effects of ultrasound. Specifically, there likely exists critical periods of brain development (Vorhees 1986) during which the fetus may be more susceptible to behavioral teratogens, such as ultrasound (Williams and Casanova 2010, 2013; Abramowicz 2012). We selected E14.5 as the ultrasound application date because it is within the critical window of mouse brain development when neurons are differentiating and migrating (Kaufman and Bard 1999).
Limitations
Abramowicz (2007) highlighted the limitations of Ang et al (2006), who showed that mice exposed to large doses of dUS in utero experienced alterations in their brain structure. Many of his comments are germane here. We acknowledge that while the mouse represents a useful research model for aspects of autistic-like behavior given its wide range of social, activity, learning, and memory behaviors, the gross physiology of a pregnant mouse differs significantly from that of a pregnant human. The ultrasound field emitted by the scan head is much more encompassing for a mouse fetus, covering the whole brain for the entire 30-minute exposure. This would not occur for a human fetus exposed to 30 minutes of ultrasound. Also, human fetal skull is thicker than mouse fetal skull, offering greater protection for the human brain. Thirty minutes of dUS therefore likely represents a significantly larger dose of ultrasound to fetal mouse brain than to fetal human brain. Moreover, mouse brain development in utero occurs over days, while that of humans in utero occurs over weeks: thirty minutes of dUS therefore covers a greater percentage of critical neuro-development time for a mouse fetus than for a human fetus. Future work will require exquisitely timed experiments to identify a definitive window of developmental vulnerability. Furthermore, exposure to dUS may have changed the ability of pregnant mice to take care of their offspring, another consideration for future work.
This study offers preliminary evidence that exposure to diagnostic ultrasound in utero can induce autistic-like social deficits and hyperactive behavior in social contexts in mice. Future work should also include additional autism-relevant behavioral tests such as tests of stereotypical behaviors and resistance to change (Crawley 2003). Also warranted is a search for an ultrasound dose-response effect, including identification of a minimum dose that does not alter behavior. Ultrasound should also be applied at different gestational time points to determine when the fetus is particularly at risk. Diagnostic ultrasound’s effect may also vary with a genetic predisposition towards autism (Williams and Casanova, 2010; 2013), perhaps studied through use of similar methods here but applied to different strains of mice (Moy et al. 2007; Yang et al. 2009). One should also consider Doppler ultrasound given its larger intensity (ter Haar and Abramowicz, 2013). Additionally, we note that we have tested only one ultrasound protocol emitted by one device out of many, any of which may yield different results.
Finally, we remark that autism is a human-specific condition. An “autistic mouse” cannot exist, and we can assess only behaviors that are perhaps relevant to a specific phenotype of autism. Our observations of autistic-like social deficits and social hyperactivity in ultrasound-exposed mice necessarily represent an extrapolation away from human applicability. Nonetheless, the social interaction assay remains an accepted metric for “autistic-like” social behaviors given the constraints of the mouse models for human behavior (Crawley 2003; Moy et al. 2007; Yang et al. 2009, 2011). Therefore, our observation of ultrasound’s ability to affect social behavior in mice requires detailed additional study, given the increasing use of ultrasound’s application to human fetuses.
Acknowledgments
We thank the Mouse Behavior Core in the Center for Human Development and Disability at the University of Washington, supported by grant P30 HD002274 from the National Institute of Child Health and Human Development, for the resources to perform the behavioral studies. We also thank Dr. Sean Murphy and Dr. Toby Cole, who manage the Mouse Behavior Core for their considerable assistance.
Contributor Information
Abbi M. McClintic, Dept. Neurological Surgery, University of Washington, 1959 NE Pacific St., Box 356470, Seattle, WA 98195
Bryan H. King, Seattle Children’s Autism Center, Seattle Children’s Hospital, 4800 Sand Point Way NE, PO Box 5371/M1-1, Seattle, WA 98105.
Sara J. Webb, Psychiatry and Behavioral Sciences, University of Washington, 1959 NE Pacific St. Box 357920, Seattle, WA 98195.
Pierre D. Mourad, Dept. Neurological Surgery, University of Washington, 1013 NE 40th St., Box 355640, Seattle, WA 98195.
References
- Abramowicz JS. Prenatal exposure to ultrasound waves: is there a risk? Ultrasound in Obstetrics and Gynecology. 2007;29:363–367. doi: 10.1002/uog.3983. [DOI] [PubMed] [Google Scholar]
- Abramowicz JS, Fowlkes JB, Skelly AC, Stratmeyer ME, Ziskin MC. Conclusions Regarding Epidemiology for Obstetric Ultrasound. Journal of Ultrasound in Medicine. 2008;27:37–644. doi: 10.7863/jum.2008.27.4.637. [DOI] [PubMed] [Google Scholar]
- Abramowicz JS. Ultrasound and Autism: Association, Link, or Coincidence? Journal of Ultrasound in Medicine. 2012;31:1261–1269. doi: 10.7863/jum.2012.31.8.1261. [DOI] [PubMed] [Google Scholar]
- Ang ES, Jr, Gluncic V, Duque A, Schafer ME, Rakic P. Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proceedings of the National Academy of Sciences of the USA. 2006;103(34):12903–10. doi: 10.1073/pnas.0605294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States 2008. MMWR Surveillance Summaries. 2012;61(3):1–19. [PubMed] [Google Scholar]
- Center for Devices and Radiological Health. Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. Food and Drug Administration; Rockville, MD: 1997. [Google Scholar]
- Dane S, Balci N. Handedness, eyedness and nasal cycle in children with autism. International Journal of Developmental Neuroscience. 2007;25(4):223–6. doi: 10.1016/j.ijdevneu.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Devi PU, Suresh R, Hande MP. Effect of Fetal Exposure to Ultrasound on the Behavior of the Adult Mouse. Radiation Research. 1995;141:314–317. [PubMed] [Google Scholar]
- Ellisman MH, Palmer DE, André MP. Diagnostic Levels of Ultrasound May Disrupt Myelination. Experimental Neurology. 1987;98:78–92. doi: 10.1016/0014-4886(87)90073-2. [DOI] [PubMed] [Google Scholar]
- Fatemi M, Ogburn PL, Jr, Greenleaf JF. Fetal stimulation by pulsed diagnostic ultrasound. J Ultrasound Med. 2001 Aug;20(8):883–9. doi: 10.7863/jum.2001.20.8.883. [DOI] [PubMed] [Google Scholar]
- FDA. Avoid Fetal ‘Keepsake’ Images, Heartbeat Monitors. 2013 Retrieved May 30, 2013, from http://www.fda.gov/forconsumers/consumerupdates/ucm095508.htm.
- Gibbs V, Cole D, Sassano A. Ultrasound Physics and Technology: How, Why, and When. Elsevier Health Sciences. 2009:94–95. [Google Scholar]
- Grether JK, Li SX, Yoshida CK, Croen LA. Antenatal ultrasound and risk of autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40(2):238–45. doi: 10.1007/s10803-009-0859-4. [DOI] [PubMed] [Google Scholar]
- Interagency Autism Coordinating Committee (IACC) IACC Strategic Plan for Autism Spectrum Disorder (ASD) Research—2012 Update. 2012 Retrieved June 20, 2013, from the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee website: http://iacc.hhs.gov/strategic-plan/2012/index.shtml.
- Jensh RP, Lewin PA, Poczobutt MT, Goldberg BB, Oler J, Goldman M, Brent RL. Effects of Prenatal Ultrasound Exposure on Adult Offspring Behavior in the Wistar Rat. Proceedings of the Society for Experimental Biology and Medicine. 1995;210:171–179. doi: 10.3181/00379727-210-43937. [DOI] [PubMed] [Google Scholar]
- Kalil K, Li L, Hutchins BI. Signaling mechanisms in cortical axon growth, guidance, and branching. Front Neuroanat. 2011 Sep 28;5:62. doi: 10.3389/fnana.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MH, Bard JBL. The Anatomical Basis of Mouse Development. San Diego: Academic Press; 1999. The brain and spinal cord; pp. 171–193. [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, Hertz-Picciotto I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):e1121–8. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KM, Hinkka-Yli-Salomäki S, Lehti V, Helenius H, Gissler M, Brown AS, Sourander A. Parental Age and Risk of Autism Spectrum Disorders in a Finnish National Birth Cohort. Journal of Autism and Developmental Disorders. 2013 Mar 12; doi: 10.1007/s10803-013-1801-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lyall K, Pauls DL, Spiegelman D, Ascherio A, Santangelo SL. Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses’ Health Study II. Autism Research. 2012;5(1):21–30. doi: 10.1002/aur.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JL, Rieske RD, Williams LW. The relationship between autism spectrum disorders and attention-deficit/hyperactivity disorder: An overview. Research in Developmental Disabilities. 2013;34(9):2475–2484. doi: 10.1016/j.ridd.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behavioral Brain Research. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham JP, Doherty DA, Kendall GE, Zubrick SR, Landau LL, Stanley FJ. Effects of repeated prenatal ultrasound examinations on childhood outcome up to 8 years of age: follow-up of a randomised controlled trial. Lancet. 2004;364(9450):2038–44. doi: 10.1016/S0140-6736(04)17516-8. [DOI] [PubMed] [Google Scholar]
- Prevalence of autism spectrum disorders in an Icelandic birth cohort. BMJ Open. 2013;3(6) doi: 10.1136/bmjopen-2013-002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rados C. FDA cautions against ultrasound ‘keepsake’ images. FDA Consumer Magazine. 2004;38:12–16. [PubMed] [Google Scholar]
- Saemundsen E, Magnússon P, Georgsdóttir I, Egilsson E, Rafnsson V, Salvesen KA. Epidemiology of diagnostic ultrasound exposure during pregnancy – European committee for medical ultrasound safety (ECMUS) European Journal of Ultrasound. 2002;15:165–171. doi: 10.1016/s0929-8266(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Sandin S, Hultman CM, Kolevzon A, Gross R, MacCabe JH, Reichenberg A. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(5):477–486.e1. doi: 10.1016/j.jaac.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Schneider-Kolsky ME, Ayobi Z, Lombardo P, Brown D, Kedang B, Gibbs ME. Ultrasound exposure of the foetal chick brain: effects on learning and memory. International Journal of Developmental Neuroscience. 2009;27(7):677–683. doi: 10.1016/j.ijdevneu.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Sheiner E, Abramaowicz JS. Clinical end users worldwide show poor knowledge regarding safety issues of ultrasound during pregnancy. Journal of Ultrasound in Medicine. 2008;27(4):499–501. doi: 10.7863/jum.2008.27.4.499. [DOI] [PubMed] [Google Scholar]
- Soper HV, Satz P, Orsini DL, Henry RR, Zvi JC, Schulman M. Handedness patterns in autism suggest subtypes. Journal of Autism and Developmental Disorders. 1986;16(2):155–67. doi: 10.1007/BF01531727. [DOI] [PubMed] [Google Scholar]
- Stalberg K, Axelsson O, Haglund B, Hultman CM, Lambe M, Kieler H. Prenatal ultrasound exposure and children’s school performance at age 15-16: follow-up of a randomized controlled trial. Ultrasound in Obstetrics and Gynecology. 2009;34(3):297–303. doi: 10.1002/uog.7332. [DOI] [PubMed] [Google Scholar]
- Stewart HD, Stewart HF, Moore RM, Garry J. Compilation of Reported Biological Effects Data and Ultrasound Exposure Levels. Journal of Clinical Ultrasound. 1985;13:167–186. doi: 10.1002/jcu.1870130304. [DOI] [PubMed] [Google Scholar]
- Stoch YK, William CJ, Granich J, Hunt AM, Landau LI, Newnham JP, Whitehouse AJO. Are Prenatal Ultrasound Scans Associated with the Autism Phenotype? Follow-up of a Randomised Controlled Trial. Journal of Autism and Developmental Disorders. 2012;42(12):2693–2701. doi: 10.1007/s10803-012-1526-8. [DOI] [PubMed] [Google Scholar]
- Suresh R, Devi PU, Ovchinnikov N, McRae A. Long-term effects of diagnostic ultrasound during fetal period on postnatal development and adult behavior of mouse. Life Sciences. 2002;71:339–350. doi: 10.1016/s0024-3205(02)01642-9. [DOI] [PubMed] [Google Scholar]
- Suresh R, Rao TR, Davis EM, Ovchinnikov N, McRae A. Effect of diagnostic ultrasound during the fetal period on learning and memory in mice. Annals of Anatomy. 2008;190:37–45. doi: 10.1016/j.aanat.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Hendrickx AG. Evaluation of the Bioeffects of Prenatal Ultrasound Exposure in the Cynomolgus Macaque (Macaca fascicularis): II. Growth and Behavior During the First Year. Teratology. 1989;39:149–162. doi: 10.1002/tera.1420390207. [DOI] [PubMed] [Google Scholar]
- ter Haar G, Abramowicz JS, Akiyama I, Evans DH, Ziskin MC, Marsal K. Do we need to restrict the use of Doppler ultrasound in the first trimester of Pregnancy? Ultrasound in Medicine and Biology. 2013;39(3):374–380. doi: 10.1016/j.ultrasmedbio.2012.11.024. [DOI] [PubMed] [Google Scholar]
- ter Haar G. Ultrasonic imaging: safety considerations. Interface Focus. 2011;1:686–689. doi: 10.1098/rsfs.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SI, Tyler WJ. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010 Jun 10;66(5):681–94. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- van der Ven E, Termorshuizen F, Laan W, Breetvelt EJ, van Os J, Selten JP. An incidence study of diagnosed autism-spectrum disorders among immigrants to the Netherlands. Acta Psychiatrica Scandinavica. 2013;128(1):54–60. doi: 10.1111/acps.12054. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Principles of Behavioral Teratology. In: Riley RP, Vorhees CV, editors. Handbook of Behavioral Teratology. New York: Plenum Press; 1986. pp. 23–48. [Google Scholar]
- Whitworth M, Bricker L, Neilson JP, Dowswell T. Ultrasound for fetal assessment in early pregnancy. Cochrane Database of Systematic Reviews. 2010;(4) doi: 10.1002/14651858.CD007058.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EL, Casanova MF. Potential teratogenic effects of ultrasound on corticogenesis: Implications for autism. Medical Hypotheses. 2010;75:53–58. doi: 10.1016/j.mehy.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Williams EL, Casanova MF. Reassessment of teratogenic risk from antenatal ultrasound. Translation Neuroscience. 2013;4(1):81–87. [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Behavioral Neuroscience. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated Three-Chambered Social Approach Task for Mice. Current Protocols in Neuroscience suppl. 2011;56:8.26.1–8.26.16. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]


