Abstract
In this study, human embryonic stem cell-derived cardiomyocytes were seeded onto controlled two-dimensional micropatterned features, and an improvement in sarcomere formation and cell alignment was observed in specific feature geometries. High-resolution photolithography techniques and microcontact printing were utilized to produce features of various rectangular geometries, with areas ranging from 2,500μm2 to 160,000μm2. The microcontact printing method was used to pattern non-adherent poly(ethylene glycol) regions on gold coated glass slides. Matrigel and fibronectin extracellular matrix (ECM) proteins were layered onto the gold-coated glass slides, providing a controlled geometry for cell adhesion. We used small molecule-based differentiation and an antibiotic purification step to produce a pure population of immature cardiomyocytes from H9 human embryonic stem cells. We then seeded this pure population of human cardiomyocytes onto the micropatterned features of various sizes and observed how the cardiomyocytes remodeled their myofilament structure in response to the feature geometries. Immunofluorescence was used to measure α-actinin expression, and phalloidin stains were used to detect actin presence in the patterned cells. Analysis of nuclear alignment was also used to determine how cell direction was influenced by the features. The seeded cells showed clear alignment with the features, dependent on the width rather than the overall aspect ratio of the features. It was determined that features with widths between 30μm and 80μm promoted highly aligned cardiomyocytes with a dramatic increase in sarcomere alignment relative to the long axis of the pattern. This creation of highly-aligned cell aggregates with robust sarcomere structures holds great potential in advancing cell-based pharmacological studies, and will help researchers to understand the means by which ECM geometries can affect myofilament structure and maturation in hESC-derived cardiomyocytes.
Keywords: Cardiomyocyte, cardiac tissue engineering, cell morphology, micropatterning, stem cell, surface modification
1. Introduction
Due to the recent emergence of regenerative and stem cell biology as leading fields of study, researchers have been able to make rapid progress in their search to uncover the basic principles underlying cellular function and development. By observing the development of various cell types from their pluripotent progenitors, scientists are beginning to understand how nature drives these cells toward their specific fates and, perhaps most importantly, how these cells can stray from their designated path to lead toward pathological outcomes. Cardiovascular disease, which continues to be the leading cause of mortality in the United States [1] and many other industrialized nations, has received particularly high levels of attention from investigators. With cardiovascular disease leading to over 800,000 deaths and approximately 610,000 new myocardial infarctions every year in the United States [2], the incentive for creating improved models, therapies, and treatments is extremely high.
Since contracting cells were first observed in human embryonic stem cell (hESC)-derived embryoid bodies [3], numerous successful attempts have been made to induce cardiac differentiation from pluripotent hESC populations. Researchers have produced cardiac phenotypes by applying growth factors Activin A and BMP4 to embryoid bodies [4] and monolayers [5,6]. More recently, improved efficiencies of differentiation have been achieved via small molecule Wnt inhibition [7,8] and extracellular matrix sandwich methods [9]. The main limitation of all of these methods is that they produce cardiomyocytes with predominantly immature properties [10]. Specifically, rather than becoming organized, aligned “barrel”-shaped cells [11] that are typically seen in native adult myocardium, the hESC-derived CMs form into randomly-aligned monolayers or clusters of contracting cells. Additionally, the multinucleation, myosin heavy chain isoform expression, Ca2+-handling, and biochemical response characteristics that are ordinarily exhibited by mature adult cardiomyocytes are not achieved by these immature cardiomyocytes [12,13].
Other studies, however, have shown that prolonged culture times of these immature cardiomyocytes over 60 days [10] and 120 days [13] do appear to lead to more mature-like phenotypes. These cardiomyocytes displayed promising changes in morphology and function, including increased multinucleation, sarcomere length, and cell size, with reduced Ca2+ time-to-peak and decay time. However, while the long-term cultured cardiomyocytes exhibited improved sarcomere alignment, they still did not achieve the level of sarcomere structure and alignment observed in native adult cardiomyocytes [14,15], and such long-term cultures would be limiting in their high cost and low throughput.
While understanding of the biology continues to improve, a wide array of engineering innovations have been developed that have enabled researchers to more accurately mimic the cells’ physiological environment while improving throughput, accuracy, and efficiency of these studies. Of these innovations, two-dimensional micropatterning and three-dimensional engineered topographies have proven to be exceptionally promising [16–19].
Two-dimensional micropatterning studies have indicated that the architecture of the extracellular environment influences cell behavior with respect to morphology [20], migration [21], cytoskeletal structure [22], nuclear shape [23], lineage determination [24], and functionality [25,26]. Several studies have been conducted in which neonatal rat and mouse cardiomyocytes were seeded onto two-dimensional micropatterns and observed. It has been consistently reported that these cells, when seeded as populations onto patterned stripes, are capable of forming myofibers with improved sarcomere organization, cell-cell junction protein expression, and contractile strength [27–29]. Additionally, micropatterned single-cell studies utilizing rat and mouse cardiomyocytes have shown trends between cell shape, sarcomere formation, and α-catenin expression [30,31]. Further engineering innovations have led to studies involving PEG-and-groove combinations [32], poly(ethylene glycol) nanotopographies [33], and highly biomimetic micropattern designs [34].
With the previously-mentioned emergence of hESC-derived cardiomyocyte differentiation protocols, researchers have recently been able to begin testing human cardiomyocytes in similar ways. A study by Zhang et al [35] has resulted in improved human cardiomyocyte maturation, E-C coupling, and sarcomere structure by culturing cell populations of varying cardiomyocyte purities onto mesh-like micropatterns. Another study has demonstrated that human cardiomyocytes can be micropatterned in arrays for high-throughput screening purposes [36].
In contrast to this prior work, we closely examined how a multi-cell micropattern shape may play a role in controlling sarcomere morphology in pure populations of human cardiomyocytes derived from embryonic stem cells. We hypothesized that the widths and aspect ratios of rectangular micropatterns may lead to changes in costamere development, which in turn could influence cell shape, alignment, polarization, and sarcomere structure. We also examined whether these varied micropattern shapes led to altered gap junction plaque formation and calcium propagation rates within the human cardiomyocyte aggregates.
2. Materials and methods
2.1. hESC Culture
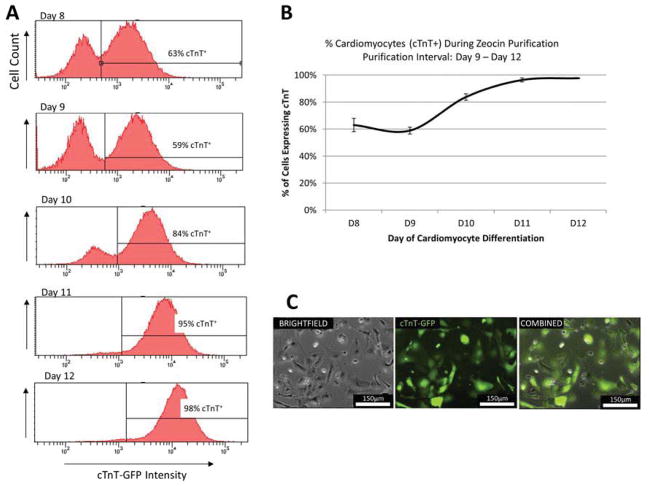
The cardiomyocytes used for all studies were derived from an engineered H9 hESC line containing a cardiac troponin T GFP (cTnT-GFP) promoter. The cTnT-GFP promoter line also exhibited resistance to the antibiotic Zeocin in conjunction with cardiac troponin T expression. This allowed for purification of cTnT-expressing cells, leading to a ~98% purity of cardiomyocyte cultures for the described studies (Figure 1).
Fig. 1.
Purification of hESC-derived cardiomyocyte population. The zeocin resistance of cTnT-expressing hESC-CMs was used to purify out a population of ~98% cardiomyocytes. Daily flow cytometry (A–B) indicated that this was achieved after three days of zeocin purification (n = 3). Epifluorescent imaging after purification reinforced this by showing that all visible cells were positive for cTnT-GFP protein (C).
Prior to differentiation, these hESCs were maintained on vitronectin-coated polystyrene plates, and E8 media [37] was replaced daily. The E8 media was produced in-house using DMEM/F-12 (Life Technologies), L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate, sodium selenite, sodium bicarbonate, holo-transferrin, insulin, βFGF (WiCell), and TGFβ (Peprotech). Unless otherwise mentioned, these reagents were purchased from Sigma-Aldrich. The cells were passaged at a 1:12 ratio every 4 days using versene (Life Technologies) and were seeded with ROCK Inhibitor (Tocris).
2.2. hESC Cardiomyocyte Differentiation
A modified version of the small molecule Wnt-agonist method of differentiation [7] was used to produce a high percentage of cardiomyocytes from the cTnT-promoting H9 hESC line (hereafter referred to as “hESC-CMs”). Briefly, the cTnT-GFP hESCs were seeded on day -3 into matrigel-coated (BD Biosciences) 12-well plates at a density of 400,000 cells per well. The seeding solution was E8 medium supplemented with 5μm ROCK Inhibitor (Tocris). On day -1, cells were fed again with E8 medium. On day 0, exactly 72 hours after the initial seed, cells were treated with RPMI (Life Technologies) supplemented with B27-insulin (Life Technologies), 10.5 μM of the Wnt agonist CHIR 99021 (Tocris), and 1μg/mL of insulin (Sigma). On day 1, cells were fed with RPMI supplemented with B27-insulin only. On day 3, cells were treated with RPMI supplemented with B27-insulin and 2.5 μM of the Wnt inhibitor IWP4 (StemGent). Cells were then fed on day 5 with RPMI containing B27-insulin and then treated with RPMI containing B27 complete on days 7,8, and 9, and every 3 days thereafter. Once contractions were observed between day 9 and day 12, zeocin treatments were applied in which 100μg/mL per day were added to the RPMI/B27 medium for 3 days to remove non-cTnT expressing cell types. The resulting cells were confirmed by flow cytometry to be ~98% cardiomyocytes.
2.3. 2D Micropattern Design and Stamp Production
The patterning technique in this study utilized a microcontact printing technique on a gold-coated glass substrate. Several steps were required to create the stamp used for printing. First, a template with the feature designs was created in AutoCAD (Autodesk) and sent to the Stanford Microfluidics Foundry for the fabrication of a photomask and a 4-inch patterned Si wafer (Stanford University, Santa Clara County, CA). Using soft photolithography techniques, the Si wafer was spin-coated with an SU-8 negative photoresist and exposed to UV light which yielded features with a height of 15 μm. The first round of testing incorporated features on the Si master consisting of rectangles with aspect ratios ranging from 1:1 – 11:1 (A). The following aspect ratios were chosen: 1:1, 3:1, 5:1, 7:1, 9:1 and 11:1 with a starting dimension of 100 μm, 200 μm, 300 μm and 400 μm, respectively. For each starting dimension, as the aspect ratio increased the surface area within the feature was held constant. The dimensions used for each feature are listed in Table 1. After the first round of testing determined that lane width, rather than overall aggregate aspect ratio, played the largest role in affecting cell behavior, a new design was created. This additional design type, shown in Figure 2B, incorporated long lanes with widths ranging from 15 μm to 115 μm. These lanes allowed calcium transient analysis to be conducted due to their long length, in addition to providing more efficient utilization of the seeding surface.
Table 1.
Dimensions for each feature. All features within each grouping contain varied aspect ratios, but identical surface areas.
| Grouping (surface area) | Feature Dimensions at Various Aspect Ratios (μm)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:1 | 1:3 | 1:5 | 1:7 | 1:9 | 1:11 | |||||||
| Width | Length | Width | Length | Width | Length | Width | Length | Width | Length | Width | Length | |
| A (2500μm2) | 50 | 50 | 29 | 87 | 22 | 112 | 19 | 132 | 17 | 150 | 15 | 166 |
| B (10,000μm2) | 100 | 100 | 58 | 173 | 45 | 224 | 38 | 265 | 33 | 300 | 30 | 332 |
| C (40,000μm2) | 200 | 200 | 115 | 346 | 89 | 447 | 76 | 529 | 67 | 600 | 60 | 663 |
| D (90,000μm2) | 300 | 300 | 173 | 520 | 134 | 671 | 113 | 794 | 100 | 900 | 90 | 995 |
| E (160,000μm2) | 400 | 400 | 231 | 693 | 179 | 894 | 151 | 1058 | 133 | 1200 | 121 | 1327 |

|
|
|
|
|
|
|||||||
Fig. 2.
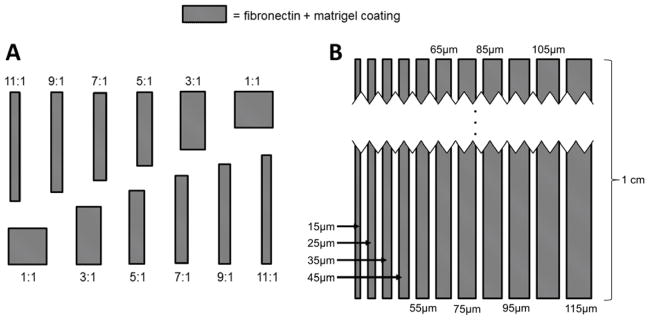
Micropattern designs. Extracellular matrix proteins were micropatterned in rectangles of varying sizes and aspect ratios. Two types of patterns were formed. (A) Features were grouped by surface area; while the features shown have varying aspect ratios, they would all have the same surface area within the same grouping. The dimensions of features within each grouping are shown in table 1. (B) Once it was determined that lane width was more important in eliciting a biological response than aspect ratio, long lanes of varying widths were formed. These lanes provided a more efficient use of space and were long enough to enable observation of calcium propagation through the hESC-CM aggregates. Dimensions shown indicate the thickness of each lane. Various spacings between neighboring lanes were attempted.
Elastomeric stamps used for microcontact printing were generated by standard soft lithographic techniques. The silicon master was rendered inert by overnight exposure in vapors of (tridecafluoro-1, 1, 2, 2-tetrahydrooctyl) trichlorosilane. Then, poly (dimethyl siloxane) (PDMS) (Sylgard 184 Kit) was used to create replicas of the silicon master by curing a 10:1 mixture of PDMS and curing agent overnight at 60°C. Fisherbrand microscope cover glass (24×50 #1) slides were cleaned by sequential washes in toluene and methanol followed by sonication for 1 minute in acetone.
2.4. μCP of ω-Mercaptoundecyl Bromoisobutyrate on Au-Coated Slides
After drying, 35 Å of titanium (Ti) followed by 180 Å of gold (Au) were deposited onto cover glass slides using a CHA-600 Telemark focused electron-beam evaporation system (Wisconsin Center for Applied Microelectronics). Au-coated glass substrates were rinsed with absolute ethanol prior to use. To make micropatterned substrates, PDMS stamps were coated with ω-mercaptoundecyl bromoisobutyrate (2mM in absolute ethanol), dried under nitrogen, and then brought in conformal contact with Ti/Au-coated glass slides. Finally, the chemically modified slides were incubated in absolute ethanol for 2h prior to being dried under nitrogen.
2.5. Surface-Initiated Activator Generated by Electron Transfer Atom Transfer Radical Polymerization (SI-AGET ATRP) of OEGMEMA
The macromonomer OEGMEMA (8g, 16.7mmol), water (7.5mL), methanol (7.5mL), copper(II) bromide (0.08mmol, 17.9mg) and 2′2-Bipyridine (0.24mmol, 37.5mg) were added to a Schlenk flask, sealed, and stirred for 30 min. Then, the flask was degassed with three freeze-vacuum-thaw cycles, and backfilled with nitrogen. Next, the mixture was transferred by syringe to a reaction flask containing micropatterned glass slides under vacuum. To start the reaction, L-ascorbic acid (0.8mmol, 140.9mg) in deionized water was injected into the flask. The reaction continued for 16h to generate micropatterned thick PEGMEMA brushes. Polymerization was stopped by adding air, and the slides were rinsed with ethanol, water, and ethanol sequentially before drying under nitrogen. Prior to cell culture, the slides were immersed in a large volume of ethanol for 2h to ensure complete removal of copper ions.
2.6. Adsorption of Adhesion Ligands
Once the slides were prepared, they were coated with a mixture of extracellular components overnight at 37°C, which allowed adsorption of attachment ligands onto the slides’ features. This controlled adsorption of adhesion ligands was caused by the PEGMEMA’s extremely limited binding affinity for these proteins in comparison to the gold-coated feature regions of the slides. The ECM solution consisted of DMEM (Life Technologies) containing 12μg/mL fibronectin from bovine plasma (Sigma) and 83μg/mL matrigel (WiCell). Through prior experimentation, this combination of extracellular matrix proteins was found to be the most successful in ensuring attachment of pure immature human cardiomyocyte cultures.
2.7. hESC-CM Seeding and Culture
Purified hESC-CMs were seeded onto micropatterns within 1 to 3 days after the purification step had been finished. Since the purification step was dependent on the time at which contractions were first observed, this seeding step typically occurred between days 13 – 16 of overall cardiomyocyte culture. The purified hESC-CMs were first washed 3 times with PBS to remove unnecessary debris that may interfere with binding onto the treated glass slides. The cells were then dissociated from their original culture dish via 15–20 minute exposure to TrypLE (Life Technologies). Every five minutes during the dissociation, the plates were gently agitated in order to help to break up the significant cell-cell binding and ECM deposits that form in these cultures. Cells were then rinsed in 10% serum-containing media and plated onto the treated glass slides in B27-supplemented RPMI medium. The hESC-CMs were seeded at a density of 30,000 cells/cm2, which allows for confluent cell attachment while avoiding excessive “piling up” of seeded cells. Spent medium was replaced with fresh RPMI+B27 every day thereafter.
2.8. Immunofluorescence and Imaging
Samples were stained and imaged after 5 days of culture. Cells were washed once with PBS, followed by a 15-minute exposure to 4% paraformaldehyde (PFA) (Electron Microscopy Sciences) at room temperature. Cells were washed once more, and were then treated for 6 minutes at room temperature with 0.1% Triton (Sigma) to permeabilize the cell membrane. Cells were washed once again with PBS and treated for 60 minutes with a blocking solution consisting of PBS, 2% FBS, 0.1% Triton, 11.2 mg/mL glycine, and 50μg/mL BSA. Cells were then treated with a primary antibody, consisting of mouse anti-α-actinin (1:60 dilution, Abcam) or mouse anti-connexin43 (1:100 dilution, BD Biosciences) overnight. The following day, cells were washed once with PBS for 5 minutes, and treated for 1 hour with blocking solution containing the secondary antibody, Cy5-conjugated goat anti-mouse (1:1000 dilution, Abcam). Cells were washed once more, and phalloidin conjugated to tetramethylrhodamine B isothiocyanate (TRITC) (Sigma) was applied at a 50μg/mL concentration to label actin filaments, and DAPI was applied at a 1:10,000 dilution to label nuclei. Cells were washed with PBS and transferred to coverslips, where they were mounted using ProLong Gold Antifade (Life Technologies).
Samples were imaged on a Nikon A1RSi Confocal Microscope with attached Photometrics CoolSNAP HQ2 camera. Nis-D Elements – Advanced Research v.3.22 software was used for image acquisition and analysis.
2.9. Measurement of Cell Nuclei Alignment
Alignment of cardiomyocytes was assessed via measurement of nuclear alignment, which is commonly used to determine the directionality of populations of cells [38]. Using the Nis-D Elements – AR software, threshold values were set within the DAPI (nuclear) channel of the confocal images. Size and shape filters were set in the Nikon Nis-D Elements software to remove debris and imaging artifacts from the analysis. Nuclei that were overlapping each other were manually separated, to keep them from being quantified as a single nucleus. The major axis of the nuclei was then determined automatically, and the angle of this axis was used to determine cell alignment. It should be noted that cardiomyocytes at this stage of maturity may often have multiple nuclei; however, this phenomenon should not play a large role in changing their directionality. Once alignments were quantified, cells were then defined as “aligned” if their major axis was within 20° of the axis of the micropatterned feature on which the cell was adhered.
2.10. Measurement of Calcium Propagation Rate
While actin, α-actinin, and nuclear staining provided characterization of the structural changes of hESC-CMs seeded on lanes of varying widths, further tests were conducted to determine the level of calcium ion conduction present in the aggregates. Thus, connexin 43 immunostaining (described above) and calcium transient analysis were conducted. For these experiments, the long micropattern designs were used (Figure 2B) since they were long enough to allow detection of the rapid calcium propagation rate throughout the aggregates. For calcium signaling assessment, the Rhod-2 acetoxymethyl (AM) ester (Life Technologies) calcium indicator dye was used. This cell-permeant dye exhibits an increase in fluorescence upon binding with cytoplasmic Ca2+, providing a direct means of observing calcium propagation through aggregates of contracting cardiomyocytes.
The Rhod-2 dye was first reconstituted to a 100× concentration of 1 mM Rhod-2 in DMSO, which was then diluted in RPMI/B27 medium and applied to the day 5 patterned cells for 15 minutes at 37°C. The cells were then washed twice with PBS, fed with RPMI/B27, and incubated for an additional 30 minutes. Dyed micropattern samples were then loaded into a heated microscope stage incubator and videos of calcium transient fluorescence were taken. The rates of these calcium transients were high enough to be undetectable by the naked eye, so ROI analysis was conducted using Nikon Nis-D Elements software on extreme ends of each aggregate (Figure 3). By comparing the Rhod-2 intensities of the two ROIs, we were able to approximate the rate of calcium propagation between the two regions.
Fig. 3.

Calcium transient analysis of hESC-CMs seeded onto lanes of varying widths. Live fluorescent imaging was conducted, and intensities were measured for ROIs at opposite ends of each lane (A). By comparing normalized calcium transient intensity over time (B) between the two ROIs, and using the distance between the ROIs, the calcium propagation rate was determined for each micropattern width. ROI=region of interest, FN=fibronectin.
3. Results
3.1. Cell Attachment and Spontaneous Contraction on Micropatterned Surfaces
Brightfield images were taken regularly to assess the level of attachment of the pure hESC-CMs onto the matrigel/fibronectin-coated features (Figure 3). As expected, there was a small amount of debris present at day 1, most of which was washed away during routine feeding. Qualitative analysis of timelapse images between day 1 and 3 revealed that the seeded cardiomyocytes changed shape slightly to fill in any empty spaces available on the micropattern (data not shown), though they did not migrate significantly after they adhered. On a select few of the micropatterns, large aggregates of cells detached at one or more locations on a feature while still remaining anchored at another point. Additionally, cells occasionally “bridged” the distance between two features, creating a linkage.
The hESC-CMs had already begun spontaneously contracting well before being seeded onto the micropattern. Immediately after seeding, however, while the cells were under stress due to dissociation, no contraction was observed. Subtle contractions were then observed 24 hours after being seeded onto the micropattern slides, and they continued to increase in strength over the next 24–48 hours. Contractions among cells within a single feature were completely synchronized. There were multiple cases in which linkage cells bridged the gap between separate features, which caused synchronization between these two features. Conversely, features did not synchronize with one another when such linkages were not present.
3.2. Actin and α-Actinin Alignment on Patterned Hydrogels
Expression of select components of the sarcomere structure was determined via phalloidin and α-actinin immunostaining and confocal imaging. Qualitative analysis of both reveals significant improvement in sarcomere alignment within micropatterned cardiomyocytes (see Figure 4). Sarcomere structures within individual cells generally aligned with each other, and the alignments of neighboring cells also tended to match direction. This phenomenon has been observed previously in rat and mouse cardiomyocytes, but this level of alignment had not yet been seen in this type of two-dimensional micropatterned structure for human cardiomyocytes.
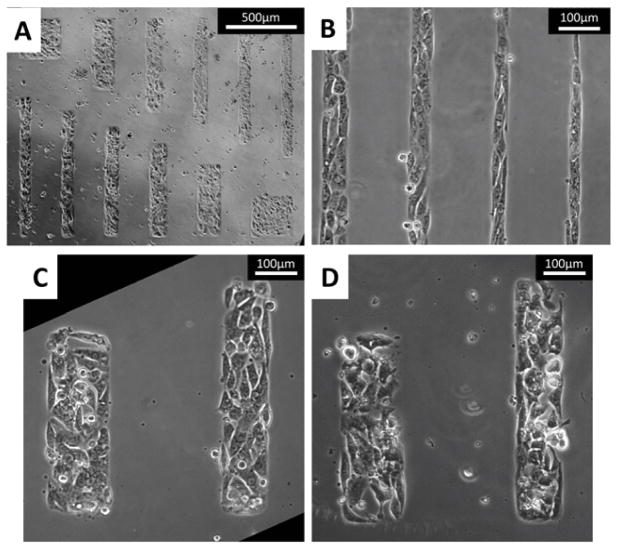
Fig. 4.
Representative brightfield images of micropatterned hESC-CMs. Cells seeded onto the micropatterned matrigel-fibronectin features matched the printed geometries very closely. (A) The first pattern design produced hESC-CM aggregates of varying aspect ratios (cells shown at day 7). All aggregates within a single grouping, as shown above, presented the same surface area. (B) The second design produced long lanes of varying widths (cells shown at day 3). As shown, the cells bound tightly to these geometric constraints and aligned clearly along the direction of the patterns. (C–D) The alignment of cells was clearly dependent on feature width, with the outermost cells aligning significantly more than cells located toward the center of each aggregate (cells shown at days 2 and 3, respectively)
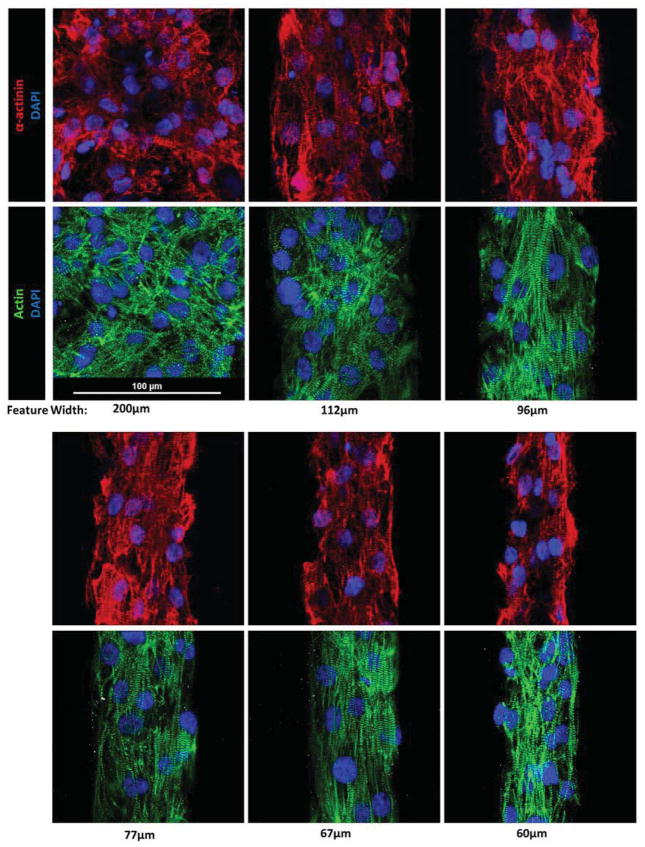
Of particular interest was the relative level of sarcomere development among the multiple feature geometries that were tested. It was clear that the aspect ratios of the features were less important in inducing well-structured sarcomeres than the widths of the features themselves. That is, while a cell’s alignment and internal structure was influenced by an edge of a micropattern, it was not heavily influenced by the aspect ratio. Specifically, it appeared that features with widths of 30μm – 80μm produced hESC-CMs with the most organized sarcomere structures.
3.3. Connexin 43 Immunofluorescence
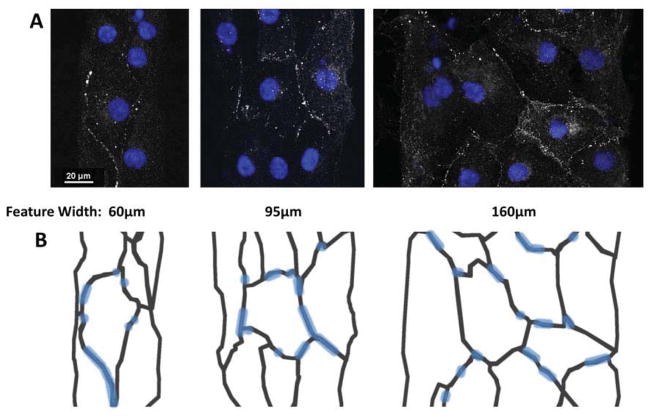
Antibody staining of connexin 43, a common gap junction protein, was conducted to assess the effects of aggregate width on cell-cell conduction. Connexin 43 gap junction channels couple cardiomyocytes in the ventricular myocardium enabling rapid electrical conduction between cells. In the normal adult ventricle, Connexin 43 gap junctions are preferentially in high abundance on the ends of cells and less on the sides. Immunostaining of monolayer cultures, as well as micropatterned aggregates of varying widths (Figure 6) revealed highly variable connexin 43 expression; unlike in sarcomere expression, there was no discernible pattern between connexin 43 expression and micropattern width. Gap junction plaques were highly punctate and occurred almost entirely within cell-cell junctions, as anticipated. These gap junctions localized to both lateral and axial edges of cardiomyocytes, however at day 5 there was no correlation found, regardless of cell shape, cell cytoskeleton orientation, or pattern width.
Fig. 6.
Connexin 43 expression of pure hESC-CMs seeded onto micropatterned matrigel/FN features of varying widths. Connexin 43 immunostaining with DAPI (A) shows a highly punctate and variable expression of gap junction plaques within the aggregates. Gap junction plaques were present along both lateral and axial edges of cardiomyocytes. A diagram (B) is included to indicate cell boundaries, as determined by phalloidin co-stain.
3.4. Nuclear Alignment on Patterned Substrates
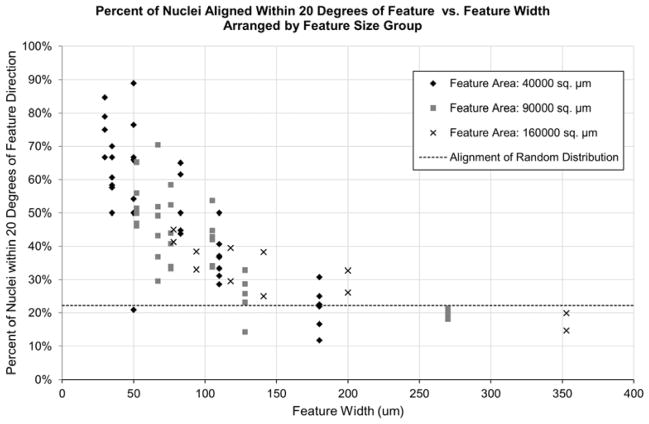
To gain quantitative insight regarding the level of directionality in the cultures, nuclear alignment was also assessed for the immature cardiomyocytes cultured on micropatterns of varying sizes and aspect ratios. As previously described, the patterns were organized into groups based on their surface area. Within each grouping, a variety of aspect ratios was examined. A nucleus was considered “aligned” if the direction of its major axis was within 20 degrees of the direction of the feature on which it was seeded. The measured directionalities of the nuclei closely matched the qualitative assessments of z-disk alignment observed in the immunostains mentioned above (see Figure 5). That is, the overall width of the micropatterns played a large role in determining cell orientation, rather than the aspect ratio of the feature as a whole.
Fig. 5.
α-actinin, actin, and DAPI stains of cardiomyocytes after 5 days of culture on features of varying widths. Both myofilament markers show clearly improved cellular alignment, sarcomere organization, and sarcomere alignment in features under ~100 μm in width. As anticipated, cells seeded onto square features (200 μm in this set) show no favor towards any specific direction. A slight difference of 0 – 5 μm was observed between the designed pattern width and the actual width of the patterned cells. This is likely due to a small amount of swelling in the PDMS stamps during the curing step, as well as some mild mismatch between the feature edges and the cells’ exact boundaries.
As shown in figure 7, the cells’ nuclei were far more aligned on features with widths in the 30μm – 80μm range. For features with widths larger than this range, the nuclei were aligned only roughly 22% of the time, which is the expected value for cells that have no directionality. Cells on the outer-most edges of the features appeared to show the highest level of alignment and nuclear elongation. Cells that were not on the outer edge of a feature still exhibited a large amount of alignment, as long as the feature width was less than approximately 100μm. We hypothesize that there is still considerable restriction in cell shape for the more centrally-located neighboring cells, created by the laterally adjacent cells that are in direct contact with the feature boundary.
Fig. 7.
Percentage of aligned nuclei of cells on varying features. It is clear that nuclei showed much stronger alignment on features with widths of 30 μm – 80 μm. The aspect ratios of the features as a whole, however, did little to affect the alignment of the hESC-CMs.
3.5. Calcium Ion Propagation Rates
Rhod-2 AM ester calcium indicator dye was used to measure the rate at which calcium signaling passes through aggregates of various sizes. A large number of lanes were tested, with widths ranging from 20μm to 110μm. The resulting calcium propagation rates, shown in figure 8, suggest that there is no clear link between aggregate width and calcium propagation rate for the observed cells. Most lanes exhibited calcium propagation rates within the range of 0.5cm/sec to 2cm/sec.
Fig. 8.
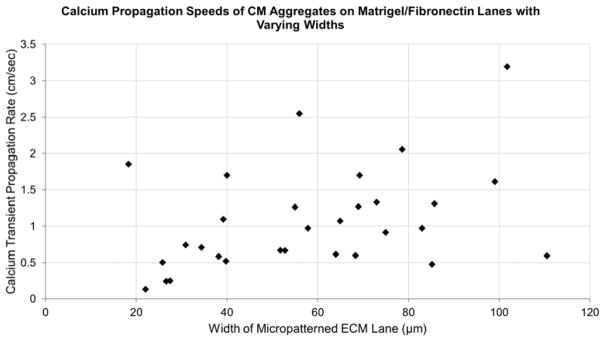
Calcium propagation speeds of hESC-CM aggregates on matrigel/fibronectin lanes with varying widths. Rhod-2 AM ester indicator dye was used to determine the rate at which calcium waves propagated through the cardiomyocyte aggregates. A compilation of all assessed lane widths indicates that there was no clear trend between lane width and calcium transient propagation rate. Calcium propagation rates typically ranged between 0.5 cm/sec and 2 cm/sec.
4. Discussion
Methods that promote cardiomyocyte alignment have the potential to lead to an exciting new range of studies on human embryonic stem cell-derived cardiomyocytes. Emerging methods of differentiating hESCs into cardiomyocytes have shown great promise in their repeatability, efficiency, and reduced cost. However, these protocols continue to only achieve an immature phenotype of cardiomyocytes, with limited cell size and elongation, low levels of multinucleation, and immature action potential characteristics. It is clear that the next challenge facing cardioregenerative biology is the achievement of a more mature phenotype that will allow for more accurate modeling of native myocardium for pharmacological studies and for potential cell therapies. We hypothesize that the mechanisms facilitating the improved sarcomere structure involve a combination of focal adhesion/costamere confinement, improved polarization due to cell elongation, and controlled directionality of cell-cell junctions and the resulting contraction-induced mechanical loading.
Focal adhesions, which are intricate protein complexes that enable binding between a cell’s cytoskeleton and the surrounding ECM, play a substantial role in the formation and alignment of actin stress fibers and force transduction. By restricting the locations at which the focal adhesions were able to form, both the cell and its stress fibers were aligned in the direction of the underlying features. In muscle cells, the laterally-located ECM-binding domains are more commonly referred to as costameres, which are highly related to focal adhesions both in function and protein composition [39]. Costameres form in conjunction with peripherally-located myofibrils along the z-disks, and their formation has been shown to be dependent on electrical coupling and mechanical loading of the cardiomyocyte [40–42].
In addition to cell-ECM binding, cell-cell contacts are also highly influenced by the micropatterned geometry. In standard two-dimensional cultures of cardiomyocytes, the cells form cell-cell junctions in all directions and are still capable of producing synchronously contracting sheets. This system is not physiologically relevant, however, since native cardiomyocytes form extremely polarized, fibrous tissue structures. The cell-cell junctions within native cardiomyocyte tissues reflect on this cellular polarity, with large differences in expression of cell-cell junction proteins in the axial versus lateral directions. Specifically, cardiomyocytes form intercalated discs with neighboring cells in the axial direction, while forming the costameres that bind to ECM ligands in the lateral direction. The intercalated discs are membrane-bound protein complexes that consist largely of adherens junctions and desmosomes to provide robust mechanical coupling between cells, as well as gap junctions to provide electrical coupling [43]. Due to the improved polarity of our cultured cardiomyocytes, there is a much higher likelihood of the formation of physiologically-relevant intercalated discs and costameres. This new platform can lead to further studies of the many pathologies related to cell-cell junction mutations, such as arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C), which has been linked to mutations in genes that encode desmosomal proteins [44].
While sarcomere formation and alignment were improved within the thin feature widths, there was no significant difference in gap junction plaque expression and calcium propagation rate among these micropatterns. The connexin 43 immunostaining revealed punctate gap junction distribution along both lateral and axial edges of cardiomyocytes, which indicates that the cells may not yet have reached the level of polarity found in mature cardiomyocytes. Additionally, calcium propagation rates ranged around 0.5cm/sec to 2cm/sec, which is significantly lower than what is typically observed in vivo in neonatal myocardium (~20 cm/sec), adult ventricles (~100 cm/sec), and adult conduction systems (~300 cm/sec). These results agree in suggesting that the calcium conduction system has yet to be fully developed at day 5 in these micropatterned systems. This also agrees with previously reported results indicating that heterogeneous connexin 43 expression, in which at least 50% of cells present limited numbers of gap junctions, may lead to slower calcium propagation rates [45]. It is possible, however, that a longer culture period within these fibrous aggregates will provide the cells with enough time to develop more robust calcium handling mechanisms.
The development of organized, structured sarcomeres is only one of many steps towards cardiomyocyte maturation that need to be better understood before a proper mature model of human cardiomyocytes can be thoroughly established. In addition to the numerous biochemical and epigenetic factors of cardiomyocyte maturation, there are other biomechanical aspects which will need to be addressed, such as substrate stiffness and mechanical loading, which were at non-physiological levels for this study. Additionally, the authors acknowledge that the use of matrigel as one of the components of the ECM substrate does provide some variability in the system; however, we believe that substituting this with laminin or other ECM components may allow for improved reproducibility and traceability. Regardless of these shortcomings, this system shows that a highly aligned, mature-like population of pure human cardiomyocytes can be produced in an exceedingly controlled manner from hESCs. We are confident that additional targeted studies of this platform will uncover fascinating mechanisms of cardiomyocyte development.
5. Conclusion
Organized cellular alignment is critical to controlling tissue micro-architecture and biological function. In this study, a pure population of human cardiomyocytes derived from embryonic stem cells was seeded onto micropatterns of varying geometries. The effects of these geometries on sarcomere development and nuclear alignment of the seeded cells were assessed and it was determined that a range of 30μm – 80μm is the ideal feature width to promote alignment of pure immature hESC-CMs, leading to improved sarcomere formation. By effectively inducing strong alignment of pure human cardiomyocyte cultures, we are now able to form much more physiologically-relevant models of heart tissues in vitro.
Acknowledgments
This research was supported with funds from the National Institutes of Health Grant K18 HL105504 from the Heart, Blood and Lung Institute and the Graduate School of the University of Wisconsin-Madison.
The authors would like to thank Professor Gary Lyons for the helpful conversations. We would like to thank Nicholas E. Propson and the Thomson Lab for providing vitronectin for regular cell maintenance. We are also grateful to Jason McNulty for technical assistance.
Footnotes
7. Disclosures
One author (TJK) holds founding shares in Cellular Dynamics International, Inc. This company produces and sells cardiomyocytes from human stem cells. This company, however, has no connection with the stated research and had no part in funding or advising the direction of this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart Disease and Stroke Statistics—2011 Update. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Kochanek KD, Murphy S, Tejada-Vera B. Deaths: final data for 2007. Hyattsville, MD: National Center for Health Statistics; [PubMed] [Google Scholar]; Natl Vital Stat Rep. 2010;58:1–135. [Google Scholar]
- 3.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8(2):228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Laflamme MA. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 7.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. P Natl Acad Sci USA. 2012;109(27):E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willems E, Spiering S, Davidovics H, Lanier M, Xia Z, Dawson M, et al. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell–derived mesoderm / novelty and significance. Circ Res. 2011;109(4):360–364. doi: 10.1161/CIRCRESAHA.111.249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111(9):1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, et al. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol-Heart C. 2003;285(6):H2355–H2363. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Teran MA, Hurle JM. Myocardial fiber architecture of the human heart ventricles. Anat Rec. 1982;204:137–147. doi: 10.1002/ar.1092040207. [DOI] [PubMed] [Google Scholar]
- 12.Binah O, Dolnikov K, Sadan O, Shilkrut M, Zeevi-Levin N, Amit M, et al. Functional and developmental properties of human embryonic stem cells–derived cardiomyocytes. J Electrocardiol. 2007;40(6, Supplement 1):S192–S196. doi: 10.1016/j.jelectrocard.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22(14):1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bird SD, Doevendans PA, van Rooijen MA, Brutel de la Riviere A, Hassink RJ, Passier R, et al. The human adult cardiomyocyte phenotype. Cardiovasc Res. 2003;58(2):423–434. doi: 10.1016/s0008-6363(03)00253-0. [DOI] [PubMed] [Google Scholar]
- 15.Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31(5):829–837. doi: 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Xiao Y, Hsieh A, Thavandiran N, Radisic M. Micro- and nanotechnology in cardiovascular tissue engineering. Nanotechnology. 2011;22(49):494003. doi: 10.1088/0957-4484/22/49/494003. [DOI] [PubMed] [Google Scholar]
- 17.Falconnet D, Csucs G, Grandin HM, Textor M. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials. 2006;27(16):3044–3063. doi: 10.1016/j.biomaterials.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20(6):573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 19.Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem-Int Edit. 2009;48(30):5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehnert D, Wehrle-Haller B, David C, Weiland U, Ballestrem C, Imhof BA, Bastmeyer M. Cell behaviour on micropatterned substrata: limits of extracellular matrix geometry for spreading and adhesion. J Cell Sci. 2004;117(1):41–52. doi: 10.1242/jcs.00836. [DOI] [PubMed] [Google Scholar]
- 21.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002;16(10):1195–1204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- 22.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. P Natl Acad Sci USA. 2002;99(4):1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. P Natl Acad Sci USA. 2010;107(11):4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaji H, Takii Y, Nishizawa M, Matsue T. Pharmacological characterization of micropatterned cardiac myocytes. Biomaterials. 2003;24(23):4239–4244. doi: 10.1016/s0142-9612(03)00275-8. [DOI] [PubMed] [Google Scholar]
- 26.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 27.Cimetta E, Pizzato S, Bollini S, Serena E, Coppi P, Elvassore N. Production of arrays of cardiac and skeletal muscle myofibers by micropatterning techniques on a soft substrate. Biomed Microdevices. 2009;11(2):389–400. doi: 10.1007/s10544-008-9245-9. [DOI] [PubMed] [Google Scholar]
- 28.McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE, et al. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J Biomed Mater Res. 2002;60(3):472–479. doi: 10.1002/jbm.1292. [DOI] [PubMed] [Google Scholar]
- 29.Feinberg AW, Alford PW, Jin H, Ripplinger CM, Werdich AA, Sheehy SP, et al. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials. 2012;33(23):5732–5741. doi: 10.1016/j.biomaterials.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskel. 2008;65(8):641–651. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chopra A, Patel A, Shieh AC, Janmey PA, Kresh JY. α-Catenin localization and sarcomere self-organization on n-cadherin adhesive patterns are myocyte contractility driven. PLoS ONE. 2012;7(10):e47592. doi: 10.1371/journal.pone.0047592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motlagh D, Hartman TJ, Desai TA, Russell B. Microfabricated grooves recapitulate neonatal myocyte connexin43 and N-cadherin expression and localization. J Biomed Mater Res A. 2003;67A(1):148–157. doi: 10.1002/jbm.a.10083. [DOI] [PubMed] [Google Scholar]
- 33.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, et al. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. P Natl Acad Sci USA. 2009;107(2):565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badie N, Satterwhite L, Bursac N. A method to replicate the microstructure of heart tissue in vitro using DTMRI-based cell micropatterning. Ann Biomed Eng. 2009;37(12):2510–2521. doi: 10.1007/s10439-009-9815-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34(23):5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serena E, Cimetta E, Zatti S, Zaglia T, Zagallo M, Keller G, et al. Micro-arrayed human embryonic stem cells-derived cardiomyocytes for in vitro functional assay. PLoS ONE. 2012;7(11):e48483. doi: 10.1371/journal.pone.0048483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. P Natl Acad Sci USA. 1997;94(3):849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quach NL, Rando TA. Focal adhesion kinase is essential for costamerogenesis in cultured skeletal muscle cells. Dev Biol. 2006;293(1):38–52. doi: 10.1016/j.ydbio.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 40.Ervasti JM. Costameres: the Achilles’ heel of herculean muscle. J Biol Chem. 2003;278(16):13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- 41.Bezakova G, Lømo T. Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (ACHR) aggregates in skeletal muscle fibers. J Cell Biol. 2001;153(7):1453–1464. doi: 10.1083/jcb.153.7.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharp WW, Simpson DG, Borg TK, Samarel AM, Terracio L. Mechanical forces regulate focal adhesion and costamere assembly in cardiac myocytes. Am J Physiol-Heart C. 1997;273(2):H546–H556. doi: 10.1152/ajpheart.1997.273.2.H546. [DOI] [PubMed] [Google Scholar]
- 43.Noorman M, van der Heyden MA, van Veen TA, Cox MG, Hauer RN, de Bakker JM, et al. Cardiac cell–cell junctions in health and disease: electrical versus mechanical coupling. J Mol Cell Cardiol. 2009;47(1):23–31. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Rampazzo A, Nava A, Malacrida S, Beffagna G, Bauce B, Rossi V, et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002;71:1200–1206. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beauchamp P, Desplantez T, McCain ML, Li W, Asimaki A, Rigoli G, et al. Electrical coupling and propagation in engineered ventricular myocardium with heterogeneous expression of connexin43. Circ Res. 2012;110(11):1445–1453. doi: 10.1161/CIRCRESAHA.111.259705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, et al. Highly elastic micropatterned hydrogel for engineering functional cardiac tissue. Adv Funct Mater. 2013;23(39):4950–4959. doi: 10.1002/adfm.201300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azarin SM, Lian X, Larson EA, Popelka HM, de Pablo JJ, Palecek SP. Modulation of Wnt/β-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials. 2012;33(7):2041–2049. doi: 10.1016/j.biomaterials.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 49.Bursac N. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91(12):45e–54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- 50.Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, et al. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2008;3(10):e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiu LLY, Janic K, Radisic M. Engineering of oriented myocardium on three-dimensional micropatterned collagen-chitosan hydrogel. Int J Artif Organs. 2012;35(4):237–250. doi: 10.5301/ijao.5000084. [DOI] [PubMed] [Google Scholar]
- 52.Dalby MJ, Riehle MO, Sutherland DS, Agheli H, Curtis ASG. Use of nanotopography to study mechanotransduction in fibroblasts – methods and perspectives. Eur J Cell Biol. 2004;83(4):159–169. doi: 10.1078/0171-9335-00369. [DOI] [PubMed] [Google Scholar]
- 53.Deutsch J, Motlagh D, Russell B, Desai TA. Fabrication of microtextured membranes for cardiac myocyte attachment and orientation. J Biomed Mater Res. 2000;53(3):267–275. doi: 10.1002/(sici)1097-4636(2000)53:3<267::aid-jbm12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 54.Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 209;326(5951):426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7(12):1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Entcheva E, Bien H. Tension development and nuclear eccentricity in topographically controlled cardiac syncytium. Biomed Microdevices. 2003;5(2):163–168. [Google Scholar]
- 57.Grosberg A, Kuo PL, Guo CL, Geisse NA, Bray MA, Adams WJ, et al. Self-organization of muscle cell structure and function. PLoS Comput Biol. 2011;7(2):e1001088. doi: 10.1371/journal.pcbi.1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim JY, Donahue HJ. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13(8):1879–1891. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 59.McDevitt TC, Woodhouse KA, Hauschka SD, Murry CE, Stayton PS. Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair. J Biomed Mater Res A. 2003;66A(3):586–595. doi: 10.1002/jbm.a.10504. [DOI] [PubMed] [Google Scholar]
- 60.Motlagh D, Senyo SE, Desai TA, Russell B. Microtextured substrata alter gene expression, protein localization and the shape of cardiac myocytes. Biomaterials. 2003;24(14):2463–2476. doi: 10.1016/s0142-9612(02)00644-0. [DOI] [PubMed] [Google Scholar]
- 61.Orlova Y, Magome N, Liu L, Chen Y, Agladze K. Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials. 2011;32(24):5615–5624. doi: 10.1016/j.biomaterials.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 62.Parrag IC, Zandstra PW, Woodhouse KA. Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol Bioeng. 2012;109(3):813–822. doi: 10.1002/bit.23353. [DOI] [PubMed] [Google Scholar]
- 63.Sasaki D, Shimizu T, Masuda S, Kobayashi J, Itoga K, Tsuda Y, et al. Mass preparation of size-controlled mouse embryonic stem cell aggregates and induction of cardiac differentiation by cell patterning method. Biomaterials. 2009;30(26):4384–4389. doi: 10.1016/j.biomaterials.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Singhvi R, Stephanopoulos G, Wang DI. Effects of substratum morphology on cell physiology. Biotechnol Bioeng. 1994;43(8):764–771. doi: 10.1002/bit.260430811. [DOI] [PubMed] [Google Scholar]