Abstract
Objective
We examined the short-term and long-term associations of serum albumin with mortality and cardiovascular disease among HIV-infected veterans.
Design
Retrospective cohort analysis using a national database of US veterans with HIV infection.
Methods
This analysis evaluated all HIV-infected veterans in the Department of Veterans Affairs HIV Clinical Case Registry (CCR), a national database consisting of demographic, clinical, laboratory, pharmaceutical, and viral status data. There were 25 522 patients enrolled between 1986 and 2007. We evaluated the associations of baseline and time-updated serum albumin levels with all-cause mortality, atherosclerotic cardiovascular disease, and heart failure by multivariate proportional hazards models.
Results
Over 21 years, there were 10 869 deaths; the cumulative mortality was 73.2 per 1000 person-years. After multivariate adjustment for covariates measured at baseline, the lowest category of serum albumin (<2.5 g/dl) was associated with a higher mortality risk compared with the highest category (>4 g/dl; hazard ratio 3.00; 2.67–3.37). When analyzed as a time-dependent model, the association strengthened substantially (15.1; 14.0–16.4). Findings were similar for atherosclerotic cardiovascular disease and heart failure. We stratified the baseline mortality model by year of follow-up and found that albumin was more strongly associated with deaths that occurred within 1 year of baseline (9.29; 7.85–11.0) than in the second (1.66; 1.18–2.33) or third (1.22; 0.77–1.96) year after measurement.
Conclusion
Among ambulatory HIV-infected patients, lower serum albumin levels are strongly predictive of mortality risk, particularly within 1 year.
Keywords: albumin, cardiovascular disease, end-stage renal disease, glomerular filtration rate, kidney
Introduction
In HIV-infected individuals, researchers have identified a constellation of diseases appearing much earlier in life than expected, including atherosclerotic cardiovascular disease (CVD) and heart failure [1,2]. Albumin is a negative acute phase response protein and marker of inflammation [3]. Lower levels of serum albumin have been associated with higher rates of atherosclerotic CVD, heart failure, and mortality in elderly noninfected persons [4,5]. Because HIV is characterized by systemic inflammation, serum albumin might have prognostic value within the HIV-infected population. Although a few studies have evaluated associations of serum albumin with mortality in the HIV-infected population [6–9], none, to our knowledge, has examined atherosclerotic CVD or heart failure. In this study, we investigated the hypothesis that low levels of serum albumin would independently predict increased risk of mortality, atherosclerotic CVD, and heart failure in a national registry of HIV-infected individuals. Moreover, we hypothesized that serum albumin would have greater value for predicting near-term adverse outcomes relative to long-term adverse outcomes.
Methods
Data sources
In this retrospective cohort analysis, we studied HIV-infected veterans in the Department of Veterans Affairs HIV Clinical Case Registry (CCR), a national database consisting of demographic, clinical, laboratory, pharmaceutical, and vital status data compiled from the Veterans Affairs electronic medical record [10]. In order to supplement demographic and clinical data, we linked the CRR data to the Veterans Affairs National Patient Care Database, the Veterans Affairs Beneficiary Identification and Records Locator Subsystem (BIRLS) Death File, and Medicare claims data.
Study participants
We included all HIV-infected patients in the Veterans Health Administration who had measurements of outpatient serum albumin, serum creatinine, and a urine dipstick between 1986 and 2007. Participants were entered into the cohort at the time of their first outpatient serum albumin measure following initial documentation of HIV infection. A total of 25 522 participants were included in our analysis.
Outcomes
The three primary outcomes were as follows: time from study entry to death; to incident atherosclerotic CVD; and to hospitalization for heart failure (Supplemental description in the appendix, http://links.lww.com/QAD/A310). Individuals were analyzed from the time of their first outpatient serum albumin measure until they were censored either by an outcome event or by the end of follow-up – 31 January 2007.
Primary predictor
Our primary predictor was baseline serum albumin, defined as an average of the first and second measurements after the initial diagnosis of HIV. We categorized serum albumin as follows: ≥4.0, 3.5–3.9, 3.0–3.4, 2.5–2.9, and less than 2.5 g/dl. For the outcomes of atherosclerotic CVD and heart failure, we combined the two lowest categories as less than 3.0 g/dl, due to small numbers of events occurring in the less than 2.5 g/dl category.
Covariates
The analysis accounted for demographic characteristics, including age, race, and sex. HIV-related prognostic indicators included CD4 cell count and viral load. Glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease (MDRD) equation. Urine dipstick results were used to quantify proteinuria. Comorbidities included BMI in kg/m2, hypertension, diabetes, dyslipidemia, hepatitis C (HCV), hepatitis B (HBV), liver disease, and smoking. Antiretroviral therapy (ART) exposure, either any or none, was an additional covariate.
Statistical analyses
For longitudinal analyses, we constructed three models. First, baseline models utilized the index value of albumin and all other covariates. We also conducted a sensitivity analysis, restricting the baseline analysis to exclude those with either kidney or liver dysfunction. Second, we conducted time-updated analyses, which updated albumin and other covariates each time they were measured, and carried the last measurement forward until the participant was censored. In these analyses, we adjusted for the recentness and frequency of albumin measurements as separate indicator variables. Third, for the mortality outcome only, we repeated the baseline model with stratification on the follow-up interval: year 1, year 2, and year 3.
We used multivariable Cox proportional hazards models to estimate the association of serum albumin levels with time to event. Tests for the proportional hazards assumption were based on the Schoenfeld residuals. We also repeated these analyses separately for the periods before and after 1996, when HAART was widely implemented in Veterans Affairs care. In order to discriminate between incident atherosclerotic CVD and heart failure, we conducted a sensitivity analysis using the competing-risks regression according to the method of Fine and Gray (1999). Inclusion of covariates in the multivariable-adjusted Cox model was based on significant univariate associations (P < 0.05) between each candidate covariate and the outcome. We determined serum albumin’s risk discrimination by the C-statistic, and compared its prognostic strength with other clinical risk factors. Analyses were conducted using Stata software version 11.0 (Stata Corp., College Station, Texas, USA).
Results
Individuals with lower baseline albumin levels were older on average and more likely to be black. Those with lower levels of albumin also had lower CD4 cell counts, higher viral load, lower estimated GFR, lower BMI, a lower prevalence of dyslipidemia, a higher prevalence of proteinuria, HCV, HBV, and liver disease, and less exposure to ART. The overall median number of albumin measures was 8 (IQR 3–18), including 10 (4–22) measures among survivors, and six (3–14) among those who died during follow-up. There were 10 689 deaths, 1083 atherosclerotic cardiovascular events, and 435 cases of heart failure. There were 347 cerebrovascular events, 652 myocardial infarctions, and 174 documented cases of peripheral vascular disease.
After multivariable adjustment for covariates measured at baseline (Table 1 Baseline), the lowest category of baseline albumin was associated with a three-fold risk of mortality (<2.5 vs. >4 g/dl), no risk of composite CVD events (hazard ratio = 0.99), and a 1.5-fold risk of congestive heart failure. In contrast, in the time-dependent model (Table 1; time-dependent covariate, TDC), the lowest albumin category had approximately a 15-fold risk for mortality, a three-fold risk for atherosclerotic cardiovascular events, and a 12-fold risk for heart failure. Similar results were seen for individuals enrolled in both the pre and post-HAART eras (Supplemental Digital Content 1 & 2, http://links.lww.com/QAD/A310). In the competing-risks analysis, associations remained similar across albumin categories (Supplementary Digital Content 3, http://links.lww.com/QAD/A310).
Table 1.
Association of serum albumin with mortality, atherosclerotic cardiovascular disease, and heart failure, 1986–2007.
| Hazard ratio (95% confidence interval) |
|||||
|---|---|---|---|---|---|
| Level of albumin (g/dl) | ≥4.0 | 3.5–3.9 | 3.0–3.4 | 2.5–2.9 | <2.5 |
| Mortality (n=10 689) | |||||
| Baseline albumin | |||||
| Demographic-adjusted | 1.0 (Reference) | 1.45 (1.38–1.52) | 2.03 (1.92–2.15) | 3.04 (2.87–3.22) | 4.49 (4.02–5.02) |
| Multivariable-adjusted | 1.0 (Reference) | 1.34 (1.27–1.40) | 1.68 (1.58–1.78) | 2.27 (2.14–2.42) | 3.00 (2.67–3.37) |
| Time-updated albumin | |||||
| Demographic-adjusted | 1.0 (Reference) | 1.93 (1.80–2.06) | 4.84 (4.52–5.17) | 12.8 (12.0–13.6) | 33.5 (31.2–36.1) |
| Multivariable-adjusted | 1.0 (Reference) | 1.65 (1.54–1.77) | 3.37 (3.15–3.61) | 7.02 (6.58–7.50) | 15.1 (14.0–16.4) |
| Composite atherosclerotic cardiovascular event (n=1083) | |||||
| Baseline albumin | |||||
| Demographic-adjusted | 1.0 (Reference) | 1.05 (0.91–1.21) | 1.13 (0.94–1.36) | 1.26 (1.01–1.56) | – |
| Multivariable-adjusted | 1.0 (Reference) | 1.01 (0.88–1.17) | 0.97 (0.80–1.18) | 0.99 (0.79–1.25) | – |
| Time-updated albumin | |||||
| Demographic-adjusted | 1.0 (Reference) | 1.34 (1.15–1.56) | 2.46 (2.07–2.91) | 3.47 (2.89–4.18) | – |
| Multivariable-adjusted | 1.0 (Reference) | 1.35 (1.16–1.58) | 2.36 (1.98–2.82) | 3.15 (2.58–3.86) | – |
| Heart failure (n=435) | |||||
| Baseline albumin | |||||
| Demographic-adjusted | 1.0 (Reference) | 1.22 (0.96–1.55) | 1.92 (1.46–2.51) | 2.40 (1.79–3.22) | – |
| Multivariable-adjusted | 1.0 (Reference) | 1.13 (0.89–1.43) | 1.45 (1.09–1.93) | 1.53 (1.10–2.11) | – |
| Time-updated albumin | |||||
| Demographic-adjusted | 1.0 (Reference) | 3.01 (2.16–4.20) | 7.91 (5.66–11.1) | 19.3 (13.9–26.6) | – |
| Multivariable-adjusted | 1.0 (Reference) | 2.75 (1.97–3.84) | 6.07 (4.31–8.55) | 11.7 (8.3–16.5) | – |
Demographic-adjusted models are adjusted for age, sex, and race; multivariable-adjusted Cox models are adjusted for factors with a P value less than 0.05 in univariate analysis. All covariates are baseline measures. For mortality, multivariate-adjusted model includes the following covariates: serum albumin, age, race, sex, estimated glomerular filtration rate (eGFR), urine dip, CD4 cell count, viral load, antiretroviral therapy, BMI, hypertension, diabetes, hepatitis C, hepatitis B, liver disease, and smoking (only dyslipidemia was not entered). For atherosclerotic cardiovascular events, multivariate-adjusted model includes the following covariates: serum albumin, age, race, sex, eGFR, urine dip, viral load, BMI, hypertension, diabetes, dyslipidemia, and smoking (antiretroviral therapy, hepatitis C, hepatitis B, liver disease, and CD4 cell count are excluded). For congestive heart failure event, multivariate-adjusted model includes the following covariates: serum albumin, age, race, sex, eGFR, urine dip, CD4 cell count, BMI, hypertension, diabetes, dyslipidemia, and smoking (antiretroviral therapy, hepatitis C, hepatitis B, liver disease, and viral load are excluded).
When sensitivity analyses were conducted for the baseline model, excluding individuals with either liver or kidney dysfunction, the hazard ratio remained similar for comparisons of less than 2.5 vs. more than 4 g/dl (hazard ratio 2.6, 2.0–3.5). Furthermore, in the time-dependent model of mortality, the hazard ratio for the less than 2.5 g/dl category was 15.5 (13.8–17.5).
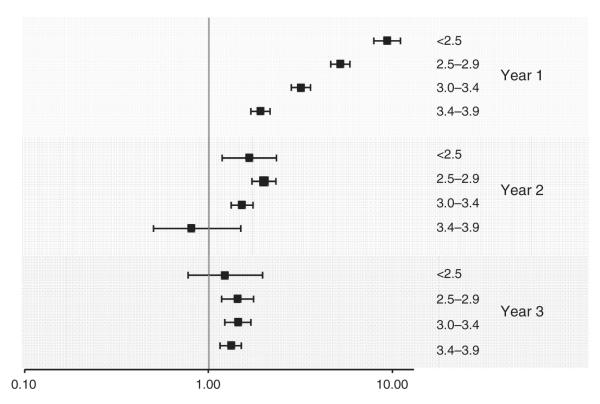
We stratified the baseline mortality model by year of follow-up (Fig. 1). The baseline albumin appeared strongly associated with deaths that occurred within 1 year (hazard ratio = 9.29 for <2.5 vs. >4 g/dl), but showed substantially weaker associations subsequently. For risk discrimination of 1-year mortality, serum albumin alone had a C-statistic of 0.74. The clinical model without albumin had a C-statistic of 0.83 (P < 0.0001), which improved to 0.86 with the addition of albumin to the model.
Fig. 1. Baseline serum albumin associations with mortality stratified by year of follow-up.
Association of baseline serum albumin categories (<2.5, 2.5–2.9, 3.0–3.4, and 3.5–3.9 g/dl) with mortality risk, stratified by year of follow-up, in multivariate-adjusted proportional hazards models.
Discussion
We found albumin to be a remarkably strong, independent predictor of 1-year mortality, atherosclerotic CVD, and heart failure. The associations of serum albumin with mortality weakened substantially after the first year of measurement. Time-dependent models of albumin demonstrated that the lowest levels of serum albumin were associated with a 15-fold risk for mortality, a three-fold risk for atherosclerotic cardiovascular events, and a 12-fold risk for heart failure. These findings were consistent in both the pre and post-HAART eras. For 1-year mortality, serum albumin was the single strongest predictor, having an individual C-statistic of 0.72, and significantly increasing the overall multivariate model from 0.83 to 0.86.
Previous smaller studies of serum albumin in HIV-infected populations have reported similar findings [9,11–13]. Feldman et al. [6,7] from the Women’s Interagency HIV Study (WIHS) reported a relative hazard of 3.1 for baseline measurements of less than 3.5 g/dl compared to concentrations greater than 4.2 g/dl, and a relative hazard of 13 for the same categories in a time-updated model. However, none of these reports spanned the pre and post-HAART eras; and none, to our knowledge, have examined serum albumin’s association with atherosclerotic cardiovascular events and heart failure.
The underlying mechanisms remain unclear. To our knowledge, there is no direct physiologic link between serum albumin and the adverse outcomes explored herein. Causes of decreased serum albumin levels are multifactorial, and include poor nutrition (decreased synthesis), liver disease (decreased production), renal disease (wasting of serum albumin in the urine), and chronic inflammation (decreased production or increased destruction). Albumin remained a strong predictor of mortality after adjusting for multiple factors. Moreover, we found in a sensitivity analysis that, when individuals with liver and kidney dysfunction were excluded, the association remained nearly identical for baseline and time-updated models. This suggests that a more transient process such as inflammation is responsible for the lower levels of albumin. HIV infection is associated with higher levels of IL-6 and high-sensitivity C-reactive protein, which also predict mortality [14,15], but neither was available in this clinical registry. It seems likely that serum albumin captures a dynamic process of inflammation in HIV infection that has clinical importance in the short-term.
Researchers have identified a number of ‘non-HIV’ markers for inclusion in a prognostic survival index in HIV infection [16]. These indices have been shown to improve discrimination of mortality within the HIV-infected population. Serum albumin could potentially add to such an index, particularly as a possible ‘early warning’ for short-term morbidity and mortality.
Our study has the following strengths. We had a large sample for our analyses, spanning a 20-year period. There were multiple measures of serum albumin for each participant, allowing time-dependent analyses and extensive covariate evaluation. Ours is also the first study, to our knowledge, to use serum albumin as a prognostic marker for atherosclerotic CVD and heart failure in HIV-infected individuals, both from baseline and in a time-updated model.
Our study also had several important limitations. First, we cannot fully account for the potential confounding effects of serum albumin measurement on our findings, as this testing may be informative about prognosis independent of the albumin concentration itself. We attempted to account for this by adjusting for the frequency and recentness of albumin measures; in fact, we found that the median number of albumin measures was greater among survivors than nonsurvivors. Second, we cannot determine the mechanisms for the association between albumin and adverse outcomes; although we hypothesize that inflammation is a likely pathway, inflammation markers are not part of routine clinical care. Third, we found a lower prevalence of smoking than has been reported in other HIV-infected populations; this raises the possibility that rates of smoking were underreported in our cohort. Fourth, given the dependency on diagnostic codes, we cannot be certain that we capture all cardiovascular events; however, we do not have reason to suspect that the albumin associations would be biased by the event capture. Fifth, our results may not be generalizable to populations poorly represented in our analysis.
Conclusion
In summary, we found that lower levels of serum albumin predict mortality, atherosclerotic cardiovascular events, and heart failure, particularly within 1 year. These findings suggest the possibility that serum albumin reflects a state of inflammation in HIV-infected individuals similar to the elderly population. Serum albumin measurements could potentially improve the prediction of short-term adverse outcomes in HIV-infected individuals.
Supplementary Material
Acknowledgements
J.L. contributed to study concept and design, and drafting of the article; R.S. contributed to analysis and interpretation of the data, and editing of the article; C.C.W. contributed to editing and formatting of the article; P.C.T. contributed to analysis and interpretation of the data, and editing of the article; C.G. contributed to analysis and interpretation of the data, and editing of the article; and M.G.S. contributed to study concept and design, analysis and interpretation of the data, and drafting of the article.
Sources of funding: 1R03AG034871–01: ‘Kidney Disease, Antiretroviral Therapy and Cardiovascular Events in HIV-Infection’ (principal investigator, M.G.S.), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, CA, USA.
1 R01 AG034853–01A2: ‘The Aging Kidney in HIV-Infection: biomarkers for early detection of injury’ (principal investigator, M.G.S.), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, CA, USA.
Footnotes
Conflicts of interest There are no conflicts of interest.
References
- 1.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–743. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 4.Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50:693–703. doi: 10.1016/s0895-4356(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 5.Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;2:1434–1436. doi: 10.1016/s0140-6736(89)92042-4. [DOI] [PubMed] [Google Scholar]
- 6.Feldman JG, Burns DN, Gange SJ, Bacchetti P, Cohen M, Anastos K, et al. Serum albumin as a predictor of survival in HIV-infected women in the Women’s Interagency HIV study. AIDS. 2000;14:863–870. doi: 10.1097/00002030-200005050-00013. [DOI] [PubMed] [Google Scholar]
- 7.Feldman JG, Gange SJ, Bacchetti P, Cohen M, Young M, Squires KE, et al. Serum albumin is a powerful predictor of survival among HIV-1-infected women. J Acquir Immune Defic Syndr. 2003;33:66–73. doi: 10.1097/00126334-200305010-00010. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez RA, Mendelson M, O’Hare AM, Hsu LC, Schoenfeld P. Determinants of survival among HIV-infected chronic dialysis patients. J Am Soc Nephrol. 2003;14:1307–1313. doi: 10.1097/01.asn.0000062963.56513.28. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SH, Astemborski J, Sterling TR, Thomas DL, Vlahov D. Serum albumin as a prognostic indicator for HIV disease progression. AIDS Res Hum Retroviruses. 2006;22:14–21. doi: 10.1089/aid.2006.22.14. [DOI] [PubMed] [Google Scholar]
- 10.Backus LI, Gavrilov S, Loomis TP, Halloran JP, Phillips BR, Belperio PS, Mole LA. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16:775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dao CN, Peters PJ, Kiarie JN, Zulu I, Muiruri P, Ong’ech J, et al. Hyponatremia, hypochloremia, and hypoalbuminemia predict an increased risk of mortality during the first year of anti-retroviral therapy among HIV-infected Zambian and Kenyan women. AIDS Res Hum Retroviruses. 2011;27:1149–1155. doi: 10.1089/AID.2010.0345. [DOI] [PubMed] [Google Scholar]
- 12.Shah S, Smith CJ, Lampe F, Youle M, Johnson MA, Phillips AN, Sabin CA. Haemoglobin and albumin as markers of HIV disease progression in the highly active antiretroviral therapy era: relationships with gender. HIV Med. 2007;8:38–45. doi: 10.1111/j.1468-1293.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 13.Sabin CA, Griffioen A, Yee TT, Emery VC, Herrero-Martinez E, Phillips AN, Lee CA. Markers of HIV-1 disease progression in individuals with haemophilia coinfected with hepatitis C virus: a longitudinal study. Lancet. 2002;360:1546–1551. doi: 10.1016/S0140-6736(02)11519-4. [DOI] [PubMed] [Google Scholar]
- 14.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger W, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 15.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justice AC, McGinnis KA, Skanderson M, Chang CC, Gilbert CL, Goetz MB, et al. Towards a combined prognostic index for survival in HIV infection: the role of ’non-HIV’ biomarkers. HIV Med. 2009;11:143–151. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.